A tertiary amine catalyzed carbocyclization sequence to furnish spirocyclo hexene systems having vicinal quaternary stereocenters†
Abstract
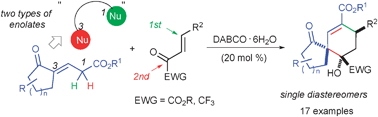
Two types of enolates can be formed stepwise from enolisable 1,3-dicarbonyl-substituted propene systems in the presence of catalytic amounts of

 Please wait while we load your content...
Please wait while we load your content...