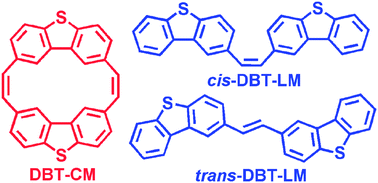
We report the synthesis and characterization of a bis-dibenzothiophene cyclic dimer containing bis-ethylene linkages (DBT-CM) and of the corresponding mono-ethylene-linked ‘linear’ cis- and trans-isomers (ciscisciscis- and transtranstrans-DBT-LM, respectively). The varied molecular architectures lead to notable differences both in terms of the solid-state packing and the molecular electronic and optical properties. X-ray crystallography reveals that the cyclic architecture of DBT-CM leads to a more densely packed stacking configuration that imparts stronger intermolecular electronic coupling for both hole and electron transport amongst adjacent molecules, while characterization of the thin-film morphology and crystallinity uncovers important temperature-dependent properties of the films as a function of the molecular architecture. Moreover, the redox, electronic structure, and optical properties of DBT-CM vary distinctly from those of its linear counterparts. The intramolecular reorganization energies for hole and electron transport for DBT-CM are markedly smaller than the linear counterparts, while the dispersion for the highest valence band (and the intermolecular electronic coupling for hole transport) is the largest for the series. The more favorable molecular packing/morphology characteristics and charge-transport properties (within the Marcus framework) of DBT-CM manifest themselves in thin-film field-effect transistor studies, where a field-effect hole-carrier mobility 0.026 cm2 V−1 s−1 is measured, a value one-order-of-magnitude larger than either linear analog.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...