Metallic CuNPs confined in hollow silicalite-1: excellent catalytic efficiency in p-nitrophenol reduction†
Rituparna
Das
,
Sourav
Ghosh
 and
Milan Kanti
Naskar
and
Milan Kanti
Naskar
 *
*
Sol–gel Division, CSIR-Central Glass and Ceramic Research Institute, Kolkata 700032, India. E-mail: milan@cgcri.res.in
First published on 24th November 2017
Abstract
Copper nanoparticles (CuNPs) confined in hollow silicalite-1 powders were synthesized by a selective “desilication–recrystallization” method using tetraethlyorthosilicate (TEOS), tetrapropylammonium hydroxide (TPAOH) and cupric chloride (CuCl2) as precursors. The synthesized product was characterized by XRD, Raman, XPS, FESEM, TEM and N2 adsorption–desorption studies. The presence of CuNPs of size 10–40 nm in the hollow silicalite-1 was confirmed by TEM. The BET surface area of the powders was 247 m2 g−1 composed of micropores and mesopores. The prepared CuNPs confined in hollow silicalite-1 showed excellent catalytic performance for the reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) with apparent rate constant and activity parameter values of 5.6 × 10−3 s−1 and 44.09 s−1 g−1, respectively for 1 mg of catalyst.
1. Introduction
Noble metal nanoparticles have attracted growing interest in chemical industries because of their fascinating properties and extensive applications in the field of catalysis, separation, and microelectronics.1–3 Among them copper nanoparticles (CuNPs) have drawn immense attention from experimentalists, theorists, and technologists during the past decade due to their lower cost and large abundance compared to silver and gold. Copper is generally used in catalysis, optical, electronic and antimicrobial applications in the most active areas of nanoscience and nanotechnology.4–7 It has been demonstrated that the intrinsic properties of CuNPs are size and shape dependent. It is well accepted that the physicochemical properties and catalytic activity of metal nanoparticles are superior to those of bulk metals. However, smaller NPs tend to aggregate due to their large surface area-to-volume ratio, which significantly reduces their actual applications.8,9 The preparation method remains a great challenge to preserve the catalytic properties and performances of metal NP catalysts. However, confined metal particles are generally monodispersed in size, isolated from each other and prevented from sintering by the Ostwald ripening mechanism which enhances the exposure of the metal active surface and exhibits size selectivity.10,11Hollow zeolites can be the ideal matrix for the confinement of metal catalysts due to their high thermal and mechanical stability, unique shape selectivity, unique pore structure with large specific surface area etc.12 On the other hand because of their brilliant resistance under corrosive conditions and molecular sieving properties, crystalline zeolitic shells can also protect the catalyst under harsh reaction condition as a selective barrier against impurities, poisons, and undesirable reactions. By introducing mesoporosity in zeolite structures, the catalytic performance can be increased with decreasing diffusion path length. Therefore, hollow mesoporous zeolites with hierarchical porosity in particular, can be an interesting candidate as a catalytic support.
The preparation of hollow zeolites has been reported by the layer-by-layer assembly technique using polystyrene spheres as the soft template and nanozeolites as the building blocks.13,14 Desilication of framework Si in alkaline media is another efficient methodology to synthesize hollow zeolites.15–17 We have recently synthesized hollow mesoporous silicalite-1 confined with biogenic AgNPs of size 10–15 nm using green carambola extract.18 Li et al. reported noble metal nanoparticles located in hollow single crystals of silicalite-1 such as Au@silicalite-1 and Pt@silicalite-1.19,20 Recently Dai et al. synthesized hollow ZSM-5 single crystals with silicon-rich exterior surfaces encapsulating iron and carbon nanotubes.21
In this present study, we report the synthesis of CuNPs confined in hollow silicalite-1 via a desilication–recrystallization method. CuNPs confined in the hollow silicalite-1 exhibited enhanced catalytic reduction of p-nitrophenol, a water pollutant present in industrial effluents. The reduced form of p-nitrophenol, i.e., p-aminophenol, may be used as a drug, photographic developer, corrosion inhibitor etc. CuNPs confined in hollow silicalite-1 revealed enhanced catalytic efficiency with a rate constant of 5.6 × 10−3 s−1 and can be reused several times without significant loss of their original activity.
