Copolymer-supported heterogeneous organocatalyst for asymmetric aldol addition in aqueous medium†
Abstract
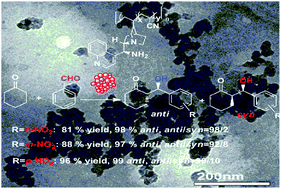
In the current study, a convenient and simple way is presented to synthesize a novel type of supported heterogeneous organocatalyst in 21–81% yield by the copolymerization of 9-amino-9-deoxy-epi-cinchonine organocatalyst with acrylonitrile using AIBN as radical initiator. The chemical compositions (x/y) and weight-average molecular weights of copolymers 1a–d were determined by 1H NMR and GPC analysis respectively. Their porous and layered structure, and surface morphology were characterized by nitrogen adsorption–desorption, XRD and TEM. In the asymmetric aldol addition of p-nitrobenzaldehyde to cyclohexanone and 1-hydroxy-2-propanone in water, all the supported organocatalysts 1a–d afforded excellent isolated yields (90.2–94.7%) and stereoselectivities (96.8–97.8%ee anti, anti/syn = 91/9). The highest catalytic property (96% yield, anti/syn = 90/10 and 99%ee anti) in water as the sole solvent was achieved under the optimized conditions. Compared with cyclohexanone, cyclopentanone and acetone showed the less desired enantioselectivities in the same aldol reactions. At the end of the aldol reaction, the copolymer-supported organocatalyst 1a was readily recovered in 95–98% yield from reaction mixture by simple filtration using an organic membrane. Even in the fifth run, there was no significant loss in catalytic activity and stereocontrol (94.3% yield, 97.2%ee anti, anti/syn = 90/10). After continuous reuse five times, there was some drop in catalytic activity and stereoselectivity.
 Please wait while we load your content...
Please wait while we load your content...