Bioinspired dynamic microcapsules
N. F. D.
AlDala'een
a,
W. N. K. W.
Mohamad
b,
N.
Alias
c,
A. M.
Ali
 c and
J.
Shaikh Mohammed
c and
J.
Shaikh Mohammed
 *a
*a
aFaculty of Innovative Design & Technology, Universiti Sultan Zainal Abidin (UniSZA), Gong Badak Campus, 21300 Kuala Terengganu, Malaysia. E-mail: javeedsm@unisza.edu.my
bSchool of Marine and Environmental Sciences, Universiti Malaysia Terengganu (UMT), 21030 Kuala Terengganu, Malaysia
cFaculty of Bioresources and Food Industry, Universiti Sultan Zainal Abidin (UniSZA), Tembila Campus, 22200 Besut, Malaysia
First published on 29th November 2017
Abstract
There is an increasing interest in bioinspired dynamic materials. Abundant illustrations of protein domains exist in nature, with remarkable ligand binding characteristics and structures that undergo conformational changes. For example, calmodulin (CaM) can have three conformational states, which are the unstructured Apo-state, Ca2+-bound ligand-exposed binding state, and compact ligand-bound state. CaM's mechanical response to biological cues is highly suitable for engineering dynamic materials. The distance between CaM globular terminals in the Ca2+-bound state is 5 nm and in the ligand-bound state is 1.5 nm. CaM's nanoscale conformational changes have been used to develop dynamic hydrogel microspheres that undergo reversible volume changes. The current work presents the fabrication and preliminary results of layer-by-layer (LbL) self-assembled Dynamic MicroCapsules (DynaMicCaps) whose multilayered shell walls are composed of polyelectrolytes and CaM. Quasi-dynamic perfusion results show that the DynaMicCaps undergo drastic volume changes, with up to ∼1500% increase, when exposed to a biochemical ligand trifluoperazine (TFP) at pH 6.3. Under similar test conditions, microcapsules without CaM also underwent volume changes, with only up to ∼290% increase, indicating that CaM's bio-responsiveness was retained within the shell walls of the DynaMicCaps. Furthermore, DynaMicCaps exposed to 0.1 M NaOH underwent volume changes, with only up to ∼580% volume increase. Therefore, DynaMicCaps represent a new class of polyelectrolyte multilayer (PEM) capsules that can potentially be used to release their payload at near physiological pH. With over 200 proteins that undergo marked, well-characterized conformational changes in response to specific biochemical triggers, several other versions of DynaMicCaps can potentially be developed.
Introduction
Recent decades have seen an increasing interest in bioinspired dynamic materials.1–4 Many of nature's soft materials undergo significant changes in their biophysicochemical properties in response to specific biochemical ligands.3 Abundant illustrations of peptide motifs and protein domains exist in nature, with remarkable ligand binding characteristics and structures that undergo conformational changes.3–5 An example is calmodulin (CaM), the primary calcium (Ca2+)-binding regulatory protein in eukaryotic cells, whose function and structure are highly dependent on Ca2+ concentration. It is a highly acidic small protein that is ubiquitous and 100% conserved among vertebrates.6–8CaM can have three conformational states, which are the unstructured globular, Apo-state (1DMO), extended dumbbell, Ca2+-bound ligand-exposed binding state (3CLN), and the compact globular, ligand-bound state (2BBM).4 Ca2+-activated CaM exposes hydrophobic pockets (surrounded by negatively charged amino acids) that promote its interaction with ligands (antipsychotic drugs, peptides, and a variety of proteins) that are hydrophobic and/or positively charged.7,9 CaM's mechanical response to biological cues is highly suitable for engineering dynamic materials.10,11 The distance between CaM globular terminals in the Ca2+-bound state is 5 nm and in the ligand-bound state is 1.5 nm.4 The Murphy group at UW-Madison has smartly used CaM's nanoscale conformational changes to develop dynamic hydrogel disks12–14 and microspheres.15–17 A CaM mutant was reacted with poly(ethylene glycol) diacrylate (PEG-diacrylate) to form acrylate-terminated PEG-CaM-PEG conjugates, which were photo-crosslinked into hydrogels that underwent reversible volume changes up to 80%13 for disks and 76 ± 10%15 for microspheres.2,3
The layer-by-layer (LbL) electrostatic self-assembly approach was elegantly exploited by Max Planck researchers to fabricate hollow polyelectrolyte multilayer (PEM) capsules.18,19 A wide range of materials including DNA, proteins, nanoparticles, lipids, viruses, and other nano-objects are suitable for use in PEMs.20–22 PEM capsules have been extensively studied as nano- and micro-carriers with potential applications in medicine, drug delivery, catalysis, biotechnology, food technology, coatings, and cosmetics.20,23,24 PEM capsules can be triggered for loading and release applications, with a wide range of internal and external stimuli including physical (temperature, light, mechanical distortion, magnetic field, electric field, ultrasound), chemical (solvent, pH, ionic strength), and biological (enzymes, receptors) stimuli.25–27
Different groups have attempted to overcome the challenge of designing PEM capsules that are stable in physiological environments but release their cargo at near physiological pH.22,28,29 For example, oligoamine ‘‘patches’’ whose overall charge could be reversibly switched in the pH range 5.5–7.5 between cationic and anionic forms by interaction with CO2 complexation and carbamate formation were used as structural interlayers of PEM capsules. The capsules showed a reversible gating of the permeation of large water-soluble polymer species when bubbled with CO2.30 Microcapsules were fabricated using an arginine-rich cationic protein, protamine.31 When triggered with trypsin (pH 7.4), the capsules disintegrated. Also, capsules were fabricated using protamine and chondroitin sulfate.32 These capsules disintegrated when triggered with trypsin (pH 7.4) or with hyaluronidase (pH 6).
