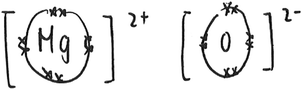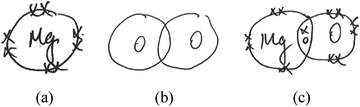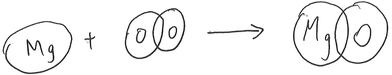Students' visualisation of chemical reactions – insights into the particle model and the atomic model
Maurice M. W.
Cheng

Division of Mathematics and Science Education, Faculty of Education, The University of Hong Kong, Hong Kong. E-mail: mwcheng@hku.hk
First published on 31st October 2017
Abstract
This paper reports on an interview study of 18 Grade 10–12 students’ model-based reasoning of a chemical reaction: the reaction of magnesium and oxygen at the submicro level. It has been proposed that chemical reactions can be conceptualised using two models: (i) the particle model, in which a reaction is regarded as the simple combination and rearrangement of reactant particles and does not involve any change in the identity of the reactants, and (ii) the atomic model, wherein a reaction involves the transformation of one chemical species into another. This paper suggests that although the particle model looks simpler than the atomic model, it can help to support the learning of some advanced chemical concepts such as energetics and collision theory. Therefore, it is postulated that students who reason using the particle model are able to demonstrate some advanced ideas about chemical reactions. The conceptualisation of reactions in terms of the atomic model and the particle model allows a flexible understanding of students’ learning. Students’ representations of the reaction between magnesium and oxygen were analysed with reference to the two models. The models were found to be useful in assessing the students’ understanding of the reaction and revealing the novel ways that the students reasoned the chemical reaction. In addition, a student who used the particle model to represent the reaction was found to explain the reaction in terms of some energetics and kinetics concepts. The study offers insights for curriculum planners and teachers into the potential of these two models to help students understand chemical reactions.
Introduction
Learning of chemical changes can be regarded as a key part of the learning of chemistry. Considerable attention has been paid by researchers to students’ learning of chemical changes. In broad terms, there are two related lines of enquiry in this area.(1) Studies that examine students’ understanding and reasoning of chemical changes. These studies reported on some ways that children conceptualise chemical changes either in general (Andersson, 1990; Hinton and Nakhleh, 1999) or with reference to specific types of chemical changes, such as combustion (BouJaoude, 1991), precipitation (Smith and Metz, 1996; Kelly et al., 2010) and redox (Garnett et al., 1990). Some studies investigated how animations could facilitate students’ learning of chemical changes (Williamson and Abraham, 1995; Ardac and Akaygun, 2004; Zhang and Linn, 2011; Kelly et al., 2017). Researchers are increasingly interested in identifying the ways that students reason about chemical changes (Chandrasegarana et al., 2007; Stains and Talanquer, 2008; Robertson and Shaffer, 2014; Yan and Talanquer, 2015; Weinrich and Talanquer, 2016).
(2) Studies that conceptualise students’ level of understanding of chemical changes. Using empirical evidence, these researchers developed theoretical frameworks to demarcate levels of achievement or progression (Ahtee and Varjola, 1998; Stavridou and Solomonidou, 1998; Liu and Lesniak, 2006; Øyehaug and Holt, 2013). These frameworks have been called ‘conceptual profiles’ (Solsona et al., 2003) or, more commonly, ‘learning progression’ (Smith et al., 2006; Johnson and Tymms, 2011; Hadenfeldt et al., 2016).
The above studies suggested directions for improving teaching and assessment. Many focused on examining students’ understanding of chemical reactions based on the interaction between ions, molecules and atoms. This paper suggests that although this model of chemical reactions – here called the atomic model of reactions – is commonly used to make sense of chemical phenomena (e.g., metal reactivity, electrochemical cells and the mechanisms of organic reactions), it is by no means the only model adopted in school chemistry curricula. The role of the model of reactions based on the simple particle model of matter – called the particle model of reactions in this paper – should not be neglected. For example, simple collision theory (introduced in chemical kinetics) is commonly represented by simple/undifferentiated particles. The aim of this study is to determine whether students’ understanding of chemical reactions can be made sense of by these two models. We also wish to determine whether the particle model of reactions can support students’ understanding of some advanced chemical concepts such as chemical kinetics and energetics. If so, it may have implications on assigning levels of understanding for these two models of reactions, and on providing an alternative way of introducing simple ideas relating to chemical kinetics and energetics.
Models of chemical reactions
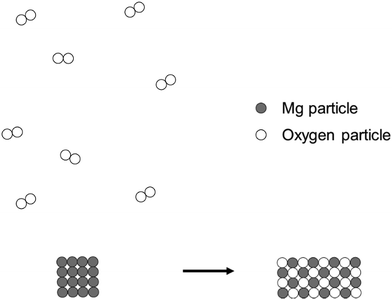
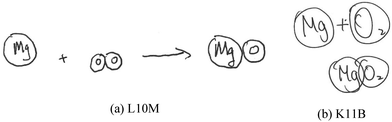
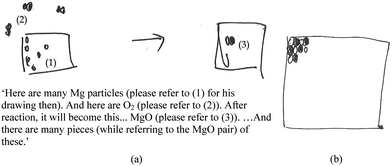
Model-based reasoning plays a key role in learning and exploring chemistry (Erduran and Duschl, 2004; Windschitl et al., 2008; Gilbert and Justi, 2016). Different models have different structural entities, and are based on different principles that govern the relationships and interactions between these structural entities. Cheng and Gilbert (2017) suggested that there are two major submicro-level models that students would come across in school chemistry.(1) Particle model of reactions. This model is based on the simple particle model of matter. The structural entities involved in this model of reactions are ‘particles’. These particles are sometimes represented as discrete circles/spheres or as clusters of connected circles or spheres in diagrams. Chemical reactions are regarded as spatial rearrangements of these particles. Lego blocks provide a common analogy for this model of reactions. Instruments that assessed students’ understanding of chemical reactions were at times based on this model (e.g., Williamson and Abraham, 1995; Ardac and Akaygun, 2004). The reaction between magnesium and oxygen can be represented as in Fig. 1.
This model does not represent changes in the identity of particles before and after reactions. In the reaction represented in Fig. 1, for example, it does not consider the formation of magnesium ions from corresponding atoms. Instead, magnesium particles are shown to remain as such after the reaction. Although this model is not entirely consistent with modern knowledge of the nature of matter (Taber, 2003), it helps the public to make sense of recent developments in technology (e.g., The Economist, 2016). This model of reactions is also represented in popular science readers (Atkins, 2013, p. 46).
