Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The development of a novel AND logic based fluorescence probe for the detection of peroxynitrite and GSH†
Adam C.
Sedgwick‡
 *a,
Hai-Hao
Han‡
b,
Jordan E.
Gardiner
*a,
Hai-Hao
Han‡
b,
Jordan E.
Gardiner
 a,
Steven D.
Bull
a,
Steven D.
Bull
 *a,
Xiao-Peng
He
*a,
Xiao-Peng
He
 *b and
Tony D.
James
*b and
Tony D.
James
 *a
*a
aDepartment of Chemistry, University of Bath, Bath, BA2 7AY, UK. E-mail: a.c.sedgwick@bath.ac.uk; s.d.bull@bath.ac.uk; t.d.james@bath.ac.uk
bKey Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, School of Chemistry and Molecular Engineering, East China University of Science and Technology, 130 Meilong Rd., Shanghai 200237, China. E-mail: xphe@ecust.edu.cn
First published on 16th March 2018
Abstract
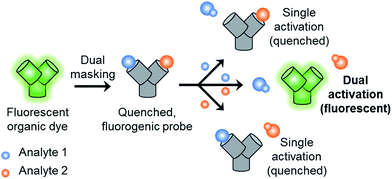
We have developed a novel AND logic based fluorescence probe for the simultaneous detection of ONOO− and GSH (GSH-PF3). The GSH-PF3 probe was synthesised over three steps starting from commercially available fluorescein. The probe was constructed by attaching the GSH reactive motif, 2,4-dinitrobenzenesulfonyl, to the previously reported boronate fluorescence based probe, PF3. GSH-PF3 produced only a small fluorescence response towards the addition of GSH or ONOO− separately. However, when the probe was exposed to both analytes, there was a significant (40-fold) fluorescence enhancement. GSH-PF3 demonstrated an excellent selectivity towards both GSH and ONOO−. In cellular imaging experiments the probe was shown to be cell permeable with no ‘turn-on’ response observed for the addition of either GSH or ONOO− separately. However, in the presence of both analytes, a clear fluorescence response was observed in live cells. GSH-PF3 was further able to monitor the co-existence of metabolically produced ONOO− and GSH by exogenous stimulation.
Peroxynitrite (ONOO−), is a highly reactive nitrogen species that is formed via the diffusion controlled reaction between superoxide anion (O2˙−) and nitric oxide (NO˙).1,2 ONOO− acts as a signalling molecule in vivo for a number of pathways.1,3 However, ONOO− is more commonly known for its deleterious properties, causing irreversible damage to a range of biological targets such as lipids, proteins and DNA.4 Therefore, ONOO− has been implicated as a key pathogenic factor for a number of diseases, which include inflammation, cancer, ischemia-reperfusion and neurodegenerative diseases.5–7 Glutathione (GSH) is a natural tripeptide (γ-L-glutamyl-L-cysteinyl-glycine), that exists in the thiol reduced form (GSH) and disulphide-oxidised (GSSG) form. GSH is the predominant form, existing at millimolar concentrations in most cells.8 However, GSH can be directly oxidised by ONOO− therefore acting as a cellular defence by serving as an ONOO− scavenger. Elevated levels of GSH are common in cells under oxidative stress and the susceptibility of a cell towards ONOO− largely depends on the concentration of intracellular GSH.1,9,10
Therefore, with this work we set out to develop a fluorescence-based probe capable of monitoring the close relationship between ONOO− and GSH. Traditionally, most fluorescence probes require a single analyte to produce a fluorescence response.11–19 However, in recent years a number of fluorescence based probes for dual or multi-analyte detection have been developed.20–27 These types of fluorescence based probes have been used for the construction of molecular logic gates or for medical diagnostics.28 AND logic based fluorescence probes require both analytes to be simultaneously present or work in tandem in order to elicit a fluorescence response. This method has a number of advantages including: being faster than serial measurements for different analytes within the same biological sample and can provide a method for monitoring bimolecular events, which may contribute to a specific disease.20
Currently, only a few reversible fluorescence based probes for the detection of ONOO− and GSH have been developed to monitor the relationship between these analytes.29,30 These include a selenium based fluorescence probe, which is oxidised by ONOO− (turn “on”) and reduced by GSH (turn “off”). The fluorescence of the CyPSe probe is initially quenched by a photoinduced electron transfer (PET) process. The presence of ONOO− results in the oxidation of selenium to CyPSe![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O causing the fluorescence emission to be “turned on”. Then in the presence of biological thiols such as cysteine and GSH, the CyPSe
O causing the fluorescence emission to be “turned on”. Then in the presence of biological thiols such as cysteine and GSH, the CyPSe![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O probe is reduced back to its non-fluorescent selenide form. A similar reversible NIR Tellurium-based fluorescence probe was later developed for monitoring the redox cycles between ONOO− and GSH. This probe was successfully applied for the visualisation of the redox cycles of ONOO− and GSH in live cells and animals.29 The probes developed by Han et al. are turn “on” with ONOO− and “off” with GSH. As far as we are aware, there are currently no AND logic based fluorescence probes for GSH “and” ONOO−.