2. Experimental
2.1. Chemicals and materials
Tetraethlyorthosilicate (TEOS), tetrapropylammonium hydroxide (TPAOH) and cupric chloride (CuCl2) were purchased from Sigma-Aldrich. Ethyl alcohol (C2H5OH), sodium borohydride (NaBH4) p-nitrophenol (4-NP) and 4A molecular sieves were acquired from Merck. All the reagents were of analytical grade and used without further purification. Deionized (DI) water was used throughout the experiment.2.2. Experimental procedure
For the synthesis of parent silicalite-1, in a typical experiment, 7.4 mL tetrapropylammonium hydroxide (TPAOH) (1 M) was added dropwise into a mixed solution of 8.3 mL tetraethylorthosilicate (TEOS), 24.7 g DI water and 8.6 mL ethanol (C2H5OH) under stirring conditions. The molar composition of the solution was maintained as 1SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2TPAOH
0.2TPAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4C2H5OH
4C2H5OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46H2O. After stirring for 6 h at room temperature, it was heated in an autoclave at 170 °C/72 h. The resulting solid was recovered by centrifugation, washed with DI water, and dried overnight at 100 °C. Finally, the template (TPAOH) was removed by calcination at 550 °C/6 h. In the next step, 1 g of calcined parent silicalite-1 (termed as S-1) was dispersed in an aqueous solution of 20 mL CuCl2 (5 mmol) under stirring for 24 h. After drying overnight at 100 °C the product was calcined at 500 °C/4 h. The calcined product (termed as CuS-1) was well dispersed in 20 mL TPAOH (0.3 M) solution followed by heating in an autoclave at 170 °C/72 h. The resulting product was recovered by centrifugation, washed with DI water, and dried overnight at 100 °C. The template was removed by calcination at 500 °C/4 h in air to obtain copper species confined in hollow silicalite-1 (termed as CuHS-1). The role of TPAOH in the desilication process toward the formation of hollow silicalite-1 is illustrated in our recent paper18 (ESI†). For the synthesis of CuNPs confined in hollow silicalite-1, the synthesized CuHS-1 was reduced under hydrogen atmosphere at 700 °C with a heating rate of 4 °C min−1 and dwelling time of 1 h (flow rate of H2 was 40 mL min−1). The reduced sample was designated as CuHS-1R.
46H2O. After stirring for 6 h at room temperature, it was heated in an autoclave at 170 °C/72 h. The resulting solid was recovered by centrifugation, washed with DI water, and dried overnight at 100 °C. Finally, the template (TPAOH) was removed by calcination at 550 °C/6 h. In the next step, 1 g of calcined parent silicalite-1 (termed as S-1) was dispersed in an aqueous solution of 20 mL CuCl2 (5 mmol) under stirring for 24 h. After drying overnight at 100 °C the product was calcined at 500 °C/4 h. The calcined product (termed as CuS-1) was well dispersed in 20 mL TPAOH (0.3 M) solution followed by heating in an autoclave at 170 °C/72 h. The resulting product was recovered by centrifugation, washed with DI water, and dried overnight at 100 °C. The template was removed by calcination at 500 °C/4 h in air to obtain copper species confined in hollow silicalite-1 (termed as CuHS-1). The role of TPAOH in the desilication process toward the formation of hollow silicalite-1 is illustrated in our recent paper18 (ESI†). For the synthesis of CuNPs confined in hollow silicalite-1, the synthesized CuHS-1 was reduced under hydrogen atmosphere at 700 °C with a heating rate of 4 °C min−1 and dwelling time of 1 h (flow rate of H2 was 40 mL min−1). The reduced sample was designated as CuHS-1R.
