Direct covalent post-synthetic chemical modification of Cr-MIL-101 using nitrating acid†
Abstract
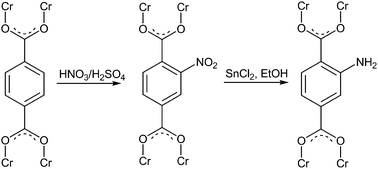
For the first time, functionality has been covalently introduced into the Cr-MIL-101 network by post-synthetic modification of the terephthalate linker molecule through

 Please wait while we load your content...
Please wait while we load your content...