O probe is reduced back to its non-fluorescent selenide form. A similar reversible NIR Tellurium-based fluorescence probe was later developed for monitoring the redox cycles between ONOO− and GSH. This probe was successfully applied for the visualisation of the redox cycles of ONOO− and GSH in live cells and animals.29 The probes developed by Han et al. are turn “on” with ONOO− and “off” with GSH. As far as we are aware, there are currently no AND logic based fluorescence probes for GSH “and” ONOO−.
With this research, we aimed to develop a GSH “and” ONOO− logic based fluorescence probe (Scheme 1). We identified a suitable mono-boronate fluorescein based fluorescence probe, PF3 that had been previously developed by Chang et. al., shown in Scheme 2. As previously reported the reactivity of boronate based fluorescence probes with ONOO−,12,14,15 are significantly greater than hypochlorite (ClO−) and H2O2.31 Therefore, we anticipated that the attachment of a GSH reactive motif to PF3 would produce a selective GSH-ONOO− AND logic based fluorescence probe, GSH-PF3 (Scheme 2). GSH-PF3 was readily synthesised in three steps. Fluorescein was triflated using N-phenyl bis(trifluoromethanesulfonamide) to afford fluorescein mono-triflate in good yield. Suzuki–Miyaura conditions were then carried out to provide fluorescein mono-boronate, PF3. The 2,4-dinitrobenzenesulfonyl unit was then attached to PF3 using 2,4-dinitrobenzenesulfonyl chloride, CH2Cl2 and NEt3 at 0 °C. Using these conditions GSH-PF3 was prepared in a reasonable yield of 52%.
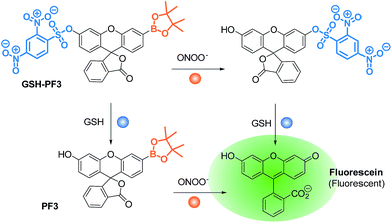 | ||
| Scheme 2 Structure of the GSH-PF3 probe and proposed sensing mechanism for the simultaneous detection of ONOO− and GSH. | ||
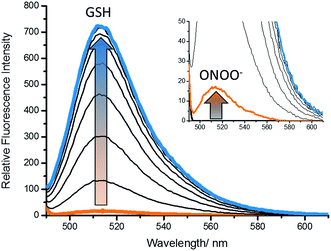
With GSH-PF3 in hand, fluorescence experiments for the detection of GSH and ONOO− were performed. As shown in Fig. 1, GSH-PF3 was initially non-fluorescent and with the addition of ONOO− (10 μM), a small fluorescence increase was observed. However, incremental additions of GSH resulted in a much larger increase in fluorescence intensity (>30-fold see ESI – Fig. S1†), clearly demonstrating the need for the addition of both GSH and ONOO− in order to achieve a full ‘turn-on’ response.
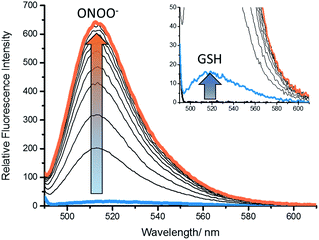
In order to demonstrate that GSH-PF3 required both GSH and ONOO− for a complete ‘turn-on’ response, the fluorescence experiments were performed the other way around. Therefore, an excess of GSH (200 μM) was added to GSH-PF3, and the probe was incubated for 10 min. Remarkably, this only led to a small increase in fluorescence intensity and the subsequent additions of ONOO− resulted in a large fluorescence increase (Fig. 2 and ESI – Fig S2†). These results confirm that the probe requires both GSH and ONOO− for a full fluorescence ‘turn-on’ response.
The selectivity of GSH-PF3 was then evaluated against a series of amino acids (L-cysteine, L-methionine, L-tryptophan, L-serine, L-lysine, L-leucine, L-glutamic acid, L-valine, L-arginine, L-histidine and L-aspartic acid) – see ESI Fig. S3.† Unsurprisingly, the probe responded to the other sulfhydryl containing amino acid cysteine. However, the biological concentrations of cysteine are low in cells.32 More importantly, GSH-PF3 displayed an excellent selectivity against other amino acids including serine, methionine and lysine. The probe demonstrated excellent selectivity for ONOO− over many other ROS including H2O2 (see ESI – Fig. S4†). The excellent selectivity for both GSH and ONOO− allowed us to evaluate GSH-PF3 in cell imaging experiments.
