Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Closely related yet different: a borylene and its dimer are non-interconvertible but connected through reactivity†
Dominic
Auerhammer
ab,
Merle
Arrowsmith
 ab,
Rian D.
Dewhurst
ab,
Rian D.
Dewhurst
 ab,
Thomas
Kupfer
ab,
Julian
Böhnke
ab and
Holger
Braunschweig
ab,
Thomas
Kupfer
ab,
Julian
Böhnke
ab and
Holger
Braunschweig
 *ab
*ab
aInstitut für Anorganische Chemie, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany. E-mail: h.braunschweig@uni-wuerzburg.de
bInstitute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
First published on 4th January 2018
Abstract
The self-stabilizing, tetrameric cyanoborylene [(cAAC)B(CN)]4 (I, cAAC = 1-(2,6-diisopropylphenyl)-3,3,5,5-tetramethylpyrrolidin-2-ylidene) and its diborene relative, [(cAAC)(CN)B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B(CN)(cAAC)] (II), both react with disulfides and diselenides to yield the corresponding cAAC-supported cyanoboron bis(chalcogenides). Furthermore, reactions of I or II with elemental sulfur and selenium in various stoichiometries provided access to a variety of cAAC-stabilized cyanoboron–chalcogen heterocycles, including a unique dithiaborirane, a diboraselenirane, 1,3-dichalcogena-2,4-diboretanes, 1,3,4-trichalcogena-2,5-diborolanes and a rare six-membered 1,2,4,5-tetrathia-3,6-diborinane. Stepwise addition reactions and solution stability studies provided insights into the mechanism of these reactions and the subtle differences in reactivity observed between I and II.
B(CN)(cAAC)] (II), both react with disulfides and diselenides to yield the corresponding cAAC-supported cyanoboron bis(chalcogenides). Furthermore, reactions of I or II with elemental sulfur and selenium in various stoichiometries provided access to a variety of cAAC-stabilized cyanoboron–chalcogen heterocycles, including a unique dithiaborirane, a diboraselenirane, 1,3-dichalcogena-2,4-diboretanes, 1,3,4-trichalcogena-2,5-diborolanes and a rare six-membered 1,2,4,5-tetrathia-3,6-diborinane. Stepwise addition reactions and solution stability studies provided insights into the mechanism of these reactions and the subtle differences in reactivity observed between I and II.
Introduction
The dimerisation of carbenes [R2C:] to form alkenes (Fig. 1A) is a fundamental reaction pattern in carbene chemistry1 and the basis of the so-called Wanzlick equilibrium.2 A similar dimerisation process has also been observed with heavier analogues of carbenes.3 However, despite the existence of isoelectronic group 13 analogues of carbenes – borylenes of the form [LRB:] (R = anionic substituent, L = neutral Lewis basic substituent) – no carbene-analogous dimerisation (Fig. 1B) has been observed for group 13 species.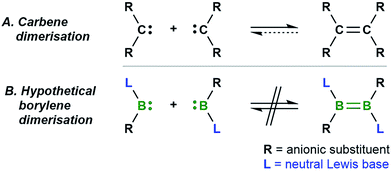 | ||
| Fig. 1 The well-known dimerisation of carbenes (A) and the currently unknown dimerisation of isoelectronic [:BRL] borylenes (B). | ||
Singly base-stabilised [LRB:] borylenes belong to the family of monovalent boron species discovered and recently reviewed by Bertrand et al.4 Perhaps the most definitive and well-defined example of this class of compounds is the linear borylene [(cAACCy)BN(SiMe3)2] (cAACCy = 2-(2,6-diisopropylphenyl)-3,3-dimethyl-2-azaspiro[4.5]decan-1-ylidene5) reported by Stephan and Bertrand.6 Over the past few years, however, our group has demonstrated that a number of doubly base-stabilized borylene species ([LL′RB:]) can act as synthons for [LRB:] borylenes, either through photodecarbonylation7,8 or the base-mediated deaggregation of tetrameric cyanoborylene [(cAAC)B(CN)]4 (I, cAAC = 1-(2,6-diisopropylphenyl)-3,3,5,5-tetramethylpyrrolidin-2-ylidene).9
The generation of [LRB:] borylenes, either stable or transient, has allowed the study of coordination chemistry at monovalent boron centers through binding of new Lewis bases, in addition to intramolecular C–H and C–C bond activations in the absence of a suitable base.6–9 The currently unrealised possibility of dimerising [LRB:] species would provide a new synthetic route to doubly base-stabilised diborenes of the form [LRB![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) BRL], which still suffer from considerable synthetic restrictions.10
BRL], which still suffer from considerable synthetic restrictions.10
Our recent synthesis of the aforementioned tetraborylene macrocycle [(cAAC)B(CN)]4 (I; Scheme 1, left)9 and the doubly base-stabilised dicyanodiborene [(cAAC)(NC)B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B(CN)(cAAC)] (II; Scheme 1, right)11 presented an interesting juxtaposition of related molecules. Macrocycle I is a tetramer (and synthetic equivalent) of the borylene [(cAAC)(CN)B:], while diborene II is a formal dimer of the same borylene. Having failed thus far to realise the interconversion of I and II through photolysis and/or heating, we were eager to test their respective reactivity with suitable reagents in order to define any differences or similarities, and hopefully gain definitive proof of their ability (or inability) to interconvert. Herein we present the reactivity of I and II with elemental chalcogens and dichalcogenides, based on our recently-reported reactions of these chalcogen reagents with B–B multiply-bonded species.12,13 Our results indicate that, although extensive similarities exist between borylene I and its formal dimer II, and they may therefore be viewed as close relatives, subtle differences in reactivity confirm that no Wanzlick-type equilibrium exists between the two.
B(CN)(cAAC)] (II; Scheme 1, right)11 presented an interesting juxtaposition of related molecules. Macrocycle I is a tetramer (and synthetic equivalent) of the borylene [(cAAC)(CN)B:], while diborene II is a formal dimer of the same borylene. Having failed thus far to realise the interconversion of I and II through photolysis and/or heating, we were eager to test their respective reactivity with suitable reagents in order to define any differences or similarities, and hopefully gain definitive proof of their ability (or inability) to interconvert. Herein we present the reactivity of I and II with elemental chalcogens and dichalcogenides, based on our recently-reported reactions of these chalcogen reagents with B–B multiply-bonded species.12,13 Our results indicate that, although extensive similarities exist between borylene I and its formal dimer II, and they may therefore be viewed as close relatives, subtle differences in reactivity confirm that no Wanzlick-type equilibrium exists between the two.