DNA-based capsules with crosslinked oligonucleotide layers using adenosine triphosphate (ATP) aptamer bridges were fabricated by the Willner group.33 When triggered with ATP (pH 7), the microcapsules dissociated through the formation of aptamer-ATP complexes. DNA-based capsules, with C–G·C+ and T–A·T triplex DNA motifs, were fabricated to induce the unlocking of the capsules at pH 5 and 7, respectively.34 Furthermore, all-DNA stabilized microcapsules consisting of nucleic acids that are reconfigured at acidic pH values (pH 5) to i-motif structures were reported.35
In another approach, microcapsules with hydrogen-bonded multilayer thin films using poly(vinylpyrrolidone) and poly(methacrylic acid) (PMA) were fabricated by the Caruso group.36–40 Thiol moieties in the films were cross-linked to stabilize the capsules at physiological pH by forming disulfide bonds. The capsules disassembled when triggered with a thiol–disulfide exchange reagent, dithiothreitol at pH 7,36 and a thiol-containing peptide, glutathione (GSH) at pH 7.2.39 Furthermore, PMA based capsules with reversible capsule permeability were fabricated.41 Poly(2-diisopropylaminoethyl methacrylate) (PDPA) based charge-shifting capsules were fabricated using hydrogen-bonding, electrostatic interactions, and click chemistry.42 The capsules stabilized with a disulfide-containing cross-linker, and degraded when triggered with GSH at intracellular acidic pH 6. Also, PDPA based capsules fabricated using non-covalent stabilization showed more significant pH-induced size responses.43 LbL-assembled degradable capsules consisting solely of polyrotaxanes degraded in the presence of GSH at pH 7.4.44 Capsules based on the metal-phenolic networks were fabricated using a one-step assembly method.45 The capsules degraded in PBS solutions at pH 5 and 6.
CaM planar films were deposited onto a micro-cantilever using the LbL method. The cantilever deflected upon binding with Ca2+ ions indicating that the CaM proteins embedded within the PEMs retained bio-responsiveness.46 Therefore, our hypothesis for the current study was that the inclusion of CaM within PEM capsule shell walls would enable nanoscale hinge motions to be translated into changes in the nanofilm structure and, in turn, lead to dynamic changes in microcapsule volume. The current work presents the fabrication and preliminary results of LbL self-assembled Dynamic MicroCapsules (DynaMicCaps) whose multilayered shell walls are composed of polyelectrolytes [PAH—poly(allylamine hydrochloride), PSS—poly(sodium 4-styrenesulfonate), and PAH-TRITC—PAH labelled with tetramethylrhodamine-5-(and-6)-isothiocyanate] and CaM. Results herein indicate that CaM's bio-responsiveness was retained within the shell walls of the DynaMicCaps and that the DynaMicCaps undergo drastic volume changes at near physiological pH.
Results and discussion
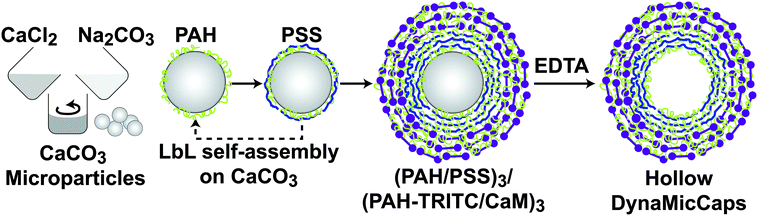
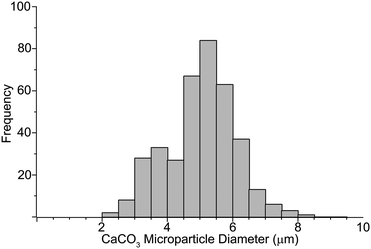
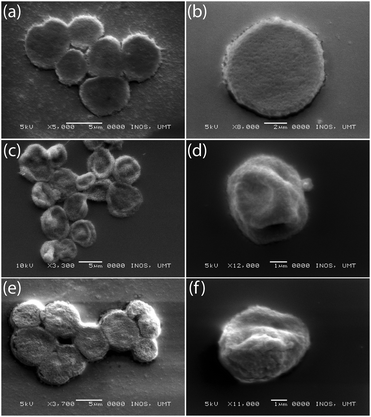
The illustration in Fig. 1 shows the processes used for the fabrication of DynaMicCaps with a PEM architecture of (PAH/PSS)3/(PAH-TRITC/CaM)3. The histogram in Fig. 2 shows the size distribution of the calcium carbonate (CaCO3) templates [with mean diameters of 5.02 ± 1.03 μm (n = 372)] used in the current study. CaCO3 templates were used here as they are easy to prepare and completely dissolvable without destroying the encapsulated macromolecules.22,24,47Fig. 3 contains the scanning electron micrographs of the DynaMicCaps, with PEM architectures of (PAH/PSS)3/(PAH-TRITC/CaM)1, (PAH/PSS)3/(PAH-TRITC/CaM)2, and (PAH/PSS)3/(PAH-TRITC/CaM)3. The shapes and surface topographies of the dried hollow microcapsules are characteristic of hollow (PSS/PAH) PEM microcapsules. An increase in surface roughness can be seen with an increase in the number of (PAH/CaM) bilayers.Fig. 4 shows the confocal fluorescence micrographs of the DynaMicCaps, with the PEM architecture of (PAH/PSS)3/(PAH-TRITC/CaM)3, in the presence of 0.1 M HCl, DI water, and 0.1 M NaOH. The equivalent circular diameters of the microcapsules were measured to be 5.12 ± 0.85 μm (n = 24) in 0.1 M HCl, 4.43 ± 0.63 μm (n = 17) in DI water, and 7.43 ± 1.42 μm (n = 39) in 0.1 M NaOH, respectively. From these measurements, it can be seen that the DynaMicCaps are swollen in both acidic and alkaline media. However, the swelling of the DynaMicCaps in the alkaline medium is more pronounced than the swelling in the acidic medium. The maximum volume change that the DynaMicCaps underwent when exposed to 0.1 M NaOH was up to ∼580% (volume increase as compared to the volume in the DI water).