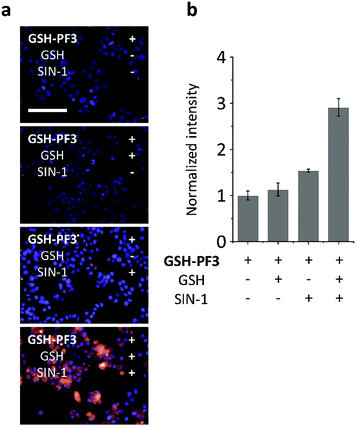
The macrophage cell line – RAW264.7 (ATCC® TIB-71™; obtained from ATCC [American Type Culture Collection]) – was used for cell imaging experiments. The cells were incubated with GSH-PF3, followed by either treatment of SIN-1 (an ONOO− donor)33 to produce intracellular ONOO− or by addition of exogenous GSH. As shown below in Fig. 3, addition of GSH separately resulted in no fluorescence response, whereas ONOO− led to a small fluorescence response in cells. This observation is believed to be due to the presence of a low levels of endogenous biological thiols in the cells, resulting in the activation of the probe's fluorescence. As predicted, treatment of cells with both GSH and SIN-1 led to a clear fluorescence increase in RAW264.7 cells with GSH-PF3, in clear agreement with the analytical data obtained on the fluorimeter.
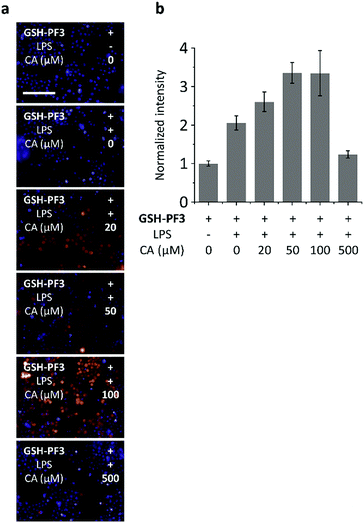
We then turned our attention to evaluate the ability of GSH-PF3 for monitoring the co-existence of metabolically produced ONOO− by lipopolysaccharide (LPS) simulation34 and GSH through a drug treatment.35 It has been reported that LPS can elicit ONOO− in macrophage, which is a signature of inflammation, whereas caffeic acid (CA) is commonly used to treat inflammation through the augmentation of intracellular GSH. Consequently, treatment with LPS, followed by the addition of increasing CA (0–100 μM) led to a gradual enhancement in the fluorescence intensity of GSH-PF3 (Fig. 4). This result is due to the co-existence of ONOO− produced by LPS stimulation as well as GSH elicited by CA. Interestingly, a further increase of the CA concentration (>100 μM) led to the suppression of probe's fluorescence, which is believed to be due to the production of an excess of GSH, resulting in quenching of the ONOO− (Fig. 4). To corroborate the presence of intracellular GSH in macrophage, a commercial GSH probe was used. The result suggested that both the exogenous addition of GSH and stimulation of metabolic GSH by treatment of CA/LPS activated the commercial probe's fluorescence (Fig. S5†). Furthermore, a cell proliferation assay indicated that GSH-PF3 is not toxic for RAW264.7 cells (Fig. S6†).
These results clearly demonstrate the potential of AND logic based fluorescence imaging probes for optimizing drug dosage in the treatment of inflammation and various other diseases. In addition this system could be used to help monitor the treatment of Alzheimer's disease (AD), since it is known that high levels of ONOO− correlate with oxidative stress and the progression of AD, while, high levels of GSH have been implicated in AD therapy.36–39 Therefore, we believe that our GSH-PF3 probe, which can detect GSH “and” ONOO− could potentially be used to monitor cellular resistance towards ROS and map therapeutic improvements in AD victims.