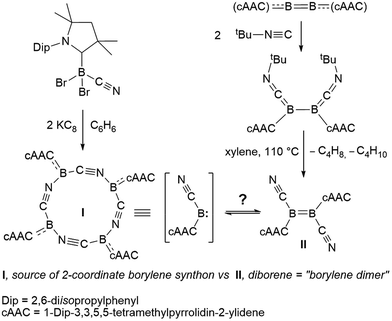 | ||
| Scheme 1 Syntheses of two boron(I) compounds of the same empirical formula, cyanoborylene I and dicyanodiborene II. | ||
Results and discussion
Synthetic studies and characterisation
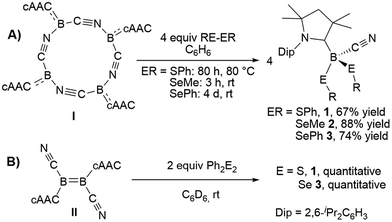
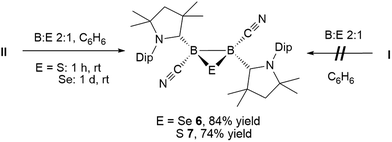
The reaction of tetrameric borylene I with four equivalents of diorganyldichalcogenides, E2R2 (E = S, Se, R = Ph; E = Se, R = Me) in benzene proceeded selectively to the corresponding cAAC-supported cyanoboron dichalcogenides, [(cAAC)B(CN)(ER)2] (ER = SPh 1, SeMe 2, SePh 3), which were isolated in moderate to good yields (Scheme 2A). Whereas the reaction with diphenyldisulfide required prolonged heating at 80 °C to proceed to completion, reactions with diselenides proceeded at room temperature, the reaction with Se2Me2 being significantly faster than that with Se2Ph2. Compounds 1–3 showed 11B NMR resonances typical of sp3 hybridized boranes, with the bis(selenides) 2 (−18.4 ppm) and 3 (−14.4 and −15.8 ppm) exhibiting shifts significantly downfield from the bis(sulfide) 1 (−9.6 ppm). Furthermore, at room temperature the bis(phenylselenide) 3 displayed highly broadened 1H NMR ligand resonances as well as two distinct, very broad 11B NMR resonances (−14.4 and −15.8 ppm), which coalesced upon heating to 70 °C, indicating hindered rotation, presumably owing to steric interactions between the bulky cAAC substituents and the large phenylselenide ligands.NMR spectroscopic data of reaction mixtures of diborene II and Ph2S2 or Ph2Se2 showed the monoboron bis(chalcogenides) 1 and 3 to be sole products of these reactions, independent of the stoichiometry used (Scheme 2B). These reactions proceeded much faster and at lower temperatures than the corresponding additions of dichalcogenides to the tetrameric borylene I. Although no intermediates were observed under these reaction conditions, it is likely that the formation of 1 and 3 from II proceeds via the successive 1,2-addition of the E–E σ-bond first across the B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B double bond and, subsequently, across the remaining B–B single bond. Although both compounds I and II reacted with Ph2Te2 at high temperatures, these reactions were rather unselective and did not yield any tractable products.
B double bond and, subsequently, across the remaining B–B single bond. Although both compounds I and II reacted with Ph2Te2 at high temperatures, these reactions were rather unselective and did not yield any tractable products.
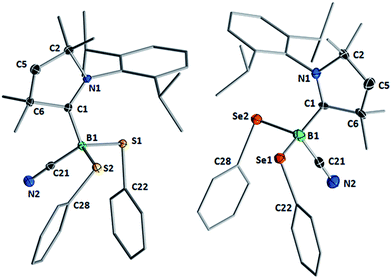
Compounds 1–3 readily crystallized from THF at room temperature, 1 as a colorless crystalline solid, and 2 and 3 as yellow crystals.‡Fig. 2 shows the crystallographically determined structures of the bis(phenylchalcogenides) 1 and 3, which crystallize in isomorphous unit cells. The B–CcAAC bond length in disulfide 1 (1.6297(19) Å) is slightly elongated compared to that in diselenide 3 (1.597(9) Å). The B–CCN bond lengths (1: 1.584(2); 3: 1.580(9)) are similar to those found in other cAAC-supported cyanoboranes (1.574(5)–1.589(3) Å).9,14 While the B–E bond lengths (B–S: 1.9248(15), 1.9578(16) Å; B–Se: 2.109(7), 2.056(8) Å) are within the typical range for Lewis-base-stabilized boron organochalcogenides, such as Marder and Norman's pyridine and phosphine adducts of 2,2′-bibenzo[d][1,3,2]dithiaboroles (B–S: 1.899(5)–1.930(2) Å)15 or Wrackmeyer's base-stabilized boron 1,2-diselenato-ortho-carboranes (B–Se: 2.031(6)–2.065(5) Å).16 While structurally unspectacular, species 1–3 represent the first examples of boron chalcogenides synthesized by the atom efficient insertion of a borylene into the E–E bond of a diorganodichalcogenide.
Stirring of a dark red suspension of tetrameric borylene I with elemental sulfur in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
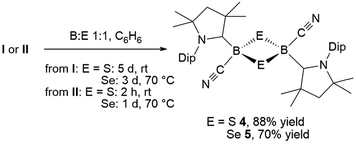
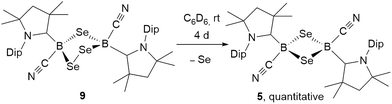
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 boron-to-sulfur ratio in benzene for 5 d at room temperature resulted in a yellow suspension, which upon filtration and slow evaporation yielded compound 4 as a yellow crystalline solid (Scheme 3). Compound 4 displayed a 11B NMR singlet at −17.9 ppm and a single set of 1H NMR resonances for the cAAC ligand, suggesting a highly symmetrical species. Despite repeated recrystallization attempts, crystalline samples of 4 always contained 5–10% of another species displaying a higher field 11B NMR resonance at −9 ppm (vide infra). The analogous reaction with elemental selenium at 60 °C for 3 d similarly provided compound 5 as an orange crystalline solid in 70% yield. Compound 5 was isolated as a single species with an 11B NMR singlet at −33.5 ppm, shifted ca. 25 ppm upfield from that of 4, and a highly shielded, broad 77Se{1H} NMR resonance at −143.1 ppm. In solution at room temperature 5 partially isomerized to a second species presenting an 11B NMR shift at −31.8 ppm and slightly shifted 1H and 13C NMR resonances. High resolution mass spectrometry experiments performed on 4 and 5 provided molecular masses consistent with dimeric compounds of the formula [(cAAC)B(CN)E]2 (E = S 4, Se 5).