(2) Atomic model of reactions. This model involves more specific structural entities such as atoms, ions, molecules, electrons and protons. These entities participate in chemical reactions and may become other chemical species as a result. Kelly et al. (2017) created an animation to represent the reaction between solid copper and silver nitrate solution at the submicro level. They emphasized on the electron exchange. Such a representation exemplifies the atomic model of reactions. Also, according to this model of reactions, the combustion of magnesium is represented as the formation of magnesium ions from magnesium atoms and the formation of oxide ions from oxygen molecules (Fig. 2). This model goes beyond the spatial rearrangement of undifferentiated chemical species. It also considers the transfer of electrons from magnesium to oxygen atoms, which forms the basis for the learning of redox reactions. Taber (2003) suggested that chemical reactions can be regarded as ‘reconfigurations of systems of nuclei (or cores) and electrons’ (p. 51). This idea is consistent with the atomic model of reactions. For example, in the same reaction, there is a change in electrons surrounding the oxygen nuclei when oxygen molecules become oxide ions. In the particle model, there was no consideration of electrons. Oxygen particles remain as oxygen particles after the reaction (Fig. 1).
 | ||
| Fig. 2 A submicro representation of the reaction between magnesium and oxygen based on the atomic model. Note that the electron arrangement of magnesium atoms as 2, 8, 2, magnesium ions as 2, 8, and that of oxygen molecules are not shown here (source: Cheng and Gilbert, 2014). | ||
In short, based on the differences in structural entities involved in reactions (e.g., without and with a consideration of electrons in the particle model and atomic model respectively), this paper differentiates the two models by changes in these structural entities before/after the reaction (e.g., Mg particles remain as Mg particles after the reaction in the particle model vs. Mg atoms become Mg ions in the atomic model). The differences between the two models are tabulated in Table 1.
| Particle model of reactions | Atomic model of reactions | |
|---|---|---|
| Structural entities involved | Particles or atoms – unitary in nature and are not made of subatomic particles. | Atoms; ions; molecules; electrons and protons as subatomic particles. |
| Process involved | Spatial rearrangement of particles or atoms. | Spatial rearrangement of the above-mentioned chemical species. Some reactions involve changes in the identity of submicro entities, e.g. from atoms to ions or from ions to molecules. |
Levels of understanding of chemical reactions
Researchers have sought to delineate levels of understanding of chemical reactions (e.g., Ahtee and Varjola, 1998; Øyehaug and Holt, 2013). Hadenfeldt et al. (2016) conceptualised the big idea ‘matter’ as being composed of four distinct ideas, namely (i) structure and composition; (ii) physical properties and change; (iii) chemical reactions; and (iv) conservation; and each of these four ideas has its five levels of progression. Level 3 (simple particle concept) and Level 4 (differentiated particle concept) of the idea ‘chemical reactions’ can be mapped onto the particle model and the atomic model, respectively. These two levels are as follows.Simple particle concept (Level 3). ‘Students describe a chemical reaction as reorganization of particles. But they have no model which allows them to describe processes during a chemical reaction’ (Hadenfeldt et al., 2016, Supporting Information, Table 2).
Differentiated particle concept (Level 4). ‘Students describe a chemical reaction as reorganization of particles and bonds… In doing so, they are able to describe elementary reactions on the basis of a differentiated particle model…’ (Hadenfeldt et al., 2016, Supporting Information, Table 2).
At Level 3, students use particles in a general way, and chemical reaction is regarded as a reorganization of these simple particles. These ideas are consistent with the particle model of reactions. A key to Level 4 is the use of differentiated particles (such as electrons, ions, atoms, molecules and protons) to describe chemical reactions, which the atomic model is consistent with.
While there is coherence, these two models cannot be reduced to these two levels of understanding. It was found that some students represented differentiated particles, but represented them as simple reorganisation for reactions. For example, one student (Cheng and Gilbert, 2017, Student B3-T1-a) represented hydrochloric acid as H+ and Cl− and magnesium as made of magnesium ions for the reaction between magnesium and hydrochloric acid. It involved the use of differentiated particles, which fit a criterion for Level 4. Meanwhile, the student represented the reaction only as the simple spatial rearrangement of these chemical species. After the reaction, according to the student, two positively charged hydrogen ions stuck together and chloride ions combined with the magnesium. Electrons were not mentioned. Therefore, the student manifested Level 3 in terms of the reorganisation of particles. In this case, it may be difficult to define the student's level of attainment. In fact, the result of Hadenfeldt et al. (2016) also suggested that students’ knowledge of differentiated particles did not guarantee their use of these differentiated particles in representing a reaction. Based on the four ideas of the ‘matter concept’, they devised a corresponding instrument that was tested with 1388 Grades 6 to 13 students. It was found that the correlation between students’ understanding of ‘structure and composition’ and their understanding of ‘chemical reactions’ was 0.29 (p. 695). It means that there was only a slight relationship (Cohen, et al., 2000, p. 202; Creswell, 2002, p. 372) between students’ understanding of ‘structure and composition’ and ‘chemical reactions’. This result implies that students capable of differentiating between different types of chemical species, such as ions, atoms and molecules (reflecting an understanding of ‘structure and composition’), may not use these chemical species to represent chemical reactions.
The lack of a strong correlation between students’ understanding of ‘structure and composition’ and their understanding of ‘chemical reactions’ may also mean that even students who do not differentiate between different chemical species (instead focusing on ‘particles’ in the Simple Particle Concept) can attain an advanced understanding of ‘chemical reactions’. This may sound implausible. However, as discussed in the next section, students who use the Simple Particle Concept in theory may acquire some advanced knowledge of chemical reactions, such as principles of energetics and kinetics.
In short, we acknowledge that levels of understanding offer an extremely useful tool for defining expected learning outcomes within the curriculum and informing the development of assessment items that map onto expected outcomes at different levels (Liu and Lesniak, 2005; Smith et al., 2006). In many instances, these levels indeed reflect students’ attainment. Nevertheless, they may not adequately model students’ understanding, when their attainment of ‘chemical reactions’ exceeds that of ‘structure and composition’ and when they represent some differentiated particles but do not use them to represent the complexity of chemical reactions.
Particle model for understanding chemical reactions
Based on the idea of the particle model and the atomic model of reactions proposed earlier on (Cheng and Gilbert, 2017), this paper suggests that although the particle model of reactions is characterised by the spatial rearrangement of reactant species, the model has a wide range of applications in helping students to learn some more advanced topics such as energetics and kinetics.In energetics, the enthalpy change of a reaction is regarded as the result of the breaking of bonds between reactant particles and the formation of bonds for products. Explaining bond strength requires an understanding of other concepts such as Coulombic electrostatics and chemical bonding. However, the particle model of reactions is often used to represent the processes of bond breaking and bond formation in an energy-level diagram. This is illustrated by a diagram from a Hong Kong textbook (Fig. 3) that represents the reaction 2H2 + O2 → 2H2O as a process of bond breaking, with HH and OO becoming discrete Hs and Os, and then bond formation, as the discrete Hs and Os combine to form H2O. This process may be based on the particle model, in which the reaction is regarded as the spatial rearrangement of reactants to form a product. Although such a representation does not explain bond strength and energy in terms of electrostatic force, it offers a simple model of energy changes in chemical reactions. In fact, similar particle representations are used in college-level chemistry textbooks to introduce the use of mean bond enthalpy to estimate the enthalpy change of reactions (e.g., Atkins and Jones, 2010, p. 277).