In summary, GSH-PF3 is an easy-to-prepare fluorescence based probe providing a platform for the development of other novel AND logic based fluorescence imaging probes for use in medical diagnostics. We are currently exploring the use of 6-amino/carboxyfluorescein, which we believe could provide the opportunity to attach additional fluorophores in order to develop ratiometric fluorescence sensors40 or for the attachment of targeting or therapeutic units.41–43
Conflicts of interest
No conflicts of interest.Acknowledgements
We would like to thank the EPSRC and the University of Bath for funding. ACS and JEG thank the EPSRC for studentships. X.-P. He thanks the National Natural Science Foundation of China (21722801 and 21572058), the Science and Technology Commission of Shanghai Municipality (15540723800), the Fundamental Research Funds for the Central Universities (222201717003) and the Shanghai Rising-Star Program (16QA1401400) for financial support. TDJ wishes to thank the Royal Society for a Wolfson Research Merit Award and ECUST for a guest professorship. The Catalysis and Sensing for our Environment (CASE) network is thanked for research exchange opportunities. NMR characterisation facilities were provided through the Chemical Characterisation and Analysis Facility (CCAF) at the University of Bath (http://www.bath.ac.uk/ccaf). The EPSRC UK National Mass Spectrometry Facility at Swansea University is thanked for analyses. All data supporting this study are provided as Electronic Supplementary Information (ESI) accompanying this paper.Notes and references
- P. Pacher, J. S. Beckman and L. Liaudet, Physiol. Rev., 2007, 87, 315 CrossRef CAS PubMed
.
- J. S. Beckman and W. H. Koppenol, Am. J. Physiol. Cell Physiol., 1996, 271, C1424 CrossRef CAS PubMed
.
- A. Weidinger and A. V. Kozlov, Biomolecules, 2015, 5, 472 CrossRef CAS PubMed
.
- P. Ascenzi, A. di Masi, C. Sciorati and E. Clementi, Biofactors, 2010, 36, 264 CrossRef CAS PubMed
.
- H. Ischiropoulos and J. S. Beckman, J. Clin. Invest., 2003, 111, 163 CrossRef CAS PubMed
.
- P. Sarchielli, F. Galli, A. Floridi and V. Gallai, Amino Acids, 2003, 25, 427 CrossRef CAS PubMed
.
- D. A. Wink, Y. Vodovotz, J. Laval, F. Laval, M. W. Dewhirst and J. B. Mitchell, Carcinogenesis, 1998, 19, 711 CrossRef CAS PubMed
.
- S. C. Lu, Mol. Aspect. Med., 2009, 30, 42 CrossRef CAS PubMed
.
- K. A. Marshall, R. Reist, P. Jenner and B. Halliwell, Free Radic. Biol. Med., 1999, 27, 515 CrossRef CAS PubMed
.
- J. P. Bolanos, S. J. R. Heales, J. M. Land and J. B. Clark, J. Neurochem., 1995, 64, 1965 CrossRef CAS PubMed
.
- M. Li, X. M. Wu, Y. Wang, Y. S. Li, W. H. Zhu and T. D. James, Chem. Commun., 2014, 50, 1751 RSC
.
- S. Palanisamy, P. Y. Wu, S. C. Wu, Y. J. Chen, S. C. Tzou, C. H. Wang, C. Y. Chen and Y. M. Wang, Biosens. Bioelectron., 2017, 91, 849 CrossRef CAS PubMed
.
- D. Cheng, Y. Pan, L. Wang, Z. B. Zeng, L. Yuan, X. B. Zhang and Y. T. Chang, J. Am. Chem. Soc., 2017, 139, 285 CrossRef CAS PubMed
.
- A. C. Sedgwick, X. L. Sun, G. Kim, J. Yoon, S. D. Bull and T. D. James, Chem. Commun., 2016, 52, 12350 RSC
.
- X. Sun, Q. Xu, G. Kim, S. E. Flower, J. P. Lowe, J. Yoon, J. S. Fossey, X. Qian, S. D. Bull and T. D. James, Chem. Sci., 2014, 5, 3368 RSC
.
- J. Yin, Y. Kwon, D. Kim, D. Lee, G. Kim, Y. Hu, J. H. Ryu and J. Yoon, J. Am. Chem. Soc., 2014, 136, 5351 CrossRef CAS PubMed
.
- Q. Xu, K.-A. Lee, S. Lee, K. M. Lee, W.-J. Lee and J. Yoon, J. Am. Chem. Soc., 2013, 135, 9944 CrossRef CAS PubMed
.
- Z. Lei, X. Li, X. Luo, H. He, J. Zheng, X. Qian and Y. Yang, Angew. Chem., Int. Ed., 2017, 56, 2979 CrossRef CAS PubMed
.
- Y. Kuriki, M. Kamiya, H. Kubo, T. Komatsu, T. Ueno, R. Tachibana, K. Hayashi, K. Hanaoka, S. Yamashita, T. Ishizawa, N. Kokudo and Y. Urano, J. Am. Chem. Soc., 2018, 140, 1767 CrossRef CAS PubMed
.
- A. Romieu, Org. Biomol. Chem., 2015, 13, 1294 CAS
.