1 boron-to-sulfur ratio in benzene for 5 d at room temperature resulted in a yellow suspension, which upon filtration and slow evaporation yielded compound 4 as a yellow crystalline solid (Scheme 3). Compound 4 displayed a 11B NMR singlet at −17.9 ppm and a single set of 1H NMR resonances for the cAAC ligand, suggesting a highly symmetrical species. Despite repeated recrystallization attempts, crystalline samples of 4 always contained 5–10% of another species displaying a higher field 11B NMR resonance at −9 ppm (vide infra). The analogous reaction with elemental selenium at 60 °C for 3 d similarly provided compound 5 as an orange crystalline solid in 70% yield. Compound 5 was isolated as a single species with an 11B NMR singlet at −33.5 ppm, shifted ca. 25 ppm upfield from that of 4, and a highly shielded, broad 77Se{1H} NMR resonance at −143.1 ppm. In solution at room temperature 5 partially isomerized to a second species presenting an 11B NMR shift at −31.8 ppm and slightly shifted 1H and 13C NMR resonances. High resolution mass spectrometry experiments performed on 4 and 5 provided molecular masses consistent with dimeric compounds of the formula [(cAAC)B(CN)E]2 (E = S 4, Se 5).
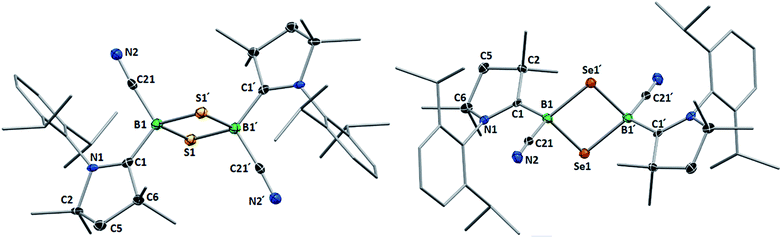
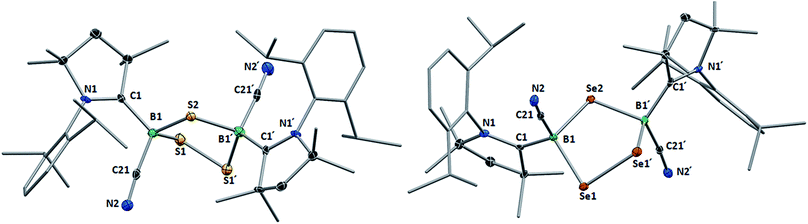
This was confirmed by X-ray crystallographic analyses, the results of which are displayed in Fig. 3. Compounds 4 and 5 crystallized in near-identical triclinic unit cells as centrosymmetric species presenting planar 1,3-dithia- and 1,3-diselena-2,4-diboretane cores, respectively. The B2S2 ring in 4 is an approximate square with four almost identical B–S bonds (1.939(3) and 1.940(3) Å) and near-perpendicular B–S–B and S–B–S angles (85.21(12) and 94.80(12)°, respectively), whereas the planar B2Se2 ring in 5 displays two slightly different B–Se bond lengths (2.069(4) and 2.100(4) Å) as well as near-perpendicular B–Se–B and Se–B–Se angles (85.13(17) and 94.87(17)°, respectively). There are only a couple of structurally characterized 1,3,2,4-dichalcogenadiboretanes in the literature, all displaying sp2 hybridized boron centers stabilized by π-donating amino substituents.17,18 Due to the lower coordination number at boron, the B–E bonds in Nöth's 2,2,6,6-tetramethylpiperidine-supported B2S2 and B2Se2 heterocycles are ca. 0.08–0.10 Å shorter than in 4 and 5, while the B–E–B and E–B–E angles are ca. 3° narrower and wider, respectively.18
For both compounds the plane consisting of the cyanoboron moiety and the π-framework of the cAAC ligand forms a ca. 80° angle with the B2E2 plane, with the respective ligands lying in trans-conformation with respect to the B2E2 core. While the solid-state structure of 5 shows a single conformational isomer, it remains unclear whether the observation of two isomers in solution is the result of a 180° rotation of one of the cAAC ligands around the B–CcAAC bond or the existence of a cis-conformer, in which both cyano ligands occupy a cis-arrangement with respect to the heterocyclic core. The B–CcAAC bond lengths (4: 1.639(4), 5: 1.606(5) Å) suggest that the cAAC ligand functions as a pure σ-donor ligand to the sp3 borane, unlike in borylene I, where a significant contribution of π-backbonding from the electron-rich B(I) center shortens the bond (ca. 1.47 Å). It is noteworthy that both the B–CcAAC and B–CCN bond lengths are significantly longer in 4 than in 5 (B–CCN: 4 1.597(4), 5 1.577(6) Å).
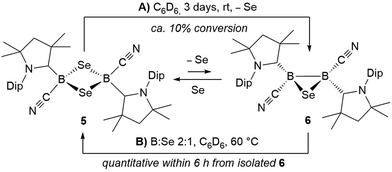
Whereas 4 proved indefinitely stable in solution at room temperature, the 11B NMR spectrum of an analytically pure sample of 5 in CD2Cl2 left at room temperature for 3 d showed the partial disappearance (<10%) of 5 concomitant with the appearance of a new boron-containing species at −22.8 ppm (Scheme 4A). A few crystals of this species could be isolated by recrystallization from diethyl ether and analyzed by mass spectroscopy, revealing a compound of the formula [(cAAC)2B2(CN)2Se] (6). X-ray crystallographic analysis of this compound showed that 6 is indeed a bis(cAAC)-stabilized 2,3-dicyano-2,3,1-diboraselenirane (Fig. 4).§ The structure of 6 is similar to that of the bis(NHC)-stabilized 2,3-dithienyl-2,3,1-diboraselenirane obtained by our group upon reaction of one equivalent of selenium with the corresponding diborene precursor,13 and likewise displays a trans-arrangement of the cyano and cAAC ligands with respect to the B2Se core. Attempts to generate 6 from I using a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 boron-to-selenium ratio failed, resulting instead in 50% conversion to the diselenadiboretane 5, with the remaining 50% of I left unreacted (Scheme 5). It thus appears that 6 is not accessible directly from borylene I but can be generated in solution at room temperature through loss of one selenium atom from 5, concomitant with B–B bond formation. This was also confirmed by closer inspection of the mass spectrum of compound 5 which showed mass patterns corresponding to 6.
1 boron-to-selenium ratio failed, resulting instead in 50% conversion to the diselenadiboretane 5, with the remaining 50% of I left unreacted (Scheme 5). It thus appears that 6 is not accessible directly from borylene I but can be generated in solution at room temperature through loss of one selenium atom from 5, concomitant with B–B bond formation. This was also confirmed by closer inspection of the mass spectrum of compound 5 which showed mass patterns corresponding to 6.