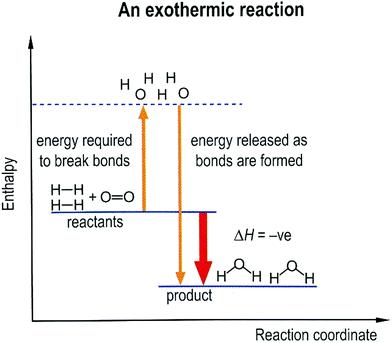 | ||
| Fig. 3 A diagram that represents the reaction as spatial rearrangement of particles/atoms, which is based on the particle model (Cheng et al., 2010, p. 159). | ||
Similarly, the collision theory is commonly used in school chemistry in Hong Kong (and perhaps elsewhere) to explain the conditions required for reactions; that is, reactant particles must possess enough energy and collide with the right orientations. Collision theory is often represented using the particle model (please refer to Fig. 4 in the same textbook). In the diagram, both A and B particles are represented in the same way before and after the reaction; only the particles with which they connect differ before (AA and BB) and after (AB) the reaction. The reaction occurs when particles collide with the correct spatial orientations to permit the spatial rearrangement of particles. Simple collision theory also addresses the effects of factors such as concentration, pressure and temperature on the rate of reaction. We acknowledge that kinetics provides much more sophisticated explanations of reactions – via reaction mechanisms and molecularity, for example. Yet collision theory, which is based on the particle model, provides a simpler and more intuitive explanation of how reactions take place.
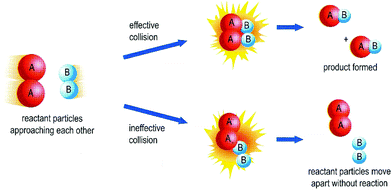 | ||
| Fig. 4 A diagram representing the collision theory, which is based on the particle model (Cheng et al., 2009, p. 35). | ||
In summary, the particle model explains reactions as the spatial rearrangement of particles. The identity of the particles involved does not change during the reaction. That is, particle As are still particle As after the reaction – they differ only in the particles with which they connect before and after the reaction (compared with the atomic model, in which magnesium atoms become magnesium ions after reacting with acids or oxygen). Nevertheless, the particle model helps to make sense of more advanced ideas such as principles of energetics and kinetics. We thus postulate that some students may simply use simple undifferentiated particles but demonstrate a more advanced understanding of chemical reactions. In this way, they may look as though they were at Level 3 of ‘chemical reactions’ (Hadenfeldt et al., 2016), but they could understand chemical reactions beyond this level.
Research questions
The broad aim of the study was to investigate students’ use of the particle model and the atomic model to reason chemical reactions. A previous study examined Grade 10–11 students’ submicro representations of the reaction between magnesium and hydrochloric acid (Cheng and Gilbert, 2017). The objective of the current study was to further illustrate the use of the particle model and the atomic model to make sense of students’ representations of chemical reactions. Another objective was to determine whether students are capable of demonstrating some advanced understanding of chemical reactions while using the particle model.The reaction under investigation was that between magnesium and oxygen. This specific reaction was chosen for three reasons. (1) Compared with other reactions, this reaction was simple enough to be represented by students at the submicro level. The students were required to consider only three reactants and products. This would minimise the possibility of their being demotivated by more chemicals in an equation. This also minimised the chance of the students’ using shorthand to represent complicated ideas. We were aware that the students might draw simple circles (a component of the particle model) as shorthand for their ideas about the atomic model. A simple reaction with fewer reactants and products would make students more likely to truthfully represent their ideas in drawings. (2) The reaction had previously been introduced to the students on at least three separate occasions: once on the topic of chemical bonding, when they learnt to draw electron diagrams of the formation of Mg2+O2−; once on the topic of metal reactivity, when they learnt and compared the reactions of oxygen with various metals; and once on the topic of empirical formulae, when they learnt to calculate the empirical formula of magnesium oxide based on given data. The students were thus not expected to find the reaction unfamiliar to the extent that it hampered their responses. (3) The students were likely to be familiar with simple reactions such as C + O2 → CO2 and C + O2 → CO. However, these reactions were less appropriate because the use of connected circles to represent reactants and products could be interpreted either as a representation of the particle model or as shorthand for the atomic model. Although students’ oral explanations went along with their drawings during the interviews, we used the reaction between Mg and O2 as a probe to prevent ambiguous diagrammatic representation. It involved magnesium ions, commonly represented as ‘Mg2+’ using the atomic model. This reaction was expected to be a more sensitive indicator of the students’ use of models.
We postulated that students who represent substances as undifferentiated particles and reactions as spatial rearrangements of these particles are nevertheless able to demonstrate some advanced ideas about chemical reactions. Grade 12 students who had completed the entire school chemistry curriculum, covering energetics and kinetics, and Grades 10 and 11 students formed our sample of informants. This study targeted at answering the following research questions:
(1) With reference to the particle model and the atomic model, how did the Grade 10–12 students represent the reaction between magnesium and oxygen at the submicro level?
(2) Did any of the students represent the particle model of reactions while demonstrating some advanced understanding of the chemical reaction? If so, how was their understanding characterised?
The answers to these questions were important for the following reasons. (1) They were expected to further testify to and validate the value of the particle model and the atomic model in modelling students’ representations of chemical reactions as proposed in Cheng and Gilbert (2017). (2) Even more importantly, an affirmative answer to the second research question would provide an empirical explanation of the low correlation between students’ understanding of ‘structure and composition’ and that of ‘chemical properties and change’. (3) In the conceptual analysis above, it was proposed that the simple particle model of matter can be used to introduce energetics and kinetics. The empirical data gathered from the students was expected to shed light on how they attempted to integrate concepts they had been taught in relation to different chemistry topics.
