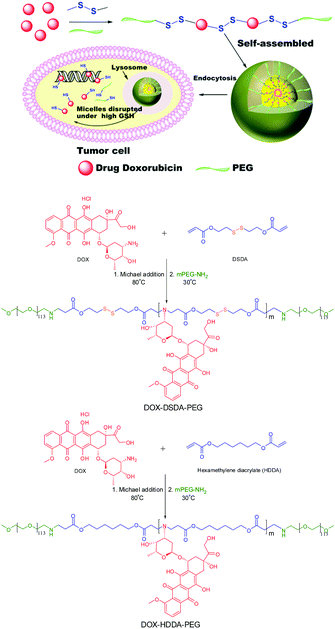
One-pot synthesis of glutathione-responsive amphiphilic drug self-delivery micelles of doxorubicin–disulfide–methoxy polyethylene glycol for tumor therapy†
Xiao
Duan
,
Ting
Bai
,
Junjie
Du
and
Jie
Kong
 *
*
MOE Key Laboratory of Space Applied Physics and Chemistry, Shaanxi Key Laboratory of Macromolecular Science and Technology, School of Science, Northwestern Polytechnical University, Xi’an, 710072, P. R. China. E-mail: kongjie@nwpu.edu.cn
First published on 30th November 2017
Abstract
We present a novel glutathione-responsive amphiphilic drug self-delivery (DSD) micelle with one-pot synthesis to synergistically address the problems of controlled drug release, degradability, drug tracing and in vivo accumulated toxicity. The anticancer drug doxorubicin (DOX), disulfide-based diacrylate (DSDA) and amino-polyethylene glycol monomethyl ether were linked by Michael addition in one-pot synthesis. The accumulative release rate of DOX analogues with drug activity from the micelles was 67.9% under pH 7.4 and GSH = 1 mg mL−1 conditions after 72 h. The cell uptake experiment showed that the micelles of DOX–DSDA–PEG were indeed taken up by A549 cells and distributed to cell nuclei. The in vitro cell viability of A549 cells was evaluated by CCK-8 and Muse Annexin V & Dead Cell Kit. The results illustrated that the completely biodegradable micelles with glutathione-responsive bonds in the backbone are an effective drug self-delivery system for tumor therapy in the future.
Drug delivery systems (DDS) are being increasingly used in tumor therapy. Most of the DDSs are actually complex therapy platforms,1–4 which include a drug molecule, target molecule, polymer carriers, fluorescent molecule, responsive bonds, etc. The DDS can generally address or ameliorate the problems of poor drug solubility, tissue damage on extravasation, unfavorable pharmacokinetics, poor biodistribution and lack of selectivity for target tissues compared with free anticancer drugs.5 However, the toxicity, degradability and complex preparation process of the carriers limit the practical applications of DDSs in clinical therapy.
Traditional conjugated or encapsulated drug delivery systems are mainly based on polymer-based carriers. The toxicity or non-biodegradability of polymers and the drug-to-polymer carrier ratio are both important considerations because the use of more polymer carrier results in poor metabolism and elimination in vivo.6 The rate of renal elimination is inversely correlated with the molecular weight of the polymers.7,8 Therefore, ideal polymers for drug delivery systems can be completely degraded into small molecules with stimulated linker bonds in the backbone.9–14 The complex preparation process is another major drawback for the application of DDSs, even if the polymer is completely degradable and non-toxic. For example, annexin V-conjugated mixed micelles were prepared in five steps using the non-biodegradable polymer of poly(2-hydroxyethyl methacrylate).15 In another study, multi-polyprodrug-arm hyperbranched amphiphiles were constructed by multi-step reactions.16 Also, dual pH-sensitive polymer–doxorubicin nanoparticles, self-assembled dual responsive micelles stabilized with protein and dual-pH sensitive charge-reversal polypeptide micelles were all synthesized by complex preparation processes.17–19
Considering the major drawbacks of complex synthetic routes and carrier toxicity or degradability, we developed a novel glutathione-responsive amphiphilic drug self-delivery micelle of doxorubicin–disulfide–methoxy polyethylene glycol (DOX–DSDA–PEG) for tumor therapy via a one-pot synthesis strategy. The drug self-delivery micelle only uses a small amount of polyethylene glycol (approved by U.S. Food and Drug Administration) as a hydrophilic chain and the drugs themselves are linked by the disulfide bonds as a hydrophobic block (DOX–DSDA). This design completely avoids the usage of toxic and non-biodegradable polymer, and the disulfide bonds can be cleaved when triggered by glutathione (GSH) in tumor cells and release small molecule drugs of DOX analogues with drug activity.20–22 The features of drug self-delivery, non-toxic polymer, GSH-responsiveness and one-pot synthesis make the drug self-delivery micelles an ideal strategy for tumor therapy.