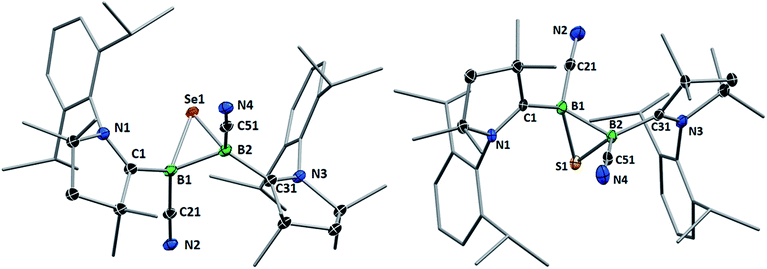 | ||
| Fig. 4 Crystallographically determined solid-state structures of the major one (88%) of the two overlapping disordered molecules of 6 in the asymmetric unit (left)§ and 7 (right). Atomic displacement ellipsoids depicted at 50% probability level. Hydrogen atoms and atomic displacement ellipsoids of peripheral substituents omitted for clarity. Selected bond lengths (Å) and angles (°) for 6: B1–C1 1.585(5), B2–C31 1.599(6), B1–C21 1.597(4), B2–C51 1.571(6), B1–B2 1.757(6), B1–Se1 2.030(4), B2–Se1 2.027(4); B1–Se1–B2 51.31(18), Se1–B1–B2 64.27(18), Se1–B2–B1 64.42(19). For 7: B1–C1 1.575(6), B2–C31 1.580(6), B1–C21 1.557(6), B2–C51 1.549(7), B1–B2 1.777(6), B1–S1 1.860(5), B2–S1 1.869(5); B1–S1–B2 56.9(2), S1–B1–B2 61.8(2), S1–B2–B1 61.3(2). | ||
Conversely, compound 6 was the only product of the reaction of diborene II with Se (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of boron to selenium) at room temperature in C6H6, as shown by the appearance of the 11B NMR shift around −22 ppm (Scheme 5). Isolated samples of 6 showed asymmetric 1H NMR cAAC resonances due to the presence of the chiral centers in B1 and B2. Attempts to detect the 77Se NMR resonance of 6 failed due to coupling to the two adjacent quadrupolar boron nuclei. Similarly, the reaction of diborene II with S (2
1 ratio of boron to selenium) at room temperature in C6H6, as shown by the appearance of the 11B NMR shift around −22 ppm (Scheme 5). Isolated samples of 6 showed asymmetric 1H NMR cAAC resonances due to the presence of the chiral centers in B1 and B2. Attempts to detect the 77Se NMR resonance of 6 failed due to coupling to the two adjacent quadrupolar boron nuclei. Similarly, the reaction of diborene II with S (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of boron to sulfur) at room temperature in C6H6 yielded the corresponding 2,3-dicyano-1,2,3-thiadiborirane 7 (Scheme 5), which presented an intense orange coloration in solution and is, to our knowledge, the first example of a thiadiborirane. The 11B NMR shift of 7, observed at −22.6 ppm, is nearly identical to that of the selenium analogue 6, suggesting little electronic influence of the chalcogen atom in these B2E heterocycles. Both 6 and 7 also displayed a near-identical set of unsymmetrical 1H NMR cAAC resonances owing to the presence of the two chiral centers at boron.
1 ratio of boron to sulfur) at room temperature in C6H6 yielded the corresponding 2,3-dicyano-1,2,3-thiadiborirane 7 (Scheme 5), which presented an intense orange coloration in solution and is, to our knowledge, the first example of a thiadiborirane. The 11B NMR shift of 7, observed at −22.6 ppm, is nearly identical to that of the selenium analogue 6, suggesting little electronic influence of the chalcogen atom in these B2E heterocycles. Both 6 and 7 also displayed a near-identical set of unsymmetrical 1H NMR cAAC resonances owing to the presence of the two chiral centers at boron.
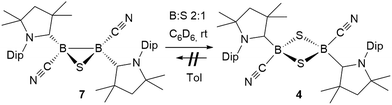
The reaction of 6 with one molar equivalent of selenium heated for 6 hours at 60 °C in benzene resulted in clean conversion to the 1,3-diselena-2,4-diboretane 5, as indicated by the 11B NMR shifts of the two isomers observed at −31.8 and −33.5 ppm (Scheme 4B). Similarly, the addition of one equivalent of elemental sulfur to thiadiborirane 7 cleanly yielded the 1,3-dithia-2,4-diboretane 4 (Scheme 6). But whereas compounds 5 and 6 are in equilibrium in the presence of selenium, with {5 + Se} favoured at room temperature and 6 favoured at elevated temperature, toluene solutions of 5 showed no evidence of ring contraction to 7 even after prolonged storage at −30 °C. The equilibrium between 5 and 6 could not be further quantified due to the poor solubility of compound 5 and elemental selenium at low temperature in toluene. It does, however, constitute a rare example of fully reversible cleavage of a B–B single bond under extremely mild conditions, and is thereby reminiscent of the reversible insertion of molecular CO into the B–B bond of the borylene borane [(cAAC)H2B–B(CO)(cAAC)], which we reported recently.19
Single crystals of 7 showed a structure very similar to that of its selenium analogue 6, displaying the same trans-arrangement of the cyano and cAAC ligands with respect to the B2S core (Fig. 4). The only notable difference lies in the slightly longer B–B bond (7 1.777(6); 6 1.757(6) Å) enforced by the significantly shorter B–E bonds (6: B–S ca. 1.86; 7: B–Se ca. 2.03 Å) and the slightly wider B–E–B angle (6: 56.9(2); 7: 51.31(18)°). Unlike the IMe-supported dithienyldiborene13 or the manganese-bound borylene [Cp(OC)2Mn![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) BtBu(IMe)] (Cp = cyclopentadienyl),20 neither compound I or II showed any reactivity toward elemental tellurium in toluene, even after prolonged heating in benzene at 80 °C.
BtBu(IMe)] (Cp = cyclopentadienyl),20 neither compound I or II showed any reactivity toward elemental tellurium in toluene, even after prolonged heating in benzene at 80 °C.
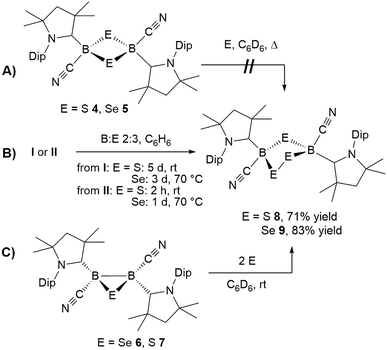
In repeated X-ray crystallographic experiments on single crystals in 4 the refinement of the data generated some residual electron density around the B2S2 ring that hinted at its overlap with a five-membered 1,2,4-dithia-3,5-diborolane ring representing less than 5% of the structure. This was consistent with the observation that recrystallized samples of 4 always showed some contamination with another species showing an 11B NMR resonance at −9 ppm, and that closer inspection of the mass spectrum of 4 revealed traces of a compound with the formula [(cAAC)2B2(CN)2S3]. Based upon this observation, attempts were made to obtain this compound by addition of sulfur to 4. However, even in the presence of excess sulfur with prolonged heating at 60 °C, only starting material was recovered (Scheme 7A).