Methodology
This study conceptualised the understanding of the reaction between magnesium and oxygen based on two models of chemical reactions. These two models and the review of the relevant literature guided the development of the research questions. An interview study was conducted to enable in-depth investigation of the ways that the students represented their ideas. We used individual interviews to collect the data to allow the students both to represent their ideas in drawings and to explain their drawings (Cheng and Gilbert, 2009). It should be useful to identify any new patterns of understanding (in relation to the second research question). I interviewed 18 students from two secondary schools in Hong Kong at the end of the academic year. By the end of Grade 10, the students had been taught different types of bonding. They had been taught to use electron diagrams to represent the formation of Mg2+ and O2− ions from Mg and O atoms in their classes on ionic bonding. They had also learnt about the reactions of different metals with oxygen gas, such as the burning of magnesium in air, in the context of a ‘metal reactivity series’. When taught the idea of ‘empirical formulae’, the reaction of magnesium and oxygen in the formation of magnesium oxide was used as an example. Teaching was focused on the drawing of electron diagrams (on the topic of ionic bonding), the use of demonstration, writing a balanced equation for the reaction (on the topic of metal reactivity) and numerical manipulation (on the topic of empirical formulae). No mention was made of models of reactions either by the teacher or in the students’ textbook. So the interview task was unlikely to be testing students to recall submicro representations. By the end of Grade 11, the students had been taught energetics, collision theory and the factors that affect reaction rate. By the end of Grade 12, they had been taught chemical kinetics, which covered activation energy and the Arrhenius equation.† The reaction between magnesium and oxygen was not used in the teaching of these ideas. Schools in the territory are classified into three bands according to students’ academic performance. The two schools sampled were from the middle band, and were different from those sampled in Cheng and Gilbert (2017). From each of Grade 10 to Grade 12 in each school, one student from the top 10th percentile, one student from the 45th to the 55th percentile and one student from the lowest 10th percentile of their final examination scores for chemistry were interviewed. Accordingly, nine students from each of the two schools (three from each of Grade 10–12) were interviewed.Interviews and transcriptions
Before conducting the interviews, consent was sought from the students and their parents. Each student was shown an interview card printed with the equation Mg (s) + O2 (g) → MgO (s) and told that the burning of magnesium was represented by the equation. They were then asked the following question. ‘Can you explain to me what the equation represents and what it tells you?’ Students whose responses addressed the submicro level were asked to produce drawings to represent their ideas. When students did not respond at the submicro level, they were asked the following question. ‘When you learn chemistry, you learn about particles like ions, atoms and molecules. We call this the microscopic world. How do you understand this chemical equation in relation to the microscopic world?’ The respondents were also asked to produce drawings to represent their ideas. The word ‘microscopic’ was chosen because it is used in the official curriculum guide to refer to the submicro level. As the above interview question included the words, ‘ions’, ‘atoms’ and ‘molecules’, the students might have been prompted to represent the atomic model. However, the use of the word ‘particles’ might equally have prompted the use of undifferentiated particles or the particle model. As the data would show, even with hints as to these chemical species, some students were found to represent undifferentiated particles. Content validity, defined as ‘the extent to which the questions on the instrument… are representative of all the possible questions that could be asked about the content…’ (Creswell, 2002, p. 184), is an important issue in this study. A strategy of establishing it is to ‘[ask] experts if the questions are representative of the area of interest’ (p. 183). In this study, the pattern of questions was the same as Cheng and Gilbert (2017). In that study, I devised the interview questions and conducted the interviews. The second author served as the expert who checked and confirmed content validity (Cohen et al., 2000, pp. 109–110). Given that the same pattern of questions was used in this current study, it was assumed that the content validity has already been established. Also, the interview questions were reported above, which should provide transparency so that readers can be assured of the content validity.All interviews were conducted by the author in Cantonese, the students’ native spoken language. The interviews were video recorded, with the camera faced the table on which the interviews were conducted. It recorded the students’ spoken responses and their process of drawing without capturing their faces. All interview videos were transcribed and translated into English. The students’ drawings were added to the transcripts for analysis. The transcripts were prepared by a research assistant fluent in both Cantonese and English. She had obtained a Bachelor's degree in chemistry in England and a PhD (also in chemistry) from an English-speaking university in Hong Kong. The author also checked the transcripts to confirm the quality of the transcription and translation.
Data analysis
The students’ representations were categorised based on the defining features of the particle model and the atomic model (please refer to the row entitled ‘Process involved’ in Table 1). The criteria for categorisation were as follows.(a) Drawings and responses that represented merely the spatial rearrangement of particles during the reaction (as in Fig. 1) were regarded as adopting the particle model.
(b) Drawings and responses that represented differentiated particles such as magnesium atoms and magnesium ions before and after the reaction (like Fig. 2) and/or involved electron transfer were regarded as based on the atomic model.
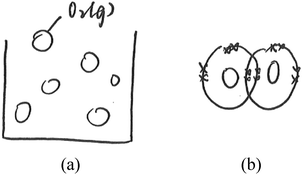
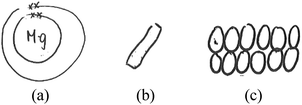
The data analysis was at times complicated by a lack of coherence between the students’ drawings and their oral labelling of the entities drawn. In particular, some students drew plain circles, which could have been interpreted either as representing undifferentiated particles or as shorthand for atoms or ions. During the interviews, the students were asked to name the circles they had drawn (please refer to Fig. 5 for an example). The data were categorised based on how the students represented the changes in submicro species during the reaction. The data were regarded as based on the atomic model if a student said that the reaction involved a change in magnesium from ‘atoms’ to ‘ions’, even when plain circles were drawn. Hence, based on the representation in Fig. 5 and what the student said earlier in the interview, the student was coded as representing the atomic model. In other words, we accepted the students’ plain circles as drawings of atoms/ions if they orally named the plain circles ‘atoms’ and then ‘ions’. However, we did not necessarily categorise drawings of ions (a positive sign inside a circle) and atoms as based on the atomic model. When these submicro entities involved only spatial rearrangement (criterion (a) above), they were categorised as based on the particle model. Two such cases were observed. These data are reported in the section entitled ‘(b) Simple combination and rearrangement of differentiated particles’.
 | ||
| Fig. 5 An excerpt of an interview with the student L10T. (* The code for students will be explained in the next section). | ||
This study focused on model usage rather than students’ misconceptions. Therefore, we did not evaluate the scientific quality of the students’ representations by, for instance, considering their decision to represent the magnesium oxide solid as discrete ion pairs or molecular pairs (Taber, 2001; Nyachwaya et al., 2011; Naah and Sanger, 2012).
To address the second research question, representations based on the particle model were further examined to determine whether the students demonstrated some advanced understanding of energetics and/or kinetics.
To ensure reliable data analysis, the data were coded independently by the author and his colleague, a specialist in chemistry education. They achieved a high level of agreement: 94% (17 of 18 students).
Results and discussion
This section begins by reporting the number of students who used the particle model and the number who used the atomic model to represent the reaction. This is followed by illustrative examples of the students’ responses and drawings. These data demonstrate qualitatively how the students represented the reaction using these two models.In response to the second research question, one student represented the reactants and product using the particle model. Meanwhile, he demonstrated some advanced understanding of the reaction in terms of some energetics and kinetics concepts. The data provided evidence that the use of the simple particle model of substances can co-exist with some higher understanding of chemical reactions.