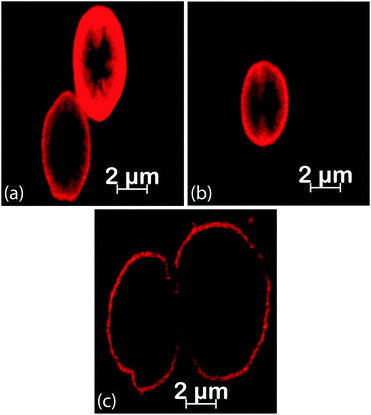 | ||
| Fig. 4 Confocal fluorescence micrographs of the DynaMicCaps, with the PEM architecture of (PAH/PSS)3/(PAH-TRITC/CaM)3, in the presence of (a) 0.1 M HCl, (b) DI water, and (c) 0.1 M NaOH. | ||
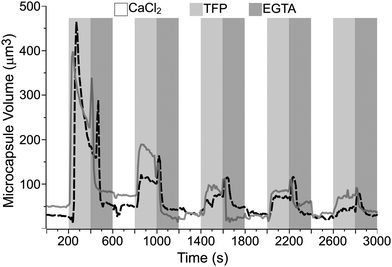
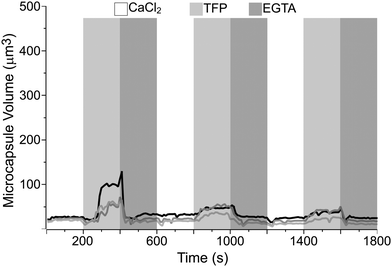
Fig. 5 shows the curves of the estimated volume of the microcapsules, with the PEM architecture of (PAH/PSS)3/(PAH-TRITC/CaM)3, over time. The dotted and solid curves represent the microcapsules that displayed the maximum volume changes from two separate quasi-dynamic perfusion experiments. The DynaMicCaps that are settled on the surface of a microchannel were sequentially perfused every 200 s with calcium chloride (CaCl2) buffer (pH 6.3, 10 mM CaCl2, 20 mM MES, 150 mM NaCl), trifluoperazine dihydrochloride (TFP) buffer (pH 6.3, 10 mM TFP, 20 mM MES, 150 mM NaCl), and ethylene glycol-bis(b-aminoethyl ether)n (EGTA) buffer (pH 6.3, 10 mM EGTA, 20 mM MES, 150 mM NaCl) solutions over five cycles. It is evident from the plot that the DynaMicCaps undergo drastic volume changes (up to ∼1500% increase) when the microcapsules are exposed to TFP buffer solution as compared to when exposed to CaCl2 or EGTA buffer solution. This phenomenon is opposite to that observed with CaM-based hydrogel disks and microspheres, where the TFP induced volume decreases.
In CaM-based hydrogels, both terminals of CaM are covalently bound to PEG. The CaM molecules collapse in the presence of TFP, resulting in the collapse of the crosslinked PEG hydrogel network. In the case of DynaMicCaps, the CaM molecules electrostatically interpenetrate the PAH-TRITC layers within the thin shell walls of the microcapsules. The inverse volume change in the DynaMicCaps compared to those in the CaM-based hydrogels can be attributed mainly to the variation in linking of the CaM molecules to the PEM thin films and the PEG hydrogel network. It is noteworthy that the DynaMicCaps undergo volume changes, both volume increase and decrease, within 100 s of the initiation of exchange (3×) of the solution in the microchannel; with ∼20 s taken to complete the 3× solution exchange procedure. Similar was the case of the TFP induced collapse in CaM-based hydrogel microspheres that occurred in less than 1 min.15–17 In fact, CaM is one of the fastest folding proteins with a folding rate of ∼2 × 105 s−1, a ligand-binding rate of ∼12.2 s−1, and a lifetime of 102 to 103 s for the bound peptide.48
Based on six separate quasi-dynamic perfusion experiments, the equivalent circular diameters of the microcapsules during the first perfusion cycle were measured to be 4.62 ± 0.39 μm (n = 98) in the presence of CaCl2 buffer and 7.37 ± 0.94 μm (n = 105) in the presence of TFP buffer. Upon comparing the equivalent circular diameters of the DynaMicCaps exposed to 0.1 M NaOH and TFP buffer (pH 6.3) solutions, it is clear that it is possible to trigger volume changes in DynaMicCaps using a biochemical ligand at near physiological pH comparable to the volume changes in the DynaMicCaps caused by alkaline solutions.