2.3. Characterization
Powder X-ray diffraction (PXRD) studies of the samples were performed using a Philips X’Pert Pro PW 3050/60 powder diffractometer using Ni-filtered Cu-Kα radiation (λ = 0.15418 nm) operated at 40 kV and 30 mA. X-ray photoelectron spectroscopy (XPS) measurements were carried out using a PHI 5000 Versaprobe II Scanning XPS microprobe (ULVAC-PHI, USA). The spectra were recorded with monochromatic AlKα (hν = 1486.6 eV) radiation with an overall energy resolution of ∼0.7 eV. The binding energy values were calibrated by using the C 1s level (284.6 eV) of the trace surface contaminants. The Raman spectrum was recorded using a RENISHAW spectrometer with 514 nm radiation from an argon laser at room temperature (514 nm). Nitrogen adsorption–desorption measurements were conducted at 77 K using a Quantachrome (ASIQ MP) instrument. The surface area was obtained using the Brunauer–Emmett–Teller (BET) method and the pore size distributions were calculated by the Barrett–Joyner–Halenda (BJH) method and density functional theory (DFT) method. The nitrogen adsorption volume at the relative pressure (p/p0) of 0.99 was used to determine the pore volume. The morphology of the particles was examined by FESEM (Model: Zeiss, Supra™ 35VP, Germany) operating with an accelerating voltage of 10 kV, and TEM using a Tecnai G2 30ST (FEI) instrument operating at 300 kV. The elemental composition of the sample was analyzed with energy dispersive analysis of X-ray spectroscopy (EDS) coupled to TEM (Mo grid was used for sample preparation). UV-Vis spectra were recorded using a UV-Vis spectrophotometer (Jasco V-730) in the wavelength range of 200–600 nm.2.4. Catalytic performance
To study the catalytic activity and recycling ability of the CuNP confined in hollow silicalite-1 sample (CuHS-1R), the reduction of p-nitrophenol (4-NP) was carried out in the presence of excess NaBH4. The catalytic reduction reaction was set up in a standard quartz cuvette with 1 cm path length and 4 mL volume. In this process, different amounts of catalyst were placed in the cuvette cell containing 4-NP (0.1 mL; 3.0 × 10−3 M), water (2.8 mL), and NaBH4 (0.1 mL; 3.0 × 10−1 M) followed by measuring time dependent absorption spectra. The molar ratio of 4-NP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaBH4 was maintained as 1
NaBH4 was maintained as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. The UV-Vis spectral changes were monitored at different time intervals. The rate constant of the reaction was estimated by the extinction coefficient of the solution at 400 nm (absorption of p-nitrophenolate ion). To reuse the catalyst, it was washed with DI water and dried at room temperature. Then the catalyst was ready to use for the next run. To optimize the dosage of the catalyst, the amount of the catalyst was varied in the range of 1–3 mg. The kinetics of the reaction are represented by ln
100. The UV-Vis spectral changes were monitored at different time intervals. The rate constant of the reaction was estimated by the extinction coefficient of the solution at 400 nm (absorption of p-nitrophenolate ion). To reuse the catalyst, it was washed with DI water and dried at room temperature. Then the catalyst was ready to use for the next run. To optimize the dosage of the catalyst, the amount of the catalyst was varied in the range of 1–3 mg. The kinetics of the reaction are represented by ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) At/A0 = −kt, where k is the pseudo-first order rate constant (apparent rate constant), t is the reaction time, A0 is the concentration of 4-NP at time t = 0, and At is the concentration of the same at time t.
At/A0 = −kt, where k is the pseudo-first order rate constant (apparent rate constant), t is the reaction time, A0 is the concentration of 4-NP at time t = 0, and At is the concentration of the same at time t.
3. Results and discusion
3.1. Characterization of CuNPs confined in silicalite-1
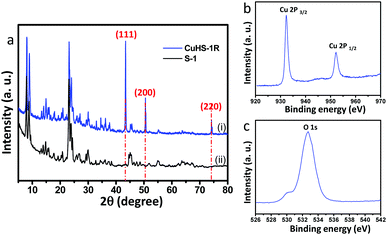
