Reaction of perfluorocyclopentene with various carbon nucleophiles—heteroaromatic lithium reagents, enolate and phosphonium ylide
Abstract
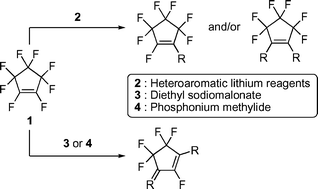
The addition of heteroaromatic lithium reagents 2 to a THF solution of perfluorocyclopentene (1) provided preferentially the corresponding monosubstituted products 5, while the addition of 1 to 2 effectively gave the 1,2-disubstituted products 6 in good to excellent yields. The reaction of 1 with sodiomalonate 3 or phosphonium ylide 4 also proceeded smoothly to form the 1,3-disubstituted product 8 or 10 in high yield, respectively.

 Please wait while we load your content...
Please wait while we load your content...