Students are coded here according to their school (K or L), their grade (10, 11 or 12) and their achievement based on their chemistry examination scores. (T represents a student from the top 10th percentile, M a student from the 45th–55th percentile and B a student from the lowest 10th percentile.) For example, the code ‘K10T’ represents a Grade 10 student from School K in the top 10th percentile.
Distribution of students adopting different models
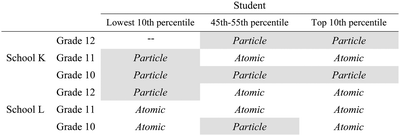
All of the students except one (K12B) demonstrated an understanding of the task requirements and drew to explain submicro diagrams in their responses. Thus, the data of the student K12B was not included in this paper. The distribution of students representing the two models is shown in Tables 2 and 3. To facilitate visual inspection, the cells representing students who adopted the particle model are shown in grey.| Particle model | Atomic model | |
|---|---|---|
| School K | 6 | 2 |
| School L | 2 | 7 |
| Total | 8 | 9 |
Due to the nature of the study and the sampling method, we cannot infer any patterns in the responses of students from different schools and different grades. The overall distribution of model usage in Table 3 was not lopsided. This allowed us to examine the different ways that the students represented the reaction using the two models. The data are reported in the next section.
Representations based on the atomic model
Nine students represented the reaction using the atomic model. Two main responses were given in this category: (a) drawing an Mg2+ and O2− ion pair (n = 6), and (b) drawing an MgO molecular pair (n = 3). The students’ responses were consistent with those reported in the literature. A typical example of each type of response is given below.We would have been unable to judge the model used by the student simply by looking at the product. The judgement had to be based also on the reactants represented. Students were regarded as using the atomic model only when the identity of the reactants and that of the products was shown to change, and the reaction was represented as more than the spatial rearrangement of particles. When probed into the meaning of the terms Mg(s) and O2(g) on the reactant side of the equation, Student L11M demonstrated flexibility in representing magnesium as a collection of magnesium atoms and in terms of its electronic structure, and in representing oxygen gas as a collection of single-unit diatomic oxygen molecules.
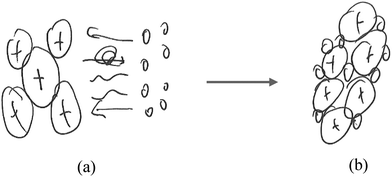
“This atom [Fig. 7a] bonds with another atom tightly to form this piece [Fig. 7b].
 | ||
| Fig. 7 Student L11M's representation of (a) a magnesium atom, (b) a lump of magnesium, and (c) a collection of magnesium atoms. | ||
This is [Fig. 7c]… one circle is one atom [pointing to Fig. 7a].
There are [referring to Fig. 8a] actually a lot. The molecules are separated, spread out.
…[this] [pointing to Fig. 8b] is one of these [referring to one of the circles in Fig. 8a].”
Based on Fig. 7c and 8a alone, the student might have been considered to represent undifferentiated particles. However, Fig. 8b indicated that those particles in Fig. 8a were merely shorthand for diatomic oxygen molecules. Magnesium atoms (Fig. 7c) were understood to be made of subatomic particles (Fig. 7a). In addition, these atoms and molecules and their electrons were represented as involved in the reaction resulting in the formation of magnesium oxide (Fig. 6). Magnesium atoms changed to ions and oxygen molecules become O2−. Therefore, this student was thought to have adopted the atomic model to represent the reaction.
“One metal mixes with oxygen, and then these two [referring to MgO in the equation on the interview card] have to attain an octet structure.”
The student's drawings of the reactants and product are shown in Fig. 9.
She explained the method of working out the electronic structure of MgO as follows.
“First, see how many outermost shell electrons are there for each thing [reactant]. And then allow them to make it into eight. Each one shares some electrons.”
She also represented changes in bonding between the reactants and product. She described the occurrence of metallic bonding between magnesium ions (Fig. 10). Despite drawing a molecular-pair diagram, the student named the bond between Mg and O an ‘ionic bond’.
 | ||
| Fig. 10 K11M's drawing of a metallic bond between Mg (note: the Chinese characters above the line means ‘metallic bond’). | ||
The aim of this study was to make sense of each student's representation by identifying the model he or she used. Although student K11M's drawing of the product was not consistent with scientific representations of MgO, we regarded her representation as akin to the atomic model. Rather than representing merely the spatial rearrangement of particles, her diagram acknowledged that electrons as subatomic particles play a role in the reaction, which is a characteristic of the atomic model.
Representations based on the particle model
Eight students represented the reaction based on the particle model. The first subsection describes two ways that the students represented the chemical reaction as a simple combination and rearrangement of undifferentiated particles. These two examples were illustrative of typical simple rearrangement representations (which were drawn by five of the eight students who adopted the particle model). The second subsection reports on two representations that might have appeared to be akin to the atomic model but were ultimately regarded as adopting the particle model. The final subsection provides evidence in support of an affirmative answer to the second research question. The student (K12T) was found to demonstrate a fairly sophisticated understanding of chemical reactions based on the particle model. This section presents data for five of eight students who represented the particle model. These examples are expected to offer useful insights into students’ overall representation of the particle model in this study.“A strip of magnesium, a strip of metal plate. When it burns, because air has oxygen, therefore, during burning, the oxygen gas… At first in the air, the two atoms might be stuck together, when burning they separate. Then the oxygen atom goes to the magnesium and [they] stick together.”
The student described the process of the reaction as follows: two oxygen atoms separate during burning, and one sticks to the magnesium atom. In the representation, each substance was thought to be made of ‘atoms’, not subatomic particles. The reaction was not presented as involving electrons. The reaction was treated as the simple spatial relocation of an oxygen atom from an O2 unit – which the student did not name as a molecule – to a magnesium atom. This representation was thus regarded as based on the particle model of reactions.
Similarly, Student K11B represented the reaction as a simple combination of Mg and O2 (Fig. 11b). ‘When they are in a reaction, they will combine together’. In neither case was the reaction presented as involving a change in the identity of the reactants. The reactant atoms or particles remained the same entities after the reaction. Both representations were thus regarded as based on the particle model of reactions.
Five students represented the particle model of reactions in a manner similar to those shown above. The representations made by the other three students who represented the particle model are reported and discussed in the following subsections.
 | ||
| Fig. 12 Student K12M's representation of (a) the interaction between magnesium and oxygen, and (b) the product magnesium oxide. | ||
“…magnesium is also made of a lot of different magnesium particle-like things… The oxygen particles are attracted by the metal magnesium.”