The glutathione-responsive and non-glutathione-responsive amphiphilic drug self-delivery micelles were synthesized via a one-pot method (Scheme 1). The hydrophobic chain was synthesized by Michael addition reaction of the double bonds. The drug molecule DOX and disulfide-based diacrylate (DSDA) or hexamethylene diacrylate (HDDA) were polymerized in anhydrous DMF at 80 °C. In order to obtain the hydrophobic polymer with DOX as a repeating unit, the secondary amine of DOX needs to be reacted with double bonds at high temperature (80 °C), because the secondary amine is less reactive at low temperature (<40 °C).23,24 After the hydrophobic polymer was synthesized, the amino-polyethylene glycol monomethyl ether was added to the reaction flask at room temperature to react with double bonds for another 24 h. The primary amine of PEG–NH2 was conjugated to the hydrophobic polymer chain at room temperature to form amphiphilic polymers (DOX–DSDA–PEG and DOX–HDDA–PEG). The GPC traces of PEG, DOX–DSDA, DOX–DSDA–PEG and DOX–HDDA–PEG are shown in Fig. S1 (ESI†). The retention times of PEG, DOX–DSDA, DOX–HDDA–PEG and DOX–DSDA–PEG were 17 min, 11.1 min, 10.6 min and 9.9 min, respectively, illustrating that the amphiphilic polymers were indeed synthesized. The 1H NMR characteristic peak of –CH2–S–S– (3.00 ppm) in DOX–DSDA is shown in Fig. S3 (ESI†). We then measured the C–H COSY spectra of DOX–DSDA and DOX to confirm that the double bonds are indeed conjugated with the secondary amine of DOX. The newly appeared C–H cross-peaks of DOX–DSDA compared with free DOX illustrated that the polymer of DOX–DSDA was successfully synthesized (Fig. S3 and S4, ESI†). The 1H NMR characteristic peaks of the amphiphilic polymer were observed at 7.0–8.0 ppm (benzene ring), 1.25 ppm (–CH3–) in DOX and 3.00 ppm (–CH2–S–S–) in DSDA (Fig. S5, ESI†).
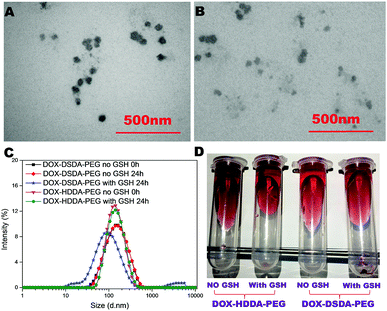
The amphiphilic polymers of DOX–DSDA–PEG and DOX–HDDA–PEG were assembled into micelles in pure water and detected by transmission electron microscopy (TEM) (Fig. 1A and B) and dynamic light scattering (DLS) (Fig. 1C). The critical micelle concentration (CMC) of DOX–DSDA–PEG was about 40 μg mL−1 (Fig. S6, ESI†). The average particle sizes of DOX–DSDA–PEG and DOX–HDDA–PEG are smaller than 175 nm, as measured by DLS, so they can selectively accumulate in tumor tissues based on the enhanced permeability and retention (EPR) effect.25,26 In general, DLS gives a hydrodynamic size that corresponds to the core and swollen corona of the micelles, whereas TEM often gives the core size of the micelles in a dried state as the corona with low electronic density is not visible. The micelles of the hydrophilic chain will be shrunk in the dried state. The longer the hydrophilic chain is, the more the shrinkage in the dried state. Thus, there is a discrepancy in the micelle sizes measured by TEM and DLS. The size distribution of DOX–HDDA–PEG did not change while the particle size of DOX–DSDA–PEG clearly changed when treated with GSH for 24 h (Fig. 1C). The stabilities of the micelles of DOX–DSDA–PEG and DOX–HDDA–PEG with or without GSH in PBS after 24 h were evaluated by a centrifugal machine at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm (Fig. 1D). For the glutathione-responsive micelles of DOX–DSDA–PEG treated with GSH (1 mg mL−1), a red sediment