The X-ray diffraction pattern of the CuNP confined in hollow silicalite-1 sample (CuHS-1R) is shown in Fig. 1a. In the XRD plot, the crystalline peaks with 2θ values at 43.29°, 50.43° and 74.13° were assigned to (111), (200) and (220) lattice planes, respectively which are in good agreement with metallic Cu (JCPDS No. 04-0836) along with the characteristic peaks of silicalite-1.5 For comparison the characteristic peaks of pure silicalite-1 (S-1) are also exhibited in Fig. 1a.22 The average crystallite size of the CuNPs estimated by X-ray diffraction line broadening of the (111) peak according to the Scherrer equation was found to be 47.5 nm. It is to be noted that the diffraction peaks of other possible impurities such as Cu2O and CuO could not be detected in this plot. However, the XRD patterns of the calcined sample synthesized before desilication (CuS-1) and after desilication (CuHS-1) method show the characteristic peaks of CuO according to the JCPDS File No. 05-0661 (Fig. S1a and b, ESI†). It shows that CuO is confined in CuS-1 and CuHS-1 samples, respectively.To investigate the composition and chemical state of Cu in the CuNPs confined in hollow silicalite-1 (CuHS-1R), X-ray photoelectron spectroscopy (XPS) was performed. Fig. 1b and c show the XPS spectra of the CuHS-1R sample representing the signals of Cu2p (Cu2p3/2 at 932.33 eV and Cu2p1/2 at 952.23 eV) and O1s (532.7 and 529.9 eV), respectively. It is difficult to identify the different oxidation states of Cu species from the Cu2p and O1s spectra as their peak positions are very close. However, the absence of the two main peaks at 954 eV (Cu2p1/2) and 934 eV (Cu2p3/2) along with shake-up satellite peaks centred at 943 eV confirmed the absence of CuO in the CuHS-1R sample. It is difficult to identify whether Cu2O is present in the CuHS-1R sample from the XPS study. From the O1s spectrum, the binding energy at 532.7 eV corroborated the presence of hydrophilic oxygen-containing groups, such as hydroxy/epoxy groups.23 More detailed results of the O1s spectrum show an oxygen band peak of Si–OH at 529.9 eV, which is related to the O1s spectra of the SiO2.24 Fig. S2 and S3, ESI† show the XPS spectra of CuS-1 and CuHS-1, respectively. Interestingly, in addition to Cu 2p signals (2p1/2 and 2p3/2), satellite peaks at higher binding energy at around 962 eV and 943 eV are observed in the Cu2p XPS spectra of the two samples, which could confirm the existence of CuO in the samples.25 It was reported that Cu(I) or Cu(0) species do not reveal any satellite peaks due to completely filled 3d shells.26 Interestingly, for the CuHS-1 particles the O1s peaks shift a little to lower energy (Fig. S3b, ESI†). The O1s spectra of SiO2 in silicalite-1 could contribute to the shifting of the peak.
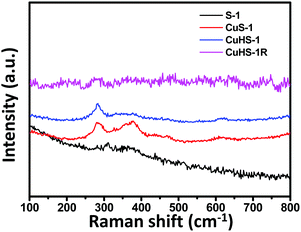
To identify the different oxidation states of Cu in silicalite-1, Raman study was performed. The Raman spectra of the samples are shown in Fig. 2. Raman peaks appeared at around 283, 333, and 622 cm−1 for the CuS-1 and CuHS-1 samples. The peak at 283 cm−1 was assigned to the Ag mode, while the peaks at 333 and 622 cm−1 correspond to Bg modes of phonon vibrations of CuO.27 Some extra peaks at around 377 and 470 cm−1 also appear due to the presence of silicalite-1 zeolite.28 Interestingly, the characteristic peaks of CuO at around 282, 330, and 612 cm−1 are missing in the Raman spectra of S-1 and CuHS-1R particles. The absence of characteristic peaks of Cu2O at 218, 306, 435 and 625 cm−1 corresponding to the second-order Raman allowed mode (2 Γ12), second order overtones (2 Γ15(1)), fourth-order overtone (4 Γ12) and infrared allowed mode (Γ15(2)), respectively29,30 could confirm that the Cu species present in the CuNPs confined in hollow silicalite-1 (CuHS-1R) are in a zero valent state. These peaks are also absent in all the Raman spectra of the materials (Fig. 2).
Fig. 3a and b show the low and high magnification FESEM images of the CuNPs confined in silicalite-1 (CuHS-1R) representing the hollowness of the particles. The TEM images (Fig. 3c and d) clearly show the particles having a hollow interior surrounded by a thin shell. It also reveals that CuNPs of size 10–40 nm are present in hollow silicalite-1. The HRTEM image of CuHS-1R shows the lattice fringes of CuNPs with a d-spacing of 0.217 nm corresponding to the (111) plane of Cu (Fig. 3e), indicating the presence of CuNPs in the sample. The selected area electron diffraction (SAED) pattern corroborated the crystalline planes of CuNPs and silicalite-1 (Fig. 3f). The presence of Cu atoms of about 0.91 at% in the sample was determined by energy dispersive X-ray spectroscopy (EDS) (Fig. 3g). It is to be noted that CuNPs may be dispersed both in the exterior and interior surfaces of hollow silicalite-1 (Fig. S4, ESI†).