Based on his drawing alone, the student might have appeared to have represented magnesium ions or charged magnesium particles, demonstrating his understanding of differentiated submicro entities and thereby meeting one of the criteria for representing the atomic model. However, the reaction was in fact presented merely as a simple combination of particles. Although the student represented charged particles, he did not represent a change in the identity of either the magnesium particle or the oxygen particle after the reaction. These product particles were represented in the same way in Fig. 12b. In addition, no subatomic particles were presented as involved in the reaction. Therefore, we categorised his representation as akin to the particle model.
The responses made by another student, K10B, further illustrate the representation of the particle model. Referring to the chemical equation, the student offered the following explanation. ‘This is a cation [while circling Mg(s) in the equation] and this is an anion [while circling O2(g) in the equation]…and synthesize to give a compound’. The names used might suggest that the student was aware of the presence of magnesium ions and oxide ions in the product. However, he was in fact referring to the identity of the reactants. His drawing represented the reaction as a simple combination of a ‘cation’ and an ‘anion’ (Fig. 13). No evidence was provided in the student's drawing or oral explanation of a change from magnesium atoms to magnesium cations and/or oxygen molecules. Therefore, student K10B's representations of the reaction were regarded as akin to the particle model.
In short, we did not identify the model that students might have used merely by referring to the types of particles drawn. More importantly, we considered the nature of the reaction – whether a simple combination/rearrangement of particles or changes to reactant particles in the formation of products with the participation of electrons. Students’ use of differentiated particles in representing substances but representing the reaction of these substances based on the particle model of reactions was also observed in an earlier study (Cheng and Gilbert, 2017). Some of the sampled students represented the reaction between magnesium and hydrochloric acid in terms of hydrogen ions, chloride ions and magnesium atoms. Yet they also represented the reaction as a simple combination (of two hydrogen ions, forming H2, and of magnesium atoms and chloride ions, forming MgCl2). Such a representation was regarded as an example of the particle model of reactions regardless of the students’ reference to differentiated particles (atoms and ions). The data presented in this section also echo the earlier finding that there was only a slight relationship (correlation = 0.29) between students’ understanding of ‘structure and composition’ and ‘chemical reactions’ (Hadenfeldt et al, 2016). In the data presented above, the students were able to represent differentiated particles (Level 4 for ‘structure and composition’); but they represented the reaction only as simple combination of particles (i.e., Level 3 for ‘chemical reactions’).
 | ||
| Fig. 14 (a) Student K12T's representation of the reaction. (b) His drawing of the product at the submicro level. | ||
In many ways, the representations were similar to those reported in the subsection entitled ‘(a) Simple combination and rearrangement of particles’ above. A simple rearrangement of particles was reported. Oxygen combined with magnesium particles in the reaction. The atoms and ions were not differentiated; the reaction involved neither electrons nor changes in magnesium from atoms to ions. Such representations were consistent with the particle model. The following two observations are worth mentioning.
(1) The student was able to draw the lattice structure of MgO in a manner similar to scientifically accepted representations. The MgO drawn in Fig. 14a was extended not only in MgO pairs but as a continuous lattice. Such a representation should usefully support the learning of scientific representations of MgO based on the atomic model.
(2) Although the student used simple particles to represent the reactants and product (Level 3 attainment for both ‘structure and composition’ and ‘chemical reactions’, using the framework proposed by Hadenfeldt et al. (2016)), he demonstrated some advanced understanding of certain reaction-related topics. Before drawing Fig. 14, he made the following remarks about the equation for the reaction.
“Magnesium metal is here and some oxygen gas is next to the magnesium metal. Then through burning, it surpasses the required conditions, the activation energy, for the reaction. Then a reaction will occur. Because the energy is enough for the reaction. So, their particles can collide. There are successful collisions and a new substance MgO is formed.”
The student seems to have used collision theory to make sense of the reaction, explaining that Mg and O2 particles must collide to form MgO. He also seemed to be aware of the role of ‘burning’ in overcoming the energy barrier (activation energy) for the reaction. Although an understanding of these ideas exceeds Level 3 attainment, the student then used the particle model to represent the reaction in a scientifically acceptable way (particularly the drawing of O2 (g) and MgO (s)). It is thus likely that the use of the particle model can coexist with some more advanced ideas of chemical reactions, such as collision theory in chemical kinetics. The data of this student may further explain the rather low correlation between students’ understanding of ‘structure and composition’ and that of ‘chemical reactions’ (Hadenfeldt et al., 2016). That is, students were unlikely to have the same level of understanding these two concepts. Take this particular student as an example, he demonstrated Level 3 attainment for ‘structure and composition’, but achieved a higher level of attainment for ‘chemical reactions’.
Conclusions
This paper was motivated by the lack of clear chemistry curricular models to represent chemical reactions. Learning-progression frameworks in this area have provided insights into curriculum, teaching and assessment. Hadenfeldt et al. (2016) provided a very useful model of students’ progression in learning matter-related concepts, delineating various levels of attainment in the categories of ‘structure and composition’, ‘physical properties and change’, ‘chemical reactions’ and ‘conservation’.Although acknowledging the contribution made by the learning-progression framework for the concept of matter, the aim of this paper was to address issues related to the learning of chemical reactions through the lens of models and modelling. We suggest that an understanding of chemical reactions can be represented in terms of the particle model and the atomic model. Such a conceptualisation allows flexibility in making sense of students’ understanding of chemical ‘structure and composition’ and ‘chemical reactions’ – this study showed that students’ understanding of chemical reactions does not have to be bundled up with their representations of structural constituents of substances (be they simple particles or differentiated particles). It also explains the low correlation between students’ understanding of chemical structures and understanding of chemical reactions. Some students (K12T in this study) who represented substances as simple particles and used the particle model of reactions also demonstrated some advanced understanding of chemical reactions. In addition, some (K12M and K10B) who represented substances in terms of differentiated particles such as atoms, ions, electrons and molecules also represented the reaction as a simple combination or spatial rearrangement of particles, which is a key feature of the particle model of reactions. We suggest that the development of learning from the simple particle model to the differentiated particle model of reactions (Level 3 to Level 4 in Hadenfeldt's learning-progression framework) may be more complicated than often assumed. Conceptualising such learning in terms of the particle model and the atomic model provides an alternative means of understanding students’ learning.
There was evidence that, over time, Grade 10–11 students demonstrated model advancement from the particle model to the atomic model in representing the reaction between magnesium and hydrochloric acid (Cheng and Gilbert, 2017). Taking the reaction between magnesium and oxygen as the example, this study further develops the idea of the particle model and proposes that some advanced ideas of reactions such as kinetics and energetics may be learnt using the particle model. It means that students advance within the particle model but may not go towards the atomic model. The data obtained in interviews with the Grade 10–12 students support this proposal.
