Oxidant- and hydrogen acceptor-free palladium catalyzed dehydrogenative cyclization of acylhydrazones to substituted oxadiazoles†
Abstract
An efficient method for the synthesis of 2,5-disubstituted 1,3,4-oxadiazoles has been developed through palladium(0) catalyzed dehydrogenative cyclization of N-arylidenearoylhydrazides. By using this method, a wide range of functionalized and potentially biologically relevant 1,3,4-oxadiazole-containing compounds have been accessed in moderate to high isolated yields. The dehydrogenative cyclization process is characterized by the nonuse of any sacrificing hydrogen acceptors or oxidants and hydrogen gas as the only by-product, and therefore circumvents the recurring problems of over-oxidation and the compatibility with easily oxidizable functionalities in oxidation protocols.
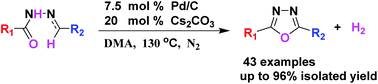


 Please wait while we load your content...
Please wait while we load your content...