Enantioselective reduction of sparingly water-soluble ketones: continuous process and recycle of the aqueous buffer system†
Abstract
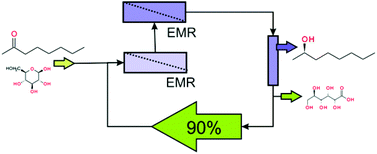
Low solubility of starting materials and products in
* Corresponding authors
a
DECHEMA-Forschungsinstitut, Theodor-Heuss-Allee 25, 60486 Frankfurt am Main, Germany
E-mail:
greiner@dechema.de
Fax: +49 69 7564 388
Tel: +49 69 7564 428
b Johnson Matthey X-Zyme, Merowingerplatz 1a, 40225 Düsseldorf, Germany
c Institut für Technische und Makromolekulare Chemie, RWTH Aachen University, Worringerweg 1, 52074 Aachen, Germany
Low solubility of starting materials and products in

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
S. Leuchs, S. Na'amnieh and L. Greiner, Green Chem., 2013, 15, 167 DOI: 10.1039/C2GC36558H
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