For comparison purposes, microcapsules without CaM (with CaM replaced with PSS) were fabricated and tested using similar approaches to those followed for the DynaMicCaps. Fig. 6 illustrates the plot of the estimated volume of the microcapsules, with the PEM architecture of (PAH/PSS)3/(PAH-TRITC/PSS)3, over time from two separate quasi-dynamic perfusion experiments. The microcapsules that are settled on the surface of a microchannel were sequentially perfused every 200 s with CaCl2, TFP, and EGTA buffer solutions over three cycles. It is clear from the curves that the microcapsules without CaM do not undergo extensive volume changes when exposed to the TFP buffer solution, as compared to the volume changes undergone by the DynaMicCaps (Fig. 5). However, it can be seen that TFP causes a volume change (up to ∼290% increase) mainly in the 1st perfusion cycle and not in the subsequent cycles. Furthermore, the gradual shrinking of DynaMicCaps observed after being triggered with TFP is absent even in the 1st perfusion cycle for the microcapsules without CaM. Based on the results shown in Fig. 5 and 6, it seems safe to assume that CaM's bio-responsiveness is retained within the shell walls of the DynaMicCaps presented here.
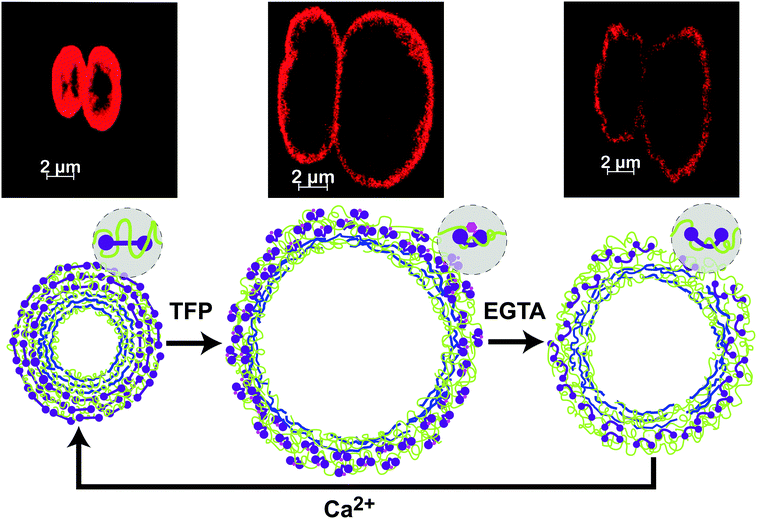
Fig. 7 displays the confocal fluorescence micrographs of the DynaMicCaps exposed to CaCl2 buffer (top-left), TFP buffer (top-center), and EGTA buffer (top-right) at 100 s, 270 s, and 470 s, respectively. The equivalent circular diameters of the more substantial volume changing DynaMicCaps (corresponding to the dotted curve in Fig. 5) in CaCl2 buffer and TFP buffer were measured to be approximately 3.81 μm and 9.61 μm, respectively. Fig. 7 (bottom) schematically illustrates the probable scenario of the DynaMicCaps undergoing a volume increase when triggered with a biochemical ligand, TFP, and a volume decrease when triggered with EGTA and CaCl2 buffers.
The (PAH/PSS)3 basement layers, used in the current work prior to depositing other multilayers, are known to be stable in various chemical environments and pervious to low molecular weight solvents and small ions.20,21 At neutral pH, weak polyelectrolytes such as PAH are more coiled.49 Also, Mw of polyelectrolytes affects the reorganization of capsule shell walls, with low Mw polyelectrolytes displaying lower entanglement of chains allowing them to reorganize easily.50–52 The current work uses a high Mw PAH, which would mean that PAH within the DynaMicCaps is highly coiled and causes higher entanglement. At pH values below 6.5, hollow PSS/PAH microcapsules are in an open state owing to the pH-responsiveness of PAH.53 In the current quasi-dynamic experiments, solutions at pH 6.3 were used, which would mean that the shell walls of the DynaMicCaps are in an open state. It should also be noted that the permeation of hollow capsules depends on the permeant charge.54,55 TFP, a biologically relevant small molecule, is a prominent phenothiazine-type antipsychotic drug that competes with target proteins for CaM binding.7,8,56–60 TFP is amphiphilic with hydrophobic phenothiazine ring systems and positively charged hydrophilic piperazine tail groups.57,61 The compact CaM-TFP structure is stabilized by van der Waals interactions between the TFP hydrophobic rings and the CaM hydrophobic pocket and through the electrostatic interactions between the positively charged TFP groups and the negatively charged CaM residues.62
When the DynaMicCaps are exposed to TFP, the TFP molecules can not only interact with the outermost CaM layer but possibly also penetrate through the porous shell wall interacting with the inner two CaM layers. CaM's nanoscale conformational changes cause lateral compression forces in its local nanoenvironment throughout the microcapsule shell wall. The fast formation of the CaM-TFP compact globular ligand-bound structures causes lateral nanoscale spatial changes that further increase the porosity of the shell walls, possibly allowing more TFP molecules to penetrate the shell walls. The abrupt excess positive charge within (and next to) the shell walls causes electrostatic repulsion between the highly coiled and entangled PAH and the positively charged groups of the free TFP molecules, enhancing their molecular movement, and is believed to cause the rapid, extensive swelling of the DynaMicCaps (1st perfusion cycle, Fig. 5);63,64 in synchrony with the lateral compressive forces due to CaM's conformational changes. The surface energy of the capsules depends on the equilibrium between hydrophilicity and hydrophobicity, causing an expansion when lateral electrostatic repulsion dominates and a shrinkage when hydrophobicity dominates.50 It is believed that after the DynaMicCaps undergo maximum expansions, the shell walls undergo layer rearrangements and the hydrophobic groups of free TFP molecules lead to a thermodynamically favored state,23,65 as evidenced from the gradual shrinking of the DynaMicCaps. During the first perfusion cycle, the thickness of the microcapsule shell wall significantly decreased with capsule expansion (refer to the fluorescence micrographs in Fig. 7).