In contrast, the room temperature reaction of tetrameric borylene I with elemental sulfur in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 boron-to-sulfur ratio yielded the corresponding yellow 1,2,4-trithia-3,5-diborolane 8 as the major reaction product in 71% isolated yield (Scheme 7B). This suggests that 8 is formed directly from I and independently of 4, rather than by insertion of a sulfur atom into the 1,3-disulfa-2,4-diboretane ring. Isolated samples of 8 showed two distinct 11B NMR resonances at −8.5 and −9.1 ppm in a 55
3 boron-to-sulfur ratio yielded the corresponding yellow 1,2,4-trithia-3,5-diborolane 8 as the major reaction product in 71% isolated yield (Scheme 7B). This suggests that 8 is formed directly from I and independently of 4, rather than by insertion of a sulfur atom into the 1,3-disulfa-2,4-diboretane ring. Isolated samples of 8 showed two distinct 11B NMR resonances at −8.5 and −9.1 ppm in a 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 ratio, ca. 9 ppm downfield from that of 4. The 1H NMR spectrum of 8 displayed two isomers in that same ratio, each presenting two sets of cAAC resonances in a 1
45 ratio, ca. 9 ppm downfield from that of 4. The 1H NMR spectrum of 8 displayed two isomers in that same ratio, each presenting two sets of cAAC resonances in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Furthermore, for each isomer, the isopropyl resonances of the Dip residue were split into two sets of asymmetric resonances. The ratio of the two isomers was temperature-independent, suggesting they may be non-exchanging diastereomers.
1 ratio. Furthermore, for each isomer, the isopropyl resonances of the Dip residue were split into two sets of asymmetric resonances. The ratio of the two isomers was temperature-independent, suggesting they may be non-exchanging diastereomers.
The analogous 1,2,4-triselena-3,5-diborolane 9 was successfully isolated as an orange-colored solid as the major product (83% yield) of the reaction of borylene I with selenium in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 boron-to-selenium ratio in benzene at 60 °C (Scheme 7B). Compound 9 presented an 11B NMR resonance at −12.3 ppm, ca. 20 ppm downfield from that of 5. Similarly to 8, the 1H NMR resonances of the isoproyl groups of the Dip residue were split into two sets, indicative of an asymmetric structure. Attempts to detect the 77Se{1H} NMR resonances of 9 failed due to strong broadening of the signal. Furthermore, while the B2S3 analogue 7 proved stable in solution, compound 9 was observed to fully decompose to the B2Se2 heterocycle 5 in benzene solution over a period of 4 days at room temperature (Scheme 8).
3 boron-to-selenium ratio in benzene at 60 °C (Scheme 7B). Compound 9 presented an 11B NMR resonance at −12.3 ppm, ca. 20 ppm downfield from that of 5. Similarly to 8, the 1H NMR resonances of the isoproyl groups of the Dip residue were split into two sets, indicative of an asymmetric structure. Attempts to detect the 77Se{1H} NMR resonances of 9 failed due to strong broadening of the signal. Furthermore, while the B2S3 analogue 7 proved stable in solution, compound 9 was observed to fully decompose to the B2Se2 heterocycle 5 in benzene solution over a period of 4 days at room temperature (Scheme 8).
Both 1,2,4-trichalcogena-3,5-diborolanes 8 and 9 could also be obtained by reacting diborene II with three molar equivalents of elemental sulfur or selenium, respectively, or from the reaction of the corresponding B2E heterocycles, 7 and 6, with two molar equivalents of sulfur or selenium, respectively (Scheme 7C).
Like their B2E2 counterparts, compounds 8 and 9 crystallized in isomorphous unit cells. For both compounds, the X-ray crystallographic structures (Fig. 5) show a five-membered central 1,2,4-trichalcogena-3,5-diborolane ring bearing one cyano and one cAAC ligand on each boron atom, arranged in a trans-configuration with respect to the B2E3 ring. While the structures thus represent a single (R,R/S,S) diastereomer, the observation of two non-exchanging isomers of 8 in solution suggests that the meso diastereomer of 8 may also be formed. The formation of a single diastereomer of 9 may be attributable to the higher reaction temperature favoring the thermodynamic (R,R/S,S) diastereomer. The structure of 8 is reminiscent of that of the bis(NHC)-stabilized 3,5-dithienyl-1,2,4-trithia-3,5-diborolane obtained by the reductive insertion of three sulfur atoms into the B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B double bond of a diborene precursor, with very similar B–S bond lengths and B–S–B and S–B–S angles.13 The 1,2,4-triselena-3,5-diborolane ring of 9 is reminiscent of that obtained by Tokitoh and co-workers upon irradiation of a boron bis(methylselenide) bound to a very bulky aryl ligand (Tbt = 2,4,6-(C(SiMe3)2H)3C6H2).21 Due to the sp3 hybridization of the cAAC-supported boron atoms in 9, however, the B–Se bonds (2.068(4), 2.086(4) Å) are slightly elongated and the Se–B–Se angle (109.4(2)°) is significantly more acute than in [(Tbt)2B2Se3] (B–Se: 1.942(7), 1.926(8) Å; Se–B–Se: 118.8(4)°).21
B double bond of a diborene precursor, with very similar B–S bond lengths and B–S–B and S–B–S angles.13 The 1,2,4-triselena-3,5-diborolane ring of 9 is reminiscent of that obtained by Tokitoh and co-workers upon irradiation of a boron bis(methylselenide) bound to a very bulky aryl ligand (Tbt = 2,4,6-(C(SiMe3)2H)3C6H2).21 Due to the sp3 hybridization of the cAAC-supported boron atoms in 9, however, the B–Se bonds (2.068(4), 2.086(4) Å) are slightly elongated and the Se–B–Se angle (109.4(2)°) is significantly more acute than in [(Tbt)2B2Se3] (B–Se: 1.942(7), 1.926(8) Å; Se–B–Se: 118.8(4)°).21
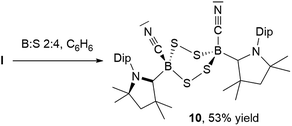
NMR spectroscopic analysis of the crystallization filtrate of 1,2,4-trithia-3,5-diborolane 8 revealed the presence of another boron-containing species presenting an 11B NMR singlet at −11.2 ppm and a single symmetrical cAAC ligand environment in its 1H NMR spectrum. Surmising that this may be a tetrathiadiborinane resulting from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 boron-to-sulfur reaction, a scaled-up reaction with this stoichiometry was carried out (Scheme 9). The resulting orange suspension was filtered and the filtrate slowly evaporated to give compound 10 as a pale yellow solid in 53% isolated yield based on boron and its formulation confirmed by LC-MS.