can clearly be observed in the centrifuge tube. The non-glutathione-responsive micelles of DOX–HDDA–PEG with or without GSH and the micelles of DOX–DSDA–PEG without GSH were quite stable in PBS even after 24 h (Fig. 1D). The micelles of DOX–DSDA–PEG were not completely disrupted at 24 h, likely due to the short degradation time (24 h) and low concentration of GSH (1 mg mL−1) and DOX–DSDA–PEG (2 mg mL−1). The undisrupted DOX–DSDA–PEG micelles can re-assemble in water and encapsulate the disrupted DOX analogues, thus there is less sediment in the centrifuge tube (Fig. 1D). As the time progressed, the micelles of DOX–DSDA–PEG were completely disrupted by 72 h, which was confirmed by the drug release curve (Fig. 2A).
000 rpm (Fig. 1D). For the glutathione-responsive micelles of DOX–DSDA–PEG treated with GSH (1 mg mL−1), a red sediment can clearly be observed in the centrifuge tube. The non-glutathione-responsive micelles of DOX–HDDA–PEG with or without GSH and the micelles of DOX–DSDA–PEG without GSH were quite stable in PBS even after 24 h (Fig. 1D). The micelles of DOX–DSDA–PEG were not completely disrupted at 24 h, likely due to the short degradation time (24 h) and low concentration of GSH (1 mg mL−1) and DOX–DSDA–PEG (2 mg mL−1). The undisrupted DOX–DSDA–PEG micelles can re-assemble in water and encapsulate the disrupted DOX analogues, thus there is less sediment in the centrifuge tube (Fig. 1D). As the time progressed, the micelles of DOX–DSDA–PEG were completely disrupted by 72 h, which was confirmed by the drug release curve (Fig. 2A).
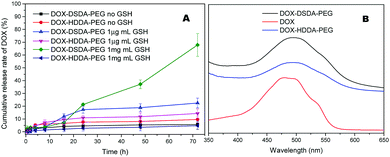
The drug loading and drug release were monitored by UV-vis at 490 nm. The absorbance curves of DOX, DOX–HDDA–PEG and DOX–DSDA–PEG in PBS are shown in Fig. 2B. The characteristic peak of DOX at 490 nm was not significantly changed after modification with DSDA, HDDA and PEG. Thus, the drug release rate and drug loading of DOX–HDDA–PEG (22%) and DOX–DSDA–PEG (25.5%) can be detected by UV-vis according to the absorbance of DOX at 490 nm. The glutathione-responsive amphiphilic self-assembled micelles were disrupted under high GSH concentration. The concentrations of GSH in tumor cells are generally in the range of 2–10 mM.27,28 In order to illustrate that controlled release can be triggered from the glutathione-responsive drug self-assembled micelle in different tumor cells, we used 3.25 mM (1 mg mL−1) as the control concentration of GSH. The cumulative drug release rate of the glutathione-responsive micelles was suddenly increased to 21.3% (24 h) from 7.2% (16 h) under the conditions of pH 7.4 & GSH = 1 mg mL−1 (Fig. 2A). The reason is that the micelles are dissolved in the solution of pH 7.4 PBS without GSH and disrupted slowly in a short time. The cumulative drug release rate of the glutathione-responsive micelles (DOX–DSDA–PEG) reached 67.9% at a high GSH concentration (GSH = 1 mg mL−1) after 72 h and the cumulative drug release rates of DOX–DSDA–PEG without GSH and with a low concentration of GSH (GSH = 1 μg mL−1) were 5.7% and 22% after 72 h, respectively. The drug release rates of the entire process of the non-glutathione-responsive micelles (DOX–HDDA–PEG) with high GSH concentration (GSH = 1 mg mL−1), low GSH concentration (GSH = 1 μg mL−1) and without GSH groups are increased slightly during 72 h (Fig. 2A).