When the DynaMicCaps are exposed to EGTA solution, the Ca2+ ions are chelated, and CaM changes to an unstructured globular apo-state, rapidly releasing the TFP molecules and causing a rapid swelling followed by a rapid, extensive shrinking of the DynaMicCaps (1st perfusion cycle, Fig. 5). It is believed that the initial rapid swelling of the DynaMicCaps, when exposed to the EGTA solution (Fig. 5), is due to the abrupt excess positive charge next to the shell walls causing an electrostatic repulsion between the highly coiled and entangled PAH and the positively charged groups of the released TFP molecules and the Ca2+ ions. The reason for a similar observation in the case of microcapsules without CaM (1st perfusion cycle of Fig. 6) is not apparent. When the DynaMicCaps are exposed to CaCl2 solutions, CaM changes back to the Ca2+-bound ligand-exposed binding state. The thickness of the microcapsule shell wall increased with capsule shrinkage. In addition, buckling of the microcapsule shell walls was observed with capsule shrinkage.
CaM-based hydrogel microspheres endured reversible volume changes when triggered with TFP over five cycles.16 However, it is clear that the magnitude of swelling and shrinking of the DynaMicCaps gradually reduces over the rest of the perfusion cycles compared to the first perfusion cycle (Fig. 5). The gradual shrinking of the DynaMicCaps observed after being triggered with TFP is not noticeable after the third perfusion cycle. It appears that with each perfusion cycle, more CaM desorption occurs from the DynaMicCaps shell walls.66
In the above discussion while explaining the probable scenarios for the observed behavior of the DynaMicCaps, it is assumed that the CaM molecules present within the shell walls of the microcapsules still bind to the TFP ligand. This assumption is in agreement with a recent report where CaM bound to a self-assembled monolayer (SAM) underwent all three conformational changes.67 Although that study was with SAMs and the current study is with PEMs, in both the cases, CaM molecules are present as the outermost layer. Interestingly, a very recent report found no evidence that the compact globular, ligand-bound state of CaM can be triggered inside a PEM.68 Nevertheless, it is noteworthy that PAH (and not CaM) is present as the outermost layer of the PEMs in that study. It will be intriguing to know whether the presence of CaM in the outermost surface of the microcapsules is the essential difference that enables the volume changes in the DynaMicCaps. Therefore, further investigations are critical for a better understanding of the mechanisms responsible for the effects observed for the hollow DynaMicCaps.
A majority of the currently available pH-responsive microcapsules capable of releasing their cargo at near physiological pH do so through the disintegration of the microcapsule shell wall. Based on the preliminary results, it is clear that the DynaMicCaps undergo volume changes at near physiological pH without being disintegrated. However, when triggered with TFP, it appears that CaM gets released from the shell walls of the DynaMicCaps. Therefore, future work should also consider crosslinking of the films to prevent the loss of CaM from the shell walls of the capsules, thereby retaining the volume changing ability of DynaMicCaps. Besides, the application of DynaMicCaps for the encapsulation and release of various compounds should be explored in the future.