4 boron-to-sulfur reaction, a scaled-up reaction with this stoichiometry was carried out (Scheme 9). The resulting orange suspension was filtered and the filtrate slowly evaporated to give compound 10 as a pale yellow solid in 53% isolated yield based on boron and its formulation confirmed by LC-MS.
Multiple recrystallizations of 10 from various solvents provided extremely thin, heavily twinned crystals, which diffracted too weakly to provide data suitable for structural discussion. However, the results of these X-ray crystallographic experiments provided conclusive proof of connectivity, confirming that 10 is indeed a bis(cAAC)-stabilized 3,6-dicyano-1,2,4,5-tetrasulfa-3,6-diborinane displaying a cis-arrangement of the cyano and cAAC ligands with respect to the central B2S4 ring, which displays a boat conformation (see Fig. S46† for the solid-state structure of 10). Attempts to insert a fourth sulfur atom into the isolated 1,2,4-trithia-3,5-diborolane 8 failed to yield 9, suggesting that, like 8, compound 9 forms directly from I, presumably by dimerization of a monomeric [(cAAC)B(CN)S2] intermediate. While 1,2,4,5-tetrasulfa-3,6-diborinanes have been postulated as minor reaction products by both Lappert and later Nöth, based solely on elemental analysis, mass spectrometry and IR spectroscopy data,22 compound 10 constitutes the first structurally and NMR-spectroscopically characterized B2S4 heterocycle.
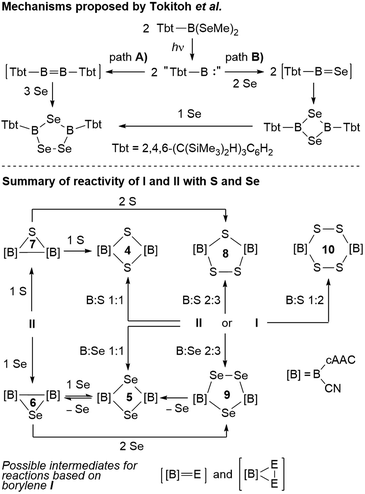
Mechanistic considerations
With all these data in hand, it was now possible to reassess the viability of the mechanism proposed by Tokitoh and co-workers for the formation of [(Tbt)2B2Se3] from [(Tbt)B(SeMe)2].21 In a first step, the authors postulated the formation of a “(Tbt)B:” borylene intermediate upon irradiation of [(Tbt)B(SeMe)2]. This monomeric borylene may then dimerize to an electron-deficient diborene [(Tbt)B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B(Tbt)] (Scheme 10, path A), which then undergoes the reductive insertion of three atoms of selenium atoms with full cleavage of the B
B(Tbt)] (Scheme 10, path A), which then undergoes the reductive insertion of three atoms of selenium atoms with full cleavage of the B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B bond. For path B, the authors proposed a monomeric [(Tbt)B
B bond. For path B, the authors proposed a monomeric [(Tbt)B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se] intermediate resulting from the reaction of “(Tbt)B:” with Se, which dimerizes to a 1,3-diselena-2,4-diboretane and finally inserts the third selenium atom. Regarding path A, numerous attempts on our part have failed to convert borylene I into its diborene relative, compound II, under thermal and/or photolytic conditions. The fact that the three-membered B2E heterocycles 6 and 7 can only be accessed directly from diborene II, but not from borylene I, is further evidence that, despite extensive overlap of reactivity outcomes, I and II do not interconvert.
Se] intermediate resulting from the reaction of “(Tbt)B:” with Se, which dimerizes to a 1,3-diselena-2,4-diboretane and finally inserts the third selenium atom. Regarding path A, numerous attempts on our part have failed to convert borylene I into its diborene relative, compound II, under thermal and/or photolytic conditions. The fact that the three-membered B2E heterocycles 6 and 7 can only be accessed directly from diborene II, but not from borylene I, is further evidence that, despite extensive overlap of reactivity outcomes, I and II do not interconvert.
Our previous report on the deaggregation of tetramer I by Lewis bases showed that only a relatively small and strong Lewis base – the NHC 1,3,4,5-tetramethylimiazol-2-ylidene – is able to break up the tetramer and generate the mixed base-stabilised [(cAAC)(NHC)B(CN)] borylene.9 This leads us to conclude that, while acting in all appearance as a source of monomeric borylene “[B]:” ([B] = (cAAC)(CN)B, Scheme 10), such a species is not, in fact, ever present in solution. Reactivity with I instead occurs by association of the reactant with the tetramer itself, ultimately causing it to deaggregate into mononuclear intermediates and products. For reactions involving I, the generation of intermediate [[B]![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) E] (E = S, Se) monomers following path B therefore seems the most likely. While the dimerization of [(cAAC)(CN)B
E] (E = S, Se) monomers following path B therefore seems the most likely. While the dimerization of [(cAAC)(CN)B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) E] via bridging chalcogen atoms provides compounds 4 and 5, the latter do not insert another chalcogen atom to form 8 and 9, respectively, under the reaction conditions employed herein (Scheme 10). This means that the addition of the third chalcogen atom must occur prior to ring-closure, leading us to propose a monomeric dichalcogenaborirane [[B]E2] intermediate, which upon reaction with [[B]
E] via bridging chalcogen atoms provides compounds 4 and 5, the latter do not insert another chalcogen atom to form 8 and 9, respectively, under the reaction conditions employed herein (Scheme 10). This means that the addition of the third chalcogen atom must occur prior to ring-closure, leading us to propose a monomeric dichalcogenaborirane [[B]E2] intermediate, which upon reaction with [[B]![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) E] yields the B2E3 heterocycles 8 and 9. Similarly, the B2S4 heterocycle 10 would result from the dimerization of [[B]S2].
E] yields the B2E3 heterocycles 8 and 9. Similarly, the B2S4 heterocycle 10 would result from the dimerization of [[B]S2].
It is noteworthy that, while the selenium-based reactions were highly selective, those based on sulfur always yielded a mixture of products. For example, based on 11B NMR spectroscopic analysis of the final reaction mixture, the reaction of I with sulfur in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 boron-to-sulfur ratio yielded 8 in 70–85% selectivity at most, alongside the smaller and larger heterocycles 4 and 10, whereas the analogous reaction with selenium provided 9 in near-quantitative yield. The increased selectivity in the formation of 9 may be ascribed to the possibility of selenium de-insertion, which allows any B2Se2 heterocycle (5) formed in the course of the reaction to lose a Se atom, forming the diboraselenirane 6, which can in turn be converted to 9 (Scheme 10). In contrast, any B2S2 (4) and B2S4 (10) heterocycles formed in the course of the reaction are inert towards sulfur de-insertion and will therefore remain as by-products. Finally, the fact that the four- and five-membered B2E2 and B2E3 heterocycles are inert towards chalcogen insertion, whereas the three-membered B2E heterocycles may be converted to both the larger B2E2 and B2E3 heterocycles, suggests that ring-expansion only proceeds by insertion of a single chalcogen atom or an E2 unit into any remaining B–B bonds.