In the cell uptake experiment, the A549 cells were treated with 15 μg mL−1 of DOX and 15 μg mL−1 of DOX in the micelles of DOX–HDDA–PEG and DOX–DSDA–PEG for 4 h and 8 h, respectively. The micelles of DOX–DSDA–PEG and DOX–HDDA–PEG were taken up by the A549 cells. The nuclei were dyed by Hoechst 33258 at 4 h and 8 h, respectively. Eight hours later, the micelles of DOX–DSDA–PEG fully overlapped with the cell nuclei in the merged image and the red fluorescence of DOX–DSDA–PEG was clearly observed in the cell nuclei. However, the DOX and DOX–HDDA–PEG were not fully distributed into the cell nuclei in the merged images even after 8 h (Fig. 3). According to the cell uptake results after 8 h, we concluded that the micelles of DOX–DSDA–PEG were fully distributed into the cell nuclei. As a result, the cell viability of DOX–DSDA–PEG was lower than that of DOX and DOX–HDDA–PEG at 24 h (Fig. 4). After uptake of DOX–DSDA–PEG by A549 cells, the micelles of DOX–DSDA–PEG were disrupted within tumor cells under high GSH concentration, releasing free DOX analogues with activity to kill tumor cells.
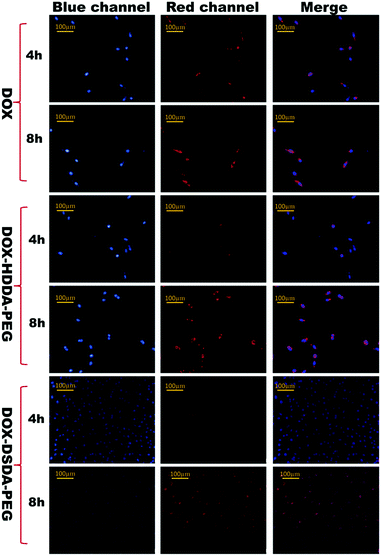 | ||
| Fig. 3 Cell uptake by A549 cells treated with DOX, DOX–HDDA–PEG and DOX–DSDA–PEG (15 μg mL−1 DOX in micelles) for different durations. | ||
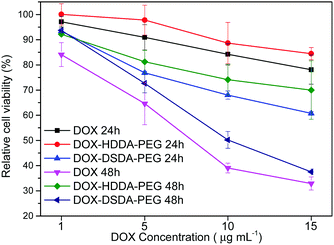 | ||
| Fig. 4 Cell viability of cells treated with DOX and micelles of DOX–DSDA–PEG and DOX–HDDA–PEG with different concentrations for 24 h and 48 h. | ||
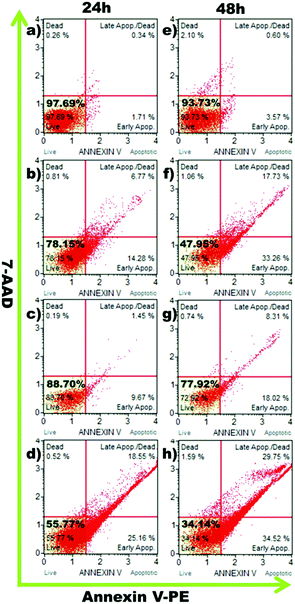
The cell viabilities of A549 cells treated with DOX, DOX–DSDA–PEG and DOX–HDDA–PEG were evaluated in vitro at 24 h and 48 h with a CCK-8 experiment. The decreased cell viability with DOX–DSDA–PEG corresponded to the increased DOX concentrations in the micelles. The cell viability of A549 cells is significantly different after being treated with high concentrations of DOX–DSDA–PEG and DOX–HDDA–PEG (Fig. 4). The controllable release of DOX in micelles from DOX–DSDA–PEG can be triggered by the presence of GSH. The cumulative drug release rates of DOX–DSDA–PEG at 24 h and 48 h were 21.3% and 37.1% at high GSH (GSH = 1 mg mL−1) concentration, respectively. The cumulative drug release rates of DOX–HDDA–PEG at 24 h and 48 h were 2.7% and 3.5% under high GSH (GSH = 1 mg mL−1) concentration, respectively (Fig. 2A). The higher the DOX dosage in the DOX–DSDA–PEG micelles is, the greater the difference in absolute drug release amount between 24 h and 48 h. When the concentration of DOX in the DOX–DSDA–PEG micelle was 15 μg mL−1, a significant difference in cell viability was observed between 24 h (60.71%) and 48 h (37.50%) (Fig. 4). The cell viability of DOX–HDDA–PEG was 84% at 24 h and 70% at 48 h. The reason for the high cell viability is that the anticancer drug cannot be