- L. Yu, S. L. Wang, K. Z. Huang, Z. G. Liu, F. Gao and W. B. Zeng, Tetrahedron, 2015, 71, 4679 CrossRef CAS
.
- G. C. Van de Bittner, C. R. Bertozzi and C. J. Chang, J. Am. Chem. Soc., 2013, 135, 1783 CrossRef CAS PubMed
.
- L. Yuan, W. Y. Lin, Y. N. Xie, B. Chen and S. S. Zhu, J. Am. Chem. Soc., 2012, 134, 1305 CrossRef CAS PubMed
.
- C. G. Dai, X. L. Liu, X. J. Du, Y. Zhang and Q. H. Song, ACS Sens., 2016, 1, 888 CrossRef CAS
.
- S. Debieu and A. Romieu, Org. Biomol. Chem., 2015, 13, 10348 CAS
.
- L. Yi, L. Wei, R. Y. Wang, C. Y. Zhang, J. Zhang, T. W. Tan and Z. Xi, Chem.–Eur. J., 2015, 21, 15167 CrossRef CAS PubMed
.
- C. Y. Ang, S. Y. Tan, S. J. Wu, Q. Y. Qu, M. F. E. Wong, Z. Luo, P. Z. Li, S. T. Selvan and Y. L. Zhao, J. Mater. Chem. C, 2016, 4, 2761 RSC
.
- S. Erbas-Cakmak, S. Kolemen, A. C. Sedgwick, T. Gunnlaugsson, T. D. James, J. Yoon and E. U. Akkaya, Chem. Soc. Rev., 2018 10.1039/c7cs00491e
.
- F. B. Yu, P. Li, B. S. Wang and K. L. Han, J. Am. Chem. Soc., 2013, 135, 7674 CrossRef CAS PubMed
.
- F. B. A. Yu, P. Li, G. Y. Li, G. J. Zhao, T. S. Chu and K. L. Han, J. Am. Chem. Soc., 2011, 133, 11030 CrossRef CAS PubMed
.
- A. Sikora, J. Zielonka, M. Lopez, J. Joseph and B. Kalyanaraman, Free Radic. Biol. Med., 2009, 47, 1401 CrossRef CAS PubMed
.
- G. Y. Wu, Y. Z. Fang, S. Yang, J. R. Lupton and N. D. Turner, J. Nutr., 2004, 134, 489 CrossRef CAS PubMed
.
- X. Li, R. R. Tao, L. J. Hong, J. Cheng, Q. Jiang, Y. M. Lu, M. H. Liao, W. F. Ye, N. N. Lu, F. Han, Y. Z. Hu and Y. H. Hu, J. Am. Chem. Soc., 2015, 137, 12296 CrossRef CAS PubMed
.
- A. Vazquez-Torres, J. Jones-Carson and E. Balish, Infect. Immun., 1996, 64, 3127 CAS
.
- M. Y. Moridani, H. Scobie, A. Jamshidzadeh, P. Salehi and P. J. O'Brien, Drug Metab. Dispos., 2001, 29, 1432 CAS
.
- M. A. Smith, P. L. R. Harris, L. M. Sayre, J. S. Beckman and G. Perry, J. Neurosci., 1997, 17, 2653 CAS
.
- E. Rojas-Gutierrez, G. Munoz-Arenas, S. Trevino, B. Espinosa, R. Chavez, K. Rojas, G. Flores, A. Diaz and J. Guevara, Synapse, 2017, 71, e21990 CrossRef PubMed
.
- C. B. Pocernich and D. A. Butterfield, Biochim. Biophys. Acta, Mol. Basis Dis., 2012, 1822, 625 CrossRef CAS PubMed
.
- I. Cacciatore, L. Baldassarre, E. Fornasari, A. Mollica and F. Pinnen, Oxid. Med. Cell. Longevity, 2012, 240146 Search PubMed
.
- A. E. Albers, V. S. Okreglak and C. J. Chang, J. Am. Chem. Soc., 2006, 128, 9640 CrossRef CAS PubMed
.
- M. H. Lee, J. Y. Kim, J. H. Han, S. Bhuniya, J. L. Sessler, C. Kang and J. S. Kim, J. Am. Chem.
Soc., 2012, 134, 12668 CrossRef CAS PubMed
.
- S. Bhuniya, S. Maiti, E. J. Kim, H. Lee, J. L. Sessler, K. S. Hong and J. S. Kim, Angew. Chem., Int. Ed., 2014, 53, 4469 CrossRef CAS PubMed
.
- X.-P. He and H. Tian, Chem, 2018, 4, 246 CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional figures, experimental section and original spectra of new compounds. See DOI: 10.1039/c8sc00733k |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2018 |