3 boron-to-sulfur ratio yielded 8 in 70–85% selectivity at most, alongside the smaller and larger heterocycles 4 and 10, whereas the analogous reaction with selenium provided 9 in near-quantitative yield. The increased selectivity in the formation of 9 may be ascribed to the possibility of selenium de-insertion, which allows any B2Se2 heterocycle (5) formed in the course of the reaction to lose a Se atom, forming the diboraselenirane 6, which can in turn be converted to 9 (Scheme 10). In contrast, any B2S2 (4) and B2S4 (10) heterocycles formed in the course of the reaction are inert towards sulfur de-insertion and will therefore remain as by-products. Finally, the fact that the four- and five-membered B2E2 and B2E3 heterocycles are inert towards chalcogen insertion, whereas the three-membered B2E heterocycles may be converted to both the larger B2E2 and B2E3 heterocycles, suggests that ring-expansion only proceeds by insertion of a single chalcogen atom or an E2 unit into any remaining B–B bonds.
Conclusions
This first comparative study on the reactivity of two boron(I) compounds of the same empirical formula, the tetrameric, self-stabilizing cyanoborylene I and its dicyanodiborene relative II, has demonstrated that, while both compounds provide access to the same products in many cases, this occurs via different pathways as shown in Scheme 11.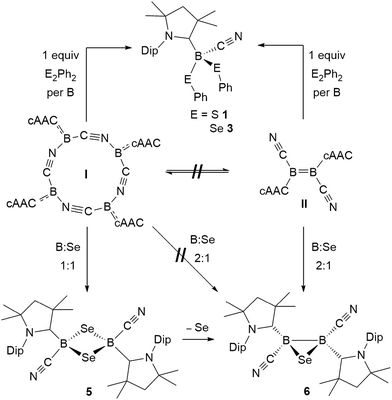 | ||
| Scheme 11 Selected reactions showing the convergent and divergent reactivity of the two boron(I) isomers I and II with dichalcogenides and elemental selenium. | ||
In reactions with dichalcogenides, both I and II yielded the mononuclear cyanoboron bis(chalcogenides) as sole products (Scheme 11). In the case of I this presumably occurs by insertion of a monomeric borylene into the chalcogen–chalcogen bond, whereas for II a successive 1,2-addition mechanism across the B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B double bond, resulting in full B–B bond cleavage is most likely at work.
B double bond, resulting in full B–B bond cleavage is most likely at work.
Reactions with elemental sulfur and selenium were found to be highly dependent on the stoichiometry used. Reactions of I or II employing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 2
1 or 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 boron-to-chalcogen ratio yielded the corresponding 1,3-dichalcogena-2,4-diboretanes or 1,2,4-trichalcogena-3,5-diborolanes, respectively. A unique 1,2,3-thiadiborirane could only be accessed from the reaction of diborene precursor II with sulfur in a 2
3 boron-to-chalcogen ratio yielded the corresponding 1,3-dichalcogena-2,4-diboretanes or 1,2,4-trichalcogena-3,5-diborolanes, respectively. A unique 1,2,3-thiadiborirane could only be accessed from the reaction of diborene precursor II with sulfur in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 boron-to-sulfur ratio, whereas the corresponding diboraselenirane was accessible both directly from the analogous reaction with selenium, and indirectly by de-insertion of one selenium atom from the four-membered 1,3-diselena-2,4-diboretane (Scheme 11). In the case of sulfur a rare example of a 1,2,4,5-tetrasulfa-3,6-diborinane was isolated from the reaction of borylene I with sulfur in a 1
1 boron-to-sulfur ratio, whereas the corresponding diboraselenirane was accessible both directly from the analogous reaction with selenium, and indirectly by de-insertion of one selenium atom from the four-membered 1,3-diselena-2,4-diboretane (Scheme 11). In the case of sulfur a rare example of a 1,2,4,5-tetrasulfa-3,6-diborinane was isolated from the reaction of borylene I with sulfur in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 boron-to-sulfur ratio.
2 boron-to-sulfur ratio.
Careful stepwise addition of chalcogen equivalents to either I or II and stability studies of the resulting heterocycles also gave insight into several mechanistic aspects of these reactions:
(i) Borylene-based reactions do not proceed via a diborene intermediate but likely via monomeric borachalcone and dichalcogenaborirane intermediates;
(ii) Ring-expansion reactions can only proceed by insertion of chalcogens into existing B–B bonds;
(iii) Ring-contraction is possible in the case of boron–selenium heterocycles only by de-insertion of Se atoms.
Although the subtle divergences in reactivity between I and II provide confirmation that no Wanzlick-type equilibrium exists between a putative monomeric form of I and its B–B bonded dimer, diborene II, the question remains as to whether this is a result of the extremely stable, tetrameric constitution of borylene I. To date, I and II represent the only existing borylene/diborene pair with the same empirical formula, however, recent advances in the synthesis of borylenes will hopefully enable a more definitive answer to the question of a possible interconversion between [LRB:] borylenes and [LRB![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) BRL] diborenes. Beyond the interest of such an interconversion from a fundamental point of view, its undeniable potential for providing a new, more reliable route towards hitherto inaccessible diborenes should continue to stimulate research into this area.
BRL] diborenes. Beyond the interest of such an interconversion from a fundamental point of view, its undeniable potential for providing a new, more reliable route towards hitherto inaccessible diborenes should continue to stimulate research into this area.
Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This project was funded by the European Research Council (ERC) under the European Union Horizon 2020 Research and Innovation Program (grant agreement no. 669054). Funding from the Alexander von Humboldt foundation (postdoctoral fellowship for M. A.) is gratefully acknowledged.Notes and references
- D. Bourissou, O. Guerret, F. P. Gabbaï and G. Bertrand, Chem. Rev., 2000, 100, 39 CrossRef CAS PubMed.
- (a) H. W. Wanzlick and H. J. Kleiner, Angew. Chem., 1961, 73, 493 CrossRef CAS; (b) H. W. Wanzlick, Angew. Chem., Int. Ed. Engl., 1962, 1, 75 CrossRef; (c) H. W. Wanzlick, F. Esser and H. J. Kleiner, Chem. Ber., 1963, 96, 1208 CrossRef CAS; (d) F. E. Hahn, L. Wittenbecher, D. L. Van and R. Fröhlich, Angew. Chem., Int. Ed., 2000, 39, 541 CrossRef CAS; (e) V. P. W. Böhm and W. A. Hermann, Angew. Chem., Int. Ed., 2000, 39, 4036 CrossRef.