Practically, conceptualising students’ learning in terms of a particle model and an atomic model offers useful guidelines for curriculum development. It opens the question of at which grade students should be taught the particle model and its associated ideas of energetics and kinetics. Given the affordances of the particle model, we should ask at which grade and why the atomic model is more suitably introduced. Students’ learning will be enhanced if Grade 10–12 teachers are sensitive to students’ possible understanding of chemical reactions based on the particle model and the atomic model, enabling appropriate support to be given to students if teachers aim for the atomic model as the expected learning outcome. The data also suggest that students who are capable of representing substances in terms of electrons, ions and atoms may not acquire the idea of the atomic model of reactions. These findings should inform classroom teachers’ efforts to support students’ use of differentiated particles in representing the atomic model of reactions.
Limitations and further research
This study examined 18 students from two secondary schools in a specific context. The two models of reactions were found to usefully capture the students’ representations. The study examined students’ representations of a single reaction only. We were unable to infer that particular students used a particular model to visualise other chemical reactions. Learning and understanding are contingent on the ways that students are taught and their prior knowledge (Taber, 2014). One model is likely to be more prevalent than another in a student's mind (based on Potvin and Cyr, 2017). Students may use one model to represent some reactions and another model for other reactions. It would thus be worthwhile to further investigate students’ use of different models of reactions in different contexts. Also, this study did not investigate how teaching might have affected students’ representations and uses of models of reactions. It would be meaningful to consider such a factor in further studies.In this study, one student used the particle model to refer to certain concepts (such as activation energy and collision theory) that are commonly taught at an advanced level of school chemistry. However, because the student had also been taught concepts related to the atomic model, we were unable to make a strong claim that the particle model alone is sufficient to support students’ learning of simple principles of chemical kinetics and energetics (exemplified in Fig. 3 and 4). Teaching-intervention studies that exclude the teaching of the atomic model would offer fuller insights into students’ learning of simple chemical kinetics and energetics ideas in the light of their understanding of the particle model.
Finally, this study did not develop a learning progression framework for the particle model and the atomic model. Further studies should be conducted to investigate the trajectory of students’ learning of these models.
Implications for teaching and curriculum planning
To enhance students’ learning of a particular model, the teaching and/or the curriculum should provide a context in which the learning and the use of such a model is warranted (Gilbert and Justi, 2016). Such a context or justification is particularly important when students have already learnt a less sophisticated model of the same phenomenon (Shiland, 1995). If we target at facilitating students to learn the atomic model of reactions, a context should be provided in which the use of the particle model is insufficient to explain a reaction. The topic of the reactivity of metals (to, for example, oxygen) is likely to provide a suitable context for the introduction of the atomic model of reactions. The particle model, which presents reactions as a simple rearrangement of particles, does not explain at the submicro level why calcium is more reactive than magnesium or why potassium is more reactive than sodium. The reactivity of metals can be explained only through consideration of the distance/attraction between the outermost shell electrons and the atomic nucleus, which requires the atomic model.The data obtained in this study suggest that the students experienced challenges in using the atomic model to scientifically represent the reaction between magnesium and oxygen. Teaching strategies for effective learning may capitalise on (1) critique, (2) interactive viewing and (3) construction. In classroom settings, students could be asked to represent the reaction of magnesium/oxygen and calcium/oxygen at the submicro levels with labelled drawings. As a strategy for critique, the students’ representations could form a basis for highlighting the limitations of the particle model. Alternatively, teachers could show an animation of the reaction of magnesium/oxygen based on the atomic model. Students could then be asked to construct a series of drawings to represent their understanding of the reaction. This study revealed that students rarely represented the role of electrons (from magnesium) in the formation of oxide ions, even when they used the atomic model. In constructing drawings, teachers should consider providing scaffolds to prompt students to consider the role of electrons in the reaction and in their drawings. Also, some students were found to represent the particle model of reactions even though they used differentiated particles. It is suggested that teachers should be sensitive about such representations, and avoid dismissing these representations simply as mistakes. If the expected learning outcome is the atomic model, teachers should help students to discern the parts of their representations that are consistent with the target model (e.g., the presence of charged magnesium particles in the product in the K12M representation, Fig. 12) and the parts that are not (e.g., the presence of neutral oxygen particles in the product, Fig. 12).
In terms of curriculum planning, because the particle model provides useful explanations of energetics and kinetics (simple collision theory), curriculum planners and teachers should acknowledge the model explicitly when addressing these two topics. This is particularly important because by the time students learn these topics, they are likely already to have learnt the atomic model of reactions. Explicit attention to the particle model should help students to see the range and limitations of different models, which may be regarded as a progression in learning chemistry (Taber, 1995). When teaching energetics and kinetics, the specific affordances of the atomic model (e.g., in making sense of the mechanisms and molecularity of reactions and the bond energy of carbon–carbon single, double and triple bonds) should be acknowledged. This will better assist students to learn and practise model-based reasoning in chemistry.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to Dr Kennedy Chan and Ms Annie KL Chan for their insightful comments on the manuscript. The research study was supported by Small Project Funding (University Research Committee, The University of Hong Kong).References
- Ahtee M. and Varjola I., (1998), Students' understanding of chemical reaction, Int. J. Sci. Educ., 20(3), 305–316.
- Andersson B., (1990), Pupils' conceptions of matter and its transformations (age 12–16), Stud. Sci. Educ., 18, 53–85.
- Ardac D. and Akaygun S., (2004), Effectiveness of multimedia-based instruction that emphasizes molecular representations on students’ understanding of chemical change, J. Res. Sci. Teach., 41(4), 317–337.
- Atkins P., (2013), What is chemistry? Oxford: Oxford University Press.
- Atkins P. and Jones L., (2010), Chemical principles: the quest for insight, 5th edn, New York: W. H. Freeman.
- BouJaoude S. B., (1991), A study of the nature of students’ understandings about the concept of burning, J. Res. Sci. Teach., 28(8), 689–704.
- Chandrasegarana A. L., Treagust D. F. and Mocerino M., (2007), The development of a two-tier multiple-choice diagnostic instrument for evaluating secondary school students’ ability to describe and explain chemical reactions using multiple levels of representation, Chem. Educ. Res. Pract., 8(3), 293–307.
- Cheng M. M. W. and Gilbert J. K., (2009), Towards a better utilization of diagrams in research into the use of representational levels in chemical education, in Gilbert J. K. and Treagust D. (ed.), Multiple representations in chemical education, Dordrecht, Netherlands: Springer, pp. 55–73.
- Cheng M. M. W. and Gilbert J. K., (2014), Teaching stoichiometry with particulate diagrams – linking macro phenomena and chemical equations, in Eilam B. and Gilbert J. K., (ed.), Science Teachers’ Use of Visual Representations, Dordrecht: Springer Science + Business Media B.V., pp. 123–142.
- Cheng M. M. W. and Gilbert J. K., (2017), Modelling students’ visualisation of chemical reaction, Int. J. Sci. Educ., 39(9), 1173–1193.