Experimental
Materials
Calmodulin bovine (Mw 19 kDa), calcium chloride (CaCl2), sodium chloride (NaCl), sodium carbonate (Na2CO3), poly(allylamine hydrochloride) (PAH, Mw 900 kDa), poly(sodium 4-styrenesulfonate) (PSS, Mw 70 kDa), poly(diallyldimethyl ammonium chloride) (PDDA), tetramethylrhodamine-5-(and-6)-isothiocyanate (TRITC), ethylene glycol-bis(b-aminoethyl ether)n (EGTA), ethylenediaminetetraacetic acid (EDTA), trifluoperazine dihydrochloride (TFP), and MES buffer were procured from Sigma-Aldrich.Fabrication of DynaMicCaps
CaCO3 microparticles were fabricated through a precipitation reaction between 20 mL solutions of 0.33 M Na2CO3 and CaCl2 as previously reported.69 Using the LbL self-assembly, positively charged PAH (2 mg mL−1, pH 6.3, 0.5 M NaCl) was deposited onto the negatively charged 2% (w/v) CaCO3 under shaking for 15 min. The modified cores were separated by centrifugation at 500 g for 5 min and rinsing with 1.5 mL of DI water (3×). Next, a negatively charged PSS (2 mg mL−1, pH 6.3, 0.5 M NaCl) was deposited using a procedure followed for PAH. The process was repeated until three (PAH/PSS) bilayers were deposited. A similar process was used to deposit PAH-TRITC (2 mg mL−1, pH 6.3, 0.5 M NaCl) and negatively charged CaM (100 μg mL−1, pH 6.3, 10 mM CaCl2, 50 mM MES, 150 mM NaCl), with 30 min shaking for CaM. The process was repeated until three (PAH-TRITC/CaM) bilayers were deposited. Hollow microcapsules were obtained by treating the modified CaCO3 cores with EDTA (0.15 M, pH 7.5) under shaking for 30 min. The microcapsules were separated by centrifugation at 1500g for 5 min and washing with fresh EDTA (0.15 M, pH 7.5) (3×) followed by washing with DI water (3×). The microcapsules were stored in DI water at 4 °C.Characterization of DynaMicCaps
Confocal Laser Scanning Microscopy (CLSM) was used for real-time observations of the DynaMicCaps under quasi-dynamic perfusion using a LSM-710 (Carl Zeiss Microscopy GmbH, Germany) inverted CLSM equipped with a 63× oil immersion objective. The microcapsules were loaded into an off-the-shelf microchannel (μ-Slide VI-Flat, ibiTreat, #1.5 polymer coverslip, ibidi GmbH, Germany) modified with positively charged PDDA (2 mg mL−1, pH 6.3, 0.5 M NaCl) for 30 min, to aid settling of the capsules on the coverslip. Using passive pumping,70 DI water was exchanged (3×) with CaCl2-buffer (pH 6.3, 10 mM CaCl2, 20 mM MES, 150 mM NaCl) and the capsules were allowed to settle down. A Zeiss LSM-710 inverted CLSM along with the ZEN 2009 light-edition software was used to capture time-lapse confocal fluorescence micrographs (63× magnification). During the time-lapse imaging, passive pumping was used to quickly exchange (3×) the CaCl2-buffer with the TFP buffer (pH 6.3, 10 mM TFP, 20 mM MES, 150 mM NaCl) and the EGTA buffer (pH 6.3, 10 mM EGTA, 20 mM MES, 150 mM NaCl). The ZEN 2012 blue-edition software was used to measure the equivalent circular diameters of the microcapsules. The black and white values in the histogram were adjusted to get the best images. The spline contour graphics tool was used to draw the circumference of the microcapsules manually, and the value of the equivalent circular diameter provided by the software was recorded and used to calculate the estimated volume of microcapsules. This procedure was repeated for each image of the time series. SciDAVis 1.D005 was used to plot the graphs. The fine surface morphological details of the DynaMicCaps were observed using a scanning electron microscope (SEM) (JSM-6360 LA JEOL, US). The microcapsules were dried at room temperature followed by the deposition of a thin coating of gold before performing SEM microscopy. Adobe CS6 was used to prepare the illustrations.Conclusions
In summary, LbL self-assembled DynaMicCaps were successfully fabricated, and evaluated under quasi-dynamic perfusion. Preliminary results indicate that biochemically triggered rapid and pronounced volume changes of the DynaMicCaps, accompanied by an increased shell wall permeability,23,71 can potentially be used for the release of drugs preloaded into the nano-engineered hollow microcarriers.53 Such a pulsed or non-uniform release of drugs would be preferable in certain medical applications, for example, in the delivery of anticancer agents, proteins, hormones, and vaccines,31,72–74 enhancing the therapeutic efficacy and minimizing toxicity.31 The DynaMicCaps presented in this work symbolize a new class of PEM capsules that can potentially be used to release their payload at near physiological pH.22 With the availability of over 200 proteins that undergo marked, well-characterized conformational changes in response to specific biochemical triggers, it is possible to develop several other versions of DynaMicCaps with CaM replacements.2,3 Future work will include testing the potential of DynaMicCaps for triggered drug release.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the Ministry of Higher Education (MOHE) of Malaysia under the Fundamental Research Grant Scheme (FRGS), Grant no. FRGS/1/2014/TK04/UNISZA/02/1.References
- J. S. Mohammed and W. L. Murphy, Adv. Mater., 2009, 21, 2361–2374 CrossRef CAS.
- W. J. King and W. L. Murphy, Polym. Chem., 2011, 2, 476–491 RSC.
- W. L. Murphy, Soft Matter, 2011, 7, 3679–3688 RSC.
- S. Banta, I. R. Wheeldon and M. Blenner, Annu. Rev. Biomed. Eng., 2010, 12, 167–186 CrossRef CAS PubMed.
- M. Kim, S. Tang and B. D. Olsen, J. Polym. Sci., Part B: Polym. Phys., 2013, 51, 587–601 CrossRef CAS.
- J. Stigler and M. Rief, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 17814–17819 CrossRef CAS PubMed.
- D. K. Menyhárd, G. M. Keseru and G. Náray-Szabó, Curr. Comput.-Aided Drug Des., 2009, 5, 264–279 CrossRef.
- N. Yamaotsu, M. Suga and S. Hirono, Biopolymers, 2001, 58, 410–421 CrossRef CAS PubMed.
- W. L. Murphy, W. S. Dillmore, J. Modica and M. Mrksich, Angew. Chem., Int. Ed., 2007, 46, 3066–3069 CrossRef CAS PubMed.
- J. D. Ehrick, S. K. Deo, T. W. Browning, L. G. Bachas, M. J. Madou and S. Daunert, Nat. Mater., 2005, 4, 298–302 CrossRef CAS PubMed.