- S. Tsutsui, K. Sakamoto and M. Kira, J. Am. Chem. Soc., 1998, 120, 9955 CrossRef CAS.
- (a) R. Kinjo, B. Donnadieu, M. A. Celik, G. Frenking and G. Bertrand, Science, 2011, 333, 610 CrossRef CAS PubMed; (b) M. Soleilhavoup and G. Bertrand, Angew. Chem., Int. Ed., 2017, 56, 10282 CrossRef CAS PubMed.
- (a) V. Lavallo, Y. Canac, C. Prasang, B. Donnadieu and G. Bertrand, Angew. Chem., Int. Ed., 2005, 44, 5705 CrossRef CAS PubMed; (b) M. Soleilhavoup and G. Bertrand, Acc. Chem. Res., 2015, 48, 256 CrossRef CAS PubMed; (c) M. Melaimi, R. Jazzar, M. Soleilhavoup and G. Bertrand, Angew. Chem., Int. Ed., 2017, 56, 10046 CrossRef CAS PubMed.
- F. Dahcheh, D. Martin, D. W. Stephan and G. Bertrand, Angew. Chem., Int. Ed., 2014, 53, 13159 CrossRef CAS PubMed.
- H. Braunschweig, R. D. Dewhurst, F. Hupp, M. Nutz, K. Radacki, C. W. Tate, A. Vargas and Q. Ye, Nature, 2015, 522, 327 CrossRef CAS PubMed.
- H. Braunschweig, I. Krummenacher, M.-A. Légaré, A. Matler, K. Radacki and Q. Ye, J. Am. Chem. Soc., 2017, 139, 1802 CrossRef CAS PubMed.
- M. Arrowsmith, D. Auerhammer, R. Bertermann, H. Braunschweig, G. Bringmann, M. A. Celik, R. D. Dewhurst, M. Finze, M. Grüne, M. Hailmann, T. Hertle and I. Krummenacher, Angew. Chem., Int. Ed., 2016, 55, 14464 CrossRef CAS PubMed.
- (a) M. Arrowsmith, H. Braunschweig and T. E. Stennett, Angew. Chem., Int. Ed., 2017, 56, 96 CrossRef CAS PubMed; (b) H. Braunschweig and R. Shang, Inorg. Chem., 2015, 54, 3099 CrossRef CAS PubMed; (c) H. Braunschweig and R. D. Dewhurst, Angew. Chem., Int. Ed., 2013, 52, 3574 CrossRef CAS PubMed; (d) H. Braunschweig, R. D. Dewhurst and V. H. Gessner, Chem. Soc. Rev., 2013, 42, 3197 RSC.
- J. Böhnke, H. Braunschweig, T. Dellermann, W. C. Ewing, T. Kramer, I. Krummenacher and A. Vargas, Angew. Chem., Int. Ed., 2015, 54, 4469 CrossRef PubMed.
- H. Braunschweig, T. Dellermann, W. C. Ewing, T. Kramer, C. Schneider and S. Ullrich, Angew. Chem., Int. Ed., 2015, 54, 10271 CrossRef CAS PubMed.
- H. Braunschweig, P. Constantinidis, T. Dellermann, W. C. Ewing, I. Fischer, M. Hess, F. R. Knight, A. Rempel, C. Schneider, S. Ullrich, A. Vargas and J. D. Woollins, Angew. Chem., Int. Ed., 2016, 55, 5606 CrossRef CAS PubMed.
- (a) M. Arrowsmith, D. Auerhammer, R. Bertermann, H. Braunschweig, G. Bringmann, M. A. Celik, J. Erdmannsdörfer, I. Krummenacher and T. Kupfer, Angew. Chem., Int. Ed., 2016, 56, 11263 CrossRef PubMed; (b) D. A. Ruiz, G. Ung, M. Melaimi and G. Bertrand, Angew. Chem., Int. Ed., 2013, 52, 7590 CrossRef CAS PubMed.
- W. Clegg, C. Dai, F. J. Lawlor, T. B. Marder, P. Nguyen, N. C. Norman, N. L. Pickett, W. P. Power and A. J. Scott, J. Chem. Soc., Dalton Trans., 1997, 839 RSC.
- (a) B. Wrackmeyer, E. V. Klimkina and W. Milius, Eur. J. Inorg. Chem., 2011, 4481 CrossRef CAS; (b) B. Wrackmeyer, E. V. Klimkina and W. Milius, Polyhedron, 2010, 29, 2324 CrossRef CAS.
- G. W. Bushnel and G. A. Rivett, Can. J. Chem., 1977, 55, 3294 CrossRef.
- (a) D. Mannig, C. K. Narula, H. Nöth and U. Wietelmann, Chem. Ber., 1985, 118, 3748 CrossRef; (b) E. Hanecker, H. Nöth and U. Wietelmann, Chem. Ber., 1986, 119, 1904 CrossRef CAS.
- M. Arrowsmith, J. Böhnke, H. Braunschweig and M. A. Celik, Angew. Chem., Int. Ed., 2017, 56, 14287 CrossRef CAS PubMed.
- S. Liu, M.-A. Légaré, D. Auerhammer, A. Hofmann and H. Braunschweig, Angew. Chem., Int. Ed., 2017, 56, 15760 CrossRef CAS PubMed.
- M. Ito, N. Tokitoh, T. Kawashima and R. Okazaki, Tetrahedron Lett., 1999, 40, 5557 CrossRef CAS.
- (a) M. F. Lappert and B. Prokai, J. Chem. Soc. A, 1967, 129 RSC; (b) H. Nöth and T. Taeger, Z. Naturforsch., B: J. Chem. Sci., 2010, 65, 173 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of new compounds, NMR spectra, crystallographic details and supplementary structures. CCDC 1582403–1582412. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc04789d |
| ‡ Compound 2 repeatedly crystallized as extremely thin, overlapping yellow plates, which could not be separated. X-ray crystallographic analysis of these provided proof of connectivity but data were of insufficient quality for further structural discussions (see ESI, Fig. S44†). |
§ The solid-state structure of molecule 6 presented a two-fold mirror disorder in a 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 ratio around the central diboraselenirane core, with the mirror plane running approximately through the plane containing (N1, Se1, N2). The disordered 2,3-dicyano-2,3-diboraselenirane cores of both parts were freely refined. Fig. S45† provides an overlay of the two parts. 12 ratio around the central diboraselenirane core, with the mirror plane running approximately through the plane containing (N1, Se1, N2). The disordered 2,3-dicyano-2,3-diboraselenirane cores of both parts were freely refined. Fig. S45† provides an overlay of the two parts. |
| This journal is © The Royal Society of Chemistry 2018 |