released from the non-glutathione-responsive micelles. The cell viability for DOX–DSDA–PEG was lower than that for free DOX at 24 h (Fig. 4), which is likely because the DOX–DSDA–PEG was fully taken up by A549 cells and distributed to the cell nuclei, while the free DOX was distributed to all cell nuclei at 8 h (Fig. 3). As the time progressed, the free DOX was distributed to the cell nuclei and triggered apoptosis. As a result, there was no significant difference in the cell viability between the DOX (15 μg mL−1) group and the DOX–DSDA–PEG group at 48 h (Fig. 4). The cell viability of the DOX–DSDA–PEG group (including concentration of DOX 15 μg mL−1) is 55.77% at 24 h and 34.14% at 48 h, as determined by the Muse Cell Analyzer (Fig. 5), and the cell viability is 60.71% at 24 h and 37.50% at 48 h, as evaluated by the CCK-8 experiment (Fig. 4). The cell apoptosis results obtained by the Muse Cell Analyzer are consistent with the cell viability results of the CCK-8 experiment. These results illustrate that the micelles of DOX–DSDA–PEG are effective DSDs for tumor therapy.
In summary, we have developed a novel glutathione-responsive amphiphilic drug self-delivery system via a one-pot synthesis method for tumor therapy. This drug self-delivery system has four advantages: (1) no toxic or non-degradable polymer; (2) controlled drug release with GSH-responsiveness; (3) DOX itself functions as a fluorescently labeled molecule and carrier and (4) one-pot synthesis. The DOX can be controllably released from the micelles of DOX–DSDA–PEG under high GSH concentration. The cell uptake and cell viability experiments further confirmed that the micelles can be taken up by A549 cells and disrupted under high GSH concentration in tumor cells. The results of this work indicate that the completely glutathione-responsive degradable micelles are feasible as a drug self-delivery system for tumor therapy.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This research work was supported by the National Natural Science Foundation of China (No. 21374089/21404085).Notes and references
- Z. Xu, S. Liu, H. Liu, C. Yang, Y. Kang and M. Wang, Chem. Commun., 2015, 51, 15768–15771 RSC.
- Y. Guo, Y. Wang, S. Li, L. Niu, D. Wei and S. Zhang, Chem. Commun., 2017, 53, 4826–4829 RSC.
- W. C. de Vries, D. Grill, M. Tesch, A. Ricker, H. Nüsse, J. Klingauf, A. Studer, V. Gerkeand and B. J. Ravoo, Angew. Chem., Int. Ed., 2017, 56, 1–6 CrossRef.
- L. H. Wang, D. C. Wu, H. X. Xu and Y. Z. You, Angew. Chem., Int. Ed., 2016, 55, 755–759 CrossRef CAS PubMed.
- T. M. Allen, Science, 2004, 303, 1818–1822 CrossRef CAS PubMed.
- X. Duan, H. Chen, L. Fan and J. Kong, ACS Biomater. Sci. Eng., 2016, 2, 2347–2354 CrossRef CAS.
- Y. Tetsuji, T. Yasuhiko and I. Yoshito, J. Pharm. Sci., 1994, 84, 601–606 Search PubMed.
