Reactivity of germanones: far removed from ketones – a computational study†
Abstract
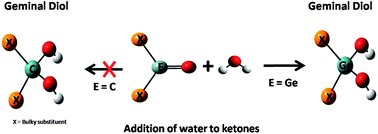
The recently isolated heavy ketone (X2E![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O; E = Ge) shows interesting reactivity towards simple addition reactions that are unknown for the organic (E
O; E = Ge) shows interesting reactivity towards simple addition reactions that are unknown for the organic (E![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) ketones. Density Functional Theory (DFT) calculations elucidate the role of the central atom and the substituent effects on the addition of acetone, carbon dioxide and water to formaldehyde, acetone, 2,4-dimethyl-3-pentanone, diphenyl ketone and their heavier (Si, Ge) analogs. NBO analysis reveals a large charge separation for heavier analogs, thereby increasing their reactivity. The activation barriers are reduced by as much as ∼30.0 to 40.0 kcal mol−1 for the nucleophilic addition to the heavy ketones as (E = Si and Ge) compared to those of an organic ketone. Comparably lower stability of the reactants and higher stability of the transition state lowers the barriers of addition reactions for the heavier ketones. Apart from a smaller barrier, the nucleophilic addition reactions which are unfavorable for ketones are favored by 20–40 kcal mol−1 for their silicon and germanium counterparts. Calculations predict that similar addition reactions must be feasible for silanones (X2Si
C) ketones. Density Functional Theory (DFT) calculations elucidate the role of the central atom and the substituent effects on the addition of acetone, carbon dioxide and water to formaldehyde, acetone, 2,4-dimethyl-3-pentanone, diphenyl ketone and their heavier (Si, Ge) analogs. NBO analysis reveals a large charge separation for heavier analogs, thereby increasing their reactivity. The activation barriers are reduced by as much as ∼30.0 to 40.0 kcal mol−1 for the nucleophilic addition to the heavy ketones as (E = Si and Ge) compared to those of an organic ketone. Comparably lower stability of the reactants and higher stability of the transition state lowers the barriers of addition reactions for the heavier ketones. Apart from a smaller barrier, the nucleophilic addition reactions which are unfavorable for ketones are favored by 20–40 kcal mol−1 for their silicon and germanium counterparts. Calculations predict that similar addition reactions must be feasible for silanones (X2Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) as well.
O) as well.

 Please wait while we load your content...
Please wait while we load your content...