- J. D. Ehrick, S. Stokes, S. Bachas-Daunert, E. A. Moschou, S. K. Deo, L. G. Bachas and S. Daunert, Adv. Mater., 2007, 19, 4024–4027 CrossRef CAS.
- W. J. King, J. S. Mohammed and W. L. Murphy, Soft Matter, 2009, 5, 2399–2406 RSC.
- Z. Sui, W. J. King and W. L. Murphy, Adv. Mater., 2007, 19, 3377–3380 CrossRef CAS.
- Z. Sui, W. J. King and W. L. Murphy, Adv. Funct. Mater., 2008, 18, 1824–1831 CrossRef CAS.
- W. J. King, M. W. Toepke and W. L. Murphy, Acta Biomater., 2011, 7, 975–985 CrossRef CAS PubMed.
- W. J. King, N. J. Pytel, K. Ng and W. L. Murphy, Macromol. Biosci., 2010, 10, 580–584 CrossRef CAS PubMed.
- W. J. King, M. W. Toepke and W. L. Murphy, Chem. Commun., 2011, 47, 526–528 RSC.
- G. B. Sukhorukov, E. Donath, S. Davis, H. Lichtenfeld, F. Caruso, V. I. Popov and H. Möhwald, Polym. Adv. Technol., 1998, 9, 759–767 CrossRef CAS.
- E. Donath, G. B. Sukhorukov, F. Caruso, S. A. Davis and H. Möhwald, Angew. Chem., Int. Ed., 1998, 37, 2201–2205 CrossRef.
- O. I. Vinogradova, O. V. Lebedeva and B.-S. Kim, Annu. Rev. Mater. Res., 2006, 36, 143–178 CrossRef CAS.
- O. I. Vinogradova, J. Phys.: Condens. Matter, 2004, 16, R1105 CrossRef CAS.
- B. G. De Geest, N. N. Sanders, G. B. Sukhorukov, J. Demeester and S. C. De Smedt, Chem. Soc. Rev., 2007, 36, 636–649 RSC.
- C. Gao, S. Leporatti, S. Moya, E. Donath and H. Möhwald, Chem. – Eur. J., 2003, 9, 915–920 CrossRef CAS PubMed.
- W. Qi, L. Duan, K. Wang, X. Yan, Y. Cui, Q. He and J. Li, Adv. Mater., 2008, 20, 601–605 CrossRef CAS.
- M. Delcea, H. Möhwald and A. G. Skirtach, Adv. Drug Delivery Rev., 2011, 63, 730–747 CrossRef CAS PubMed.
- A. S. Timin, D. J. Gould and G. B. Sukhorukov, Expert Opin. Drug Delivery, 2017, 14, 583–587 CrossRef PubMed.
- A. S. Timin, H. Gao, D. V. Voronin, D. A. Gorin and G. B. Sukhorukov, Adv. Mater. Interfaces, 2017, 4, 1600338 CrossRef.
- S. De Koker, L. J. De Cock, P. Rivera-Gil, W. J. Parak, R. Auzély Velty, C. Vervaet, J. P. Remon, J. Grooten and B. G. De Geest, Adv. Drug Delivery Rev., 2011, 63, 748–761 CrossRef CAS PubMed.
- B. G. De Geest, G. B. Sukhorukov and H. Möhwald, Expert Opin. Drug Delivery, 2009, 6, 613–624 CrossRef CAS PubMed.
- L. Hartmann, M. Bedard, H. G. Börner, H. Möhwald, G. B. Sukhorukov and M. Antonietti, Soft Matter, 2008, 4, 534–539 RSC.
- K. Radhakrishnan and A. M. Raichur, Chem. Commun., 2012, 48, 2307–2309 RSC.
- K. Radhakrishnan, J. Tripathy and A. M. Raichur, Chem. Commun., 2013, 49, 5390–5392 RSC.
- W.-C. Liao, C.-H. Lu, R. Hartmann, F. Wang, Y. S. Sohn, W. J. Parak and I. Willner, ACS Nano, 2015, 9, 9078–9086 CrossRef CAS PubMed.
- W.-C. Liao, M. Riutin, W. J. Parak and I. Willner, ACS Nano, 2016, 10, 8683–8689 CrossRef CAS PubMed.
- F. Huang, W.-C. Liao, Y. S. Sohn, R. Nechushtai, C.-H. Lu and I. Willner, J. Am. Chem. Soc., 2016, 138, 8936–8945 CrossRef CAS PubMed.
- A. N. Zelikin, J. F. Quinn and F. Caruso, Biomacromolecules, 2006, 7, 27–30 CrossRef CAS PubMed.
- A. N. Zelikin, Q. Li and F. Caruso, Angew. Chem., Int. Ed., 2006, 45, 7743–7745 CrossRef CAS PubMed.
- A. N. Zelikin, A. L. Becker, A. P. Johnston, K. L. Wark, F. Turatti and F. Caruso, ACS Nano, 2007, 1, 63–69 CrossRef CAS PubMed.
- A. N. Zelikin, Q. Li and F. Caruso, Chem. Mater., 2008, 20, 2655–2661 CrossRef CAS.
- A. L. Becker, A. P. R. Johnston and F. Caruso, Small, 2010, 6, 1836–1852 CAS.
- K. Kempe, K. F. Noi, S. L. Ng, M. Müllner and F. Caruso, Polymer, 2014, 55, 6451–6459 CrossRef CAS.