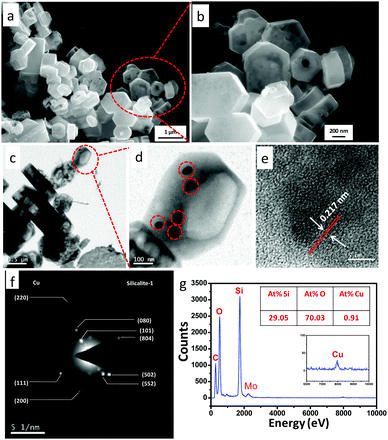 | ||
| Fig. 3 (a and b) FESEM images, (c and d) TEM images, (e) HRTEM image, (f) SAED patterns and (g) EDS of CuNPs confined in hollow silicalite-1 (CuHS-1R). | ||
The line scan spectra of the elemental analysis confirmed the almost uniform distribution of Cu atom concentration (blue line) along a line representing both exterior and interior surfaces of hollow silicalite-1 (Fig. S5, ESI†). Before desilication of the sample CuS-1, the pseudo-hexagonal prismatic shaped particles of silicalite-1 were revealed by FESEM (Fig. S6a and b, ESI†) and TEM images (Fig. S6c and d ESI†). The characteristic SAED image of silicalite-1 is shown in Fig. S6e, ESI.† The EDS analysis indicated the presence of 1.49 at% Cu atoms in the sample (Fig. S7, ESI†). Interestingly, during the desilication process on the CuS-1 sample, the silicate oligomers are leached from the interior of the crystals and crystallize on the crystal surface, leading to regular hollow crystals with well-defined cavities and walls. Fig. S8a and b and c and d, ESI† show the FESEM and TEM images of CuHS-1, respectively after desilication of CuS-1. The SAED image of the CuHS-1 sample indicates the single crystalline nature of silicalite-1 (Fig. S8e, ESI†). The EDS of CuHS-1 shows the presence of 1.22 at% Cu atoms in the sample (Fig. S9, ESI†). It is to be pointed out that during desilication, some amount of CuO is dissolved in the alkaline medium rendering lower at% of Cu in CuHS-1.
The N2 adsorption–desorption isotherms and pore size distributions (PSDs) of the CuS-1 and CuHS-1 samples are revealed in Fig. S10 and S11 (ESI†), respectively. In the CuS-1 sample (Fig. S10a, ESI†), a steep rise in the isotherm at lower relative pressure, around p/p0 = 0.1, indicated the abundance of micropores in the sample. The mesoporosity in the sample was evidenced by the BJH PSD curve (Fig. S10b, ESI†).
The DFT pore size distribution curve reveals micropores generated at around 6 Å, which is the characteristic pore of silicalite-1 zeolite. The BET isotherms and PSDs of the CuHS-1 sample are shown in Fig. S11, ESI.† In this isotherm the hysteresis loop shifted towards higher p/p0 at around 0.5 indicating the abundance of mesopores (Fig. S11a, ESI†). It can be concluded that the mesoporosity is generated in the sample due to dissolution of silica with 0.3 M TPAOH treatment. The increase in mesoporosity in this sample was verified by the corresponding BJH pore size distributions (Fig. S11b, ESI†). The N2 adsorption–desorption isotherms of CuHS-1R are shown in Fig. 4a. The presence of mesoporosity and microporosity of silicalite-1 in the sample was confirmed by the pore size distribution (PSD) curves determined by the BJH (Fig. 4b) and DFT (Fig. 4c) methods, respectively. It is reflected from the isotherms that there is no significant change in the isotherms in both the CuHS-1 and CuHS-1R samples. From the isotherm, it is evident that at higher relative pressure H2 type hysteresis loops appeared indicating ink-bottle like pores. The large hysteresis loops at around p/p0 = 0.5 indicated the profusion of mesoporosity in the sample which was also evidenced by the BJH pore size distribution curve. The textural properties (BET surface area, total pore volume, and pore size) of the samples are shown in Table 1. The BET surface area, pore volume and pore size of CuHS-1R were found to be 247 m2 g−1, 0.387 cm3 g−1 and 6.2 nm, respectively. Table 1 indicates that the BET surface area and microporous surface area decreased in the order of CuS-1 > CuHS-1 > CuHS-1R. The total specific surface area is determined by the BET method, while the microporous contribution is examined by the difference between the BET surface area and the external surface area, i.e., the mesoporous surface area (derived from the slope of the t (statistical thickness)-plot).31 The t-plot graphs and linear fitted BET plots of CuS-1, CuHS-1 and CuHS-1R are shown in Fig. S12, S13 and S14, respectively, ESI.† For microporous materials like silicalite-1, the linear BET region occurs at p/p0 < 0.1, while the linear t-plot range is obtained at higher p/p0. The total surface area and the micropore surface area are gradually decreased; however, the ratio of the mesoporous surface area to the microporous surface area increased in the order of CuS-1 < CuHS-1 < CuHS-1R (Table 1).
