Anion-induced isomerization of fluorescent semi(thio)carbazones†
Abstract
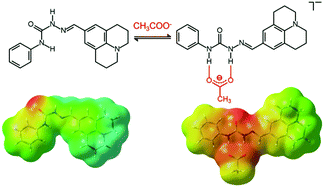
In this work, we have investigated the properties of novel fluorescent semi(thio)carbazone systems with anions by UV-vis, spectrofluorimetric and NMR studies, in acetonitrile and DMSO solutions. Conformational preferences were determined by theoretical calculations within the DFT approach. For the free receptors, these studies pointed out a marked prevalence of anti conformations, which are stabilized by intramolecular H-bonds between the (thio)urea NH group and the imino nitrogen atom. For the julolidine-based thiosemicarbazone system, intermolecular interactions involving the thiourea NH groups and the S atoms of two receptor molecules were also found in the crystals, leading to the formation of supramolecular dimers. Theoretical studies showed that anion binding involved a change of the conformation of the receptors from anti to syn. In the syn isomers, two NH groups actually point in the same direction, thus favouring the binding of anions, e.g. the Y-shaped acetate ion. This thorough study on the novel semi(thio)carbazones provides useful information for the application of this type of molecule in all the fields of chemistry, in which urea-based systems are employed (e.g. anion recognition and transport, catalysis, soft materials, etc.).


 Please wait while we load your content...
Please wait while we load your content...