- K. Liang, G. K. Such, Z. Zhu, Y. Yan, H. Lomas and F. Caruso, Adv. Mater., 2011, 23, H273–H277 CrossRef CAS PubMed.
- K. Liang, G. K. Such, A. P. Johnston, Z. Zhu, H. Ejima, J. J. Richardson, J. Cui and F. Caruso, Adv. Mater., 2014, 26, 1901–1905 CrossRef CAS PubMed.
- H. H. Dam and F. Caruso, Langmuir, 2013, 29, 7203–7208 CrossRef CAS PubMed.
- Y. Ping, J. Guo, H. Ejima, X. Chen, J. J. Richardson, H. Sun and F. Caruso, Small, 2015, 11, 2032–2036 CrossRef CAS PubMed.
- X. Yan, K. Hill, H. Gao and H.-F. Ji, Langmuir, 2006, 22, 11241–11244 CrossRef CAS PubMed.
- A. I. Petrov, A. A. Antipov and G. B. Sukhorukov, Macromolecules, 2003, 36, 10079–10086 CrossRef CAS.
- J. P. Junker, F. Ziegler and M. Rief, Science, 2009, 323, 633–637 CrossRef CAS PubMed.
- H. Bysell, R. Månsson, P. Hansson and M. Malmsten, Adv. Drug Delivery Rev., 2011, 63, 1172–1185 CrossRef CAS PubMed.
- K. Köhler, H. Möhwald and G. B. Sukhorukov, J. Phys. Chem. B, 2006, 110, 24002–24010 CrossRef PubMed.
- K. Köhler, D. G. Shchukin, H. Möhwald and G. B. Sukhorukov, J. Phys. Chem. B, 2005, 109, 18250–18259 CrossRef PubMed.
- J. Yu, B. M. Meharg and I. Lee, Polymer, 2017, 109, 297–306 CrossRef CAS.
- B.-S. Kim and J.-W. Choi, Biotechnol. Bioprocess Eng., 2007, 12, 323 CrossRef CAS.
- F. Li, Y. Yan, D. Wang, J. Zhang and S. Guo, J. Microencapsulation, 2014, 31, 567–572 CrossRef CAS PubMed.
- A. M. Pavlov, G. B. Sukhorukov and D. J. Gould, J. Controlled Release, 2013, 172, 22–29 CrossRef CAS PubMed.
- W. Caetano and M. Tabak, J. Colloid Interface Sci., 2000, 225, 69–81 CrossRef CAS PubMed.
- A. Broniec, A. U. Gjerde, A. B. Ølmheim and H. Holmsen, Langmuir, 2007, 23, 694–699 CrossRef CAS PubMed.
- M. Asghar and I. Khan, Braz. J. Med. Biol. Res., 2008, 41, 455–461 CrossRef CAS PubMed.
- N. Seedher, Indian J. Pharm. Sci., 2000, 62, 16 CAS.
- M. Vandonselaar, R. A. Hickie, J. W. Quail and L. Delbaere, Nat. Struct. Biol., 1994, 1, 795–801 CrossRef CAS PubMed.
- R. Sharma and R. K. Mahajan, RSC Adv., 2012, 2, 9571–9583 RSC.
- N. Matsushima, N. Hayashi, Y. Jinbo and Y. Izumi, Biochem. J., 2000, 347, 211–215 CrossRef CAS PubMed.
- X. Wang, J. Shi, Z. Jiang, Z. Li, W. Zhang, X. Song, Q. Ai and H. Wu, Biomacromolecules, 2013, 14, 3861–3869 CrossRef CAS PubMed.
- P. M. Biesheuvel, T. Mauser, G. B. Sukhorukov and H. Möhwald, Macromolecules, 2006, 39, 8480–8486 CrossRef CAS.
- C. Déjugnat and G. B. Sukhorukov, Langmuir, 2004, 20, 7265–7269 CrossRef PubMed.
- Z. She, M. N. Antipina, J. Li and G. B. Sukhorukov, Biomacromolecules, 2010, 11, 1241–1247 CrossRef CAS PubMed.
- H. Noguchi, T. Adachi, A. Nakatomi, M. Yazawa and K. Uosaki, J. Phys. Chem. C, 2016, 120, 16035–16041 CAS.
- S. Cinar, S. Möbitz, S. Al-Ayoubi, B.-K. Seidlhofer and C. Czeslik, Langmuir, 2017, 33, 3982–3990 CrossRef CAS PubMed.
- D. V. Volodkin, A. I. Petrov, M. Prevot and G. B. Sukhorukov, Langmuir, 2004, 20, 3398–3406 CrossRef CAS PubMed.
- G. M. Walker and D. J. Beebe, Lab Chip, 2002, 2, 131–134 RSC.
- W. Tong, C. Gao and H. Möhwald, Colloid Polym. Sci., 2008, 286, 1103 CAS.
- B. G. De Geest, C. Déjugnat, G. B. Sukhorukov, K. Braeckmans, S. C. De Smedt and J. Demeester, Adv. Mater., 2005, 17, 2357–2361 CrossRef CAS.
- B. G. De Geest, C. Déjugnat, M. Prevot, G. B. Sukhorukov, J. Demeester and S. C. De Smedt, Adv. Funct. Mater., 2007, 17, 531–537 CrossRef CAS.
- W. Tong and C. Gao, J. Mater. Chem., 2008, 18, 3799–3812 RSC.
| This journal is © The Royal Society of Chemistry 2018 |