Anomeric oxygen to carbon rearrangements of alkynyl tributylstannane derivatives of furanyl (γ)- and pyranyl (δ)-lactols
Abstract
Tetrahydropyran and tetrahydrofuran containing natural products, drugs and agrochemicals often possess carbon–carbon bonds adjacent to the heteroatom. Consequently, new methods for the construction of anomeric carbon–carbon bonds are of considerable importance. We have devised a new strategy to access these systems that requires the treatment of O-glycoside alkynyl tributylstannane derivatives of furanyl and pyranyl lactols with Lewis acid to effect oxygen to carbon rearrangements. This leads to the formation of the corresponding carbon linked alkynol products that can be further manipulated to produce key structural motifs and building blocks for the assembly of complex molecules.
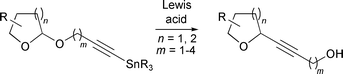

 Please wait while we load your content...
Please wait while we load your content...