Novel bisimidazolium pincers as low loading ligands for in situ palladium-catalyzed Suzuki–Miyaura reaction in the ambient atmosphere†
Abstract
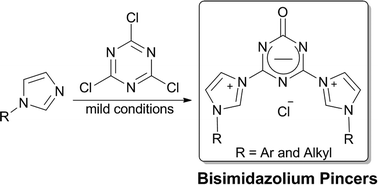
A series of novel triazinonide-bridged bisimidazolium pincers were easily synthesized by quaternization of functionalized N-phenylimidazoles with highly reactive cyanuric chloride under mild conditions. The pincer 3c was proven to be a very efficient ligand for in situ Pd-catalyzed Suzuki–Miyaura reaction with ppm-level catalyst loading.

 Please wait while we load your content...
Please wait while we load your content...