Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow†‡
Abstract
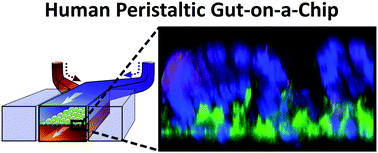
Development of an in vitro living cell-based model of the intestine that mimics the mechanical, structural, absorptive, transport and pathophysiological properties of the human gut along with its crucial microbial symbionts could accelerate pharmaceutical development, and potentially replace animal testing. Here, we describe a biomimetic ‘human gut-on-a-chip’ microdevice composed of two microfluidic channels separated by a porous flexible membrane coated with extracellular matrix (ECM) and lined by human intestinal epithelial (Caco-2) cells that mimics the complex structure and physiology of living intestine. The gut microenvironment is recreated by flowing fluid at a low rate (30 μL h−1) producing low shear stress (0.02 dyne cm−2) over the microchannels, and by exerting cyclic strain (10%; 0.15 Hz) that mimics physiological peristaltic motions. Under these conditions, a columnar epithelium develops that polarizes rapidly, spontaneously grows into folds that recapitulate the structure of intestinal villi, and forms a high integrity barrier to small molecules that better mimics whole intestine than cells in cultured in static Transwell models. In addition, a normal intestinal microbe (Lactobacillus rhamnosus GG) can be successfully co-cultured for extended periods (>1 week) on the luminal surface of the cultured epithelium without compromising epithelial cell viability, and this actually improves barrier function as previously observed in humans. Thus, this gut-on-a-chip recapitulates multiple dynamic physical and functional features of human intestine that are critical for its function within a controlled microfluidic environment that is amenable for transport, absorption, and toxicity studies, and hence it should have great value for drug testing as well as development of novel intestinal disease models.
- This article is part of the themed collection: Focus on USA

 Please wait while we load your content...
Please wait while we load your content...