- Y. Tetsuji, T. Yasuhiko and I. Yoshito, J. Pharm. Sci., 1995, 84, 349–354 CrossRef.
- X. Duan, Y. Wu, M. Ma, J. Du, S. Zhang, H. Chen and J. Kong, J. Mater. Sci., 2017, 52, 9430–9440 CrossRef CAS.
- N. Liu, J. Vignolle, J. M. Vincent, F. Robert, Y. Landais, H. Cramail and D. Taton, Macromolecules, 2014, 47, 1532–1542 CrossRef CAS.
- R. G. Parmar, M. Busuek, E. S. Walsh, K. R. Leander, B. J. Howell, L. Sepp-Lorenzino, E. Kemp, L. S. Crocker, A. Leone, C. J. Kochansky, B. A. Carr, R. M. Garbaccio, S. L. Colletti and W. Wang, Bioconjugate Chem., 2013, 24, 640–647 CrossRef CAS PubMed.
- C. Saptarshi and S. Ramakrishnan, Macromolecules, 2011, 44, 4658–4664 CrossRef.
- H. Chen, J. Jia, X. Duan, Z. Yang and J. Kong, J. Polym. Sci., Part A: Polym. Chem., 2015, 53, 2374–2380 CrossRef CAS.
- Y. Zhuang, H. Deng, Y. Su, L. He, R. Wang, G. Tong, D. He and X. Zhu, Biomacromolecules, 2016, 17, 2050–2062 CrossRef CAS PubMed.
- Y. Pan, X. Ren, S. Wang, X. Li, X. Luoand and Z. Yin, Biomacromolecules, 2017, 28, 1470–1480 Search PubMed.
- P. Sun, D. Chen, H. Deng, N. Wang, P. Huang, X. Jin and X. Zhu, Bioconjugate Chem., 2013, 24, 640–647 CrossRef PubMed.
- J. Du, X. Du, C. Maoand and J. Wang, J. Am. Chem. Soc., 2011, 133, 17560–17563 CrossRef CAS PubMed.
- S. Han, Z. Li, J. Zhu, K. Han, Z. Zeng, W. Hong, W. Li, H. Jia, Y. Liu, R. Zhuo and X. Zhang, Small, 2015, 11, 2543–2554 CrossRef CAS PubMed.
- M. R. AjiAlex, C. Nehate, S. Veeranarayanan, S. Kumar, R. Kulshreshtha and V. Koul, Biomaterials, 2017, 133, 94–106 CrossRef CAS PubMed.
- D. Sun, J. Ding, C. Xiao, J. Chen, X. Zhuang and X. Chen, ACS Appl. Mater. Interfaces, 2014, 6, 21202–21214 CAS.
- P. J. Burke and T. H. Koch, J. Med. Chem., 2004, 47, 1193–1206 CrossRef CAS PubMed.
- G. C. Post, B. L. Barthel, D. J. Burkhart, J. R. Hagadorn and T. H. Koch, J. Med. Chem., 2005, 48, 7648–7657 CrossRef CAS PubMed.
- C. Hong, Y. You, D. Wu, Y. Liuand and C. Pan, J. Am. Chem. Soc., 2007, 129, 5354–5355 CrossRef CAS PubMed.
- Y. Z. You, C. Y. Hong and C. Y. Pan, Macromolecules, 2009, 42, 573–575 CrossRef CAS.
- H. Maeda, H. Nakamura and J. Fang, Adv. Drug Delivery Rev., 2013, 65, 71–79 CrossRef CAS PubMed.
- J. Si, S. Shao, Y. Shen and K. Wang, Small, 2016, 12, 5108–5119 CrossRef CAS PubMed.
- G. I. Roshchupkina, A. A. Bobko, A. Bratasz, V. A. Reznikov, P. Kuppusamy and V. V. Khramtsov, Free Radical Biol. Med., 2008, 45, 312–320 CrossRef CAS PubMed.
- R. Cheng, F. Feng, F. Meng, C. Deng, J. Feijen and Z. Zhong, J. Controlled Release, 2011, 152, 2–12 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7tb02817b |
| This journal is © The Royal Society of Chemistry 2018 |