- Cheng E., Chow J., Chow Y. F., Kai A., Lai K. K. and Wong W. H., (2009), Chemistry: a modern view (book4), Hong Kong: Aristo.
- Cheng E., Chow J., Chow Y. F., Kai A., Lai K. K. and Wong W. H., (2010), Chemistry: a modern view (book3), Hong Kong: Aristo.
- Cohen L., Manion L. & Morrison K., (2000), Research methods in education, 5th edn, London; New York: Routledge/Falmer.
- Creswell J. W., (2002), Educational research: planning, conducting, and evaluating quantitative and qualitative research, Upper Saddle River, N.J.; Columbus, OH: Merrill/Prentice Hall.
- Erduran S. and Duschl R. A., (2004), Interdisciplinary characterization of models and the nature of chemical knowledge in the classroom, Stud. Sci. Educ., 40, 105–138.
- Garnett P. J., Garnett P. J. and Treagust D., (1990), Implication of research on students’ understanding of electrochemistry for improving science curricula and classroom practice, Int. J. Sci. Educ., 12(2), 147–156.
- Gilbert J. K. and Justi R., (2016), Modelling-based teaching in science education, Switzerland: Springer.
- Hadenfeldt J. C., Neumann K., Bernholt S., Liu X. and Parchmann I., (2016), Students’ progression in understanding the matter concept, J. Res. Sci. Teach., 53(5), 683–708.
- Hinton M. E. and Nakhleh M. B., (1999), Students’ microscopic, macroscopic, and symbolic representations of chemical reactions, Chem. Educ., 4, 158–167.
- Johnson P. and Tymms P., (2011), The emergence of a learning progression in middle school chemistry, J. Res. Sci. Teach., 48(8), 849–877.
- Kelly R. M., Barrera J. H. and Mohamed S. C., (2010), An analysis of undergraduate general chemistry students’ misconceptions of the submicroscopic level of precipitation reactions, J. Chem. Educ., 87(1), 113–118.
- Kelly R. M., Akaygun S., Hansen S. J. R. and Villalta-Cerdas A., (2017), The effect that comparing molecular animations of varying accuracy has on students’ submicroscopic explanations, Chem. Educ. Res. Pract., 18(4), 582–600.
- Liu X. and Lesniak K. M., (2005), Students’ progression of understanding the matter concept from elementary to high school, Sci. Educ., 89(3), 433–450.
- Liu X. and Lesniak K., (2006), Progression in children's understanding of the matter concept from elementary to high school, J. Res. Sci. Teach., 43(3), 320–347.
- Naah B. M. and Sanger M. J., (2012), Student misconceptions in writing balanced equations for dissolving ionic compounds in water, Chem. Educ. Res. Pract., 13(3), 186–194.
- Nyachwaya J. M., Mohamed A.-R., Roehrig G. H., Wood N. B., Kern A. L. and Schneiderd J. L., (2011), The development of an open-ended drawing tool: an alternative diagnostic tool for assessing students’ understanding of the particulate nature of matter, Chem. Educ. Res. Pract., 12, 121–132.
- Øyehaug A. B. and Holt A., (2013), Students’ understanding of the nature of matter and chemical reactions – a longitudinal study of conceptual restructuring, Chem. Educ. Res. Pract., 14(4), 450–467.
- Potvin P. and Cyr G., (2017), Toward a durable prevalence of scientific conceptions: tracking the effects of two interfering misconceptions about buoyancy from preschoolers to science teachers, J. Res. Sci. Teach., 54(9), 1121–1142.
- Robertson A. D. and Shaffer P. S., (2014), “Combustion always produces carbon dioxide and water”: a discussion of university chemistry students’ use of rules in place of principles, Chem. Educ. Res. Pract., 15(4), 763–776.
- Shiland T. W., (1995), What's the use of all this theory? The role of quantum mechanics in high school chemistry textbooks, J. Chem. Educ., 72(3), 215–219.
- Smith K. J. and Metz P. A., (1996), Evaluating student understanding of solution chemistry through microscopic representations, J. Chem. Educ., 73(3), 233–235.
- Smith C. L., Wiser M., Anderson C. W. and Krajcik J., (2006), Implications for children's learning for assessment: a proposed learning progression for matter and the atomic molecular theory, Measurement: Interdisciplinary Research and Perspectives, 14(1–2), 1–98.
- Solsona N., Izquierdo M. and De Jong O., (2003), Exploring the development of students' conceptual profiles of chemical change, Int. J. Sci. Educ., 25(1), 3–12.
- Stains M. and Talanquer V., (2008), Classification of chemical reactions: stages of expertise, J. Res. Sci. Teach., 45(7), 771–793.
- Stavridou H. and Solomonidou C., (1998), Conceptual reorganization and the construction of the chemical reaction concept during secondary education, Int. J. Sci. Educ., 20(2), 205–221.
- Taber K. S., (1995), An analogy for discussing progression in learning chemistry, Sch. Sci. Rev., 76(276), 91–95.
- Taber K. S., (2001), Building the structural concepts of chemistry: some considerations from educational research, Chem. Educ.: Res. Pract., 2(2), 123–158.
- Taber K. S., (2003), The atom in the chemistry curriculum: fundamental concept, teaching model or epistemological obstacle? Found. Chem., 5(1), 43–84.
- Taber K. S., (2014), Student thinking and learning in science: perspectives on the nature and development of learners' ideas, New York, N.Y.: Routledge.
- The Economist, (2016), Atoms and the voids – Individual atoms offer ultra-dense information storage, Economist, 420(8999), 61.
- Weinrich M. L. and Talanquer V., (2016), Mapping students’ modes of reasoning when thinking about chemical reactions used to make a desired product, Chem. Educ. Res. Pract., 17(2), 394–406.
- Williamson V. M. and Abraham M. R., (1995), The effects of computer animation on the particulate mental models of college chemistry students, J. Res. Sci. Teach., 32(5), 521–534.
- Windschitl M., Thompson J. and Braaten M., (2008), Beyond the scientific method: model-based inquiry as a new paradigm of preference for school science investigations, Sci. Educ., 92(5), 941–967.
- Yan F. and Talanquer V., (2015), Students’ ideas about how and why chemical reactions happen: mapping the conceptual landscape, Int. J. Sci. Educ., 37(18), 3066–3092.
- Zhang Z. H. and Linn M. C., (2011), Can generating representations enhance learning with dynamic visualizations? J. Res. Sci. Teach., 48(10), 1177–1198.
Footnote |
| † The curriculum can be found in the Chemistry Curriculum and Assessment Guide (secondary 4–6), available at http://www.edb.gov.hk/attachment/en/curriculum…/Chem_C&A_Guide_updated_e.pdf. |
| This journal is © The Royal Society of Chemistry 2018 |