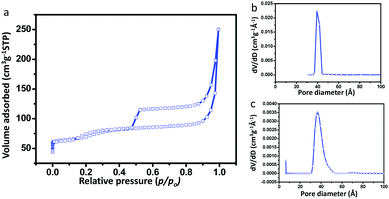 | ||
| Fig. 4 (a) N2 adsorption and desorption isotherms, and pore size distributions (PSD) by the (b) BJH and (c) DFT method of CuNPs confined in hollow silicalite-1 (CuHS-1R). | ||
| Sample Id | S BET (m2 g−1) | S mic (m2 g−1) | S ext (m2 g−1) | S ext/Smicd | V p-total (cm3 g−1) | d P (nm) |
|---|---|---|---|---|---|---|
| a BET surface area. b Micropore surface area. c External surface area. d Ratio of external surface area to micropore surface area. e Total pore volume. f Average pore size. | ||||||
| CuS-1 | 315 | 242 | 73 | 0.3016 | 0.205 | 2.6 |
| CuHS-1 | 280 | 203 | 77 | 0.3793 | 0.389 | 5.5 |
| CuHS-1R | 247 | 164 | 83 | 0.5060 | 0.387 | 6.2 |
This could be due to the structural distortion32 during desilication followed by the second and third calcination steps. Interestingly, the average pore size also significantly increased after desilication of silicalite-1. Furthermore, for the CuHS-1R sample, the increase in average pore diameter and reduction of surface area were attributed to the development of partial strain generated during the formation of CuNPs.33 The total pore volume of the CuHS-1 and CuHS-1R samples increased due to the presence of higher mesoporosity in the samples compared to that of the CuS-1 sample.
3.2. Catalytic activity of the samples
In order to evaluate the catalytic performance of the CuNPs confined in hollow silicalite-1 (CuHS-1R), reduction of p-nitrophenol (4-NP) to p-aminophenol (4-AP) in the presence of excess of NaBH4 was performed. 4-NP shows a distinct spectral profile with an absorption maximum at 317 nm in water, which shifted to 400 nm in the presence of NaBH4 due to the formation of p-nitrophenolate ions.34 It is reported that in the absence of catalyst, the peak at 400 nm remains unchanged with time indicating no reduction.18 It could be attributed that the high kinetic barrier between mutually repelling negative ions of 4-NP (p-nitrophenolate) and BH4− inhibits the further reduction of 4-NP35 in the absence of catalyst.
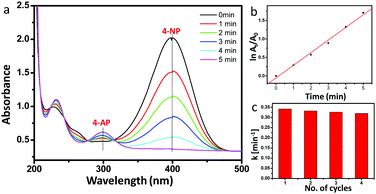
Fig. 5a shows that after addition of 1 mg catalyst, the absorbance at 400 nm of the p-nitrophenolate ion was reduced within 5 min accompanied by the simultaneous increase of the absorption peak at 300 nm which corresponds to the formation of 4-AP at room temperature. From the logarithm plot of the absorbance (−ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) At/A0) versus reaction time, the pseudo-first order rate constant (apparent rate constant) was calculated as 5.6 × 10−3 s−1 for 1 mg of catalyst at room temperature (Fig. 5b). The recyclability test of the catalyst was performed. Fig. 5c shows that the catalytic efficiency remained almost the same after reusing at least four times, which is reflected by nearly the same rate constant values.
At/A0) versus reaction time, the pseudo-first order rate constant (apparent rate constant) was calculated as 5.6 × 10−3 s−1 for 1 mg of catalyst at room temperature (Fig. 5b). The recyclability test of the catalyst was performed. Fig. 5c shows that the catalytic efficiency remained almost the same after reusing at least four times, which is reflected by nearly the same rate constant values.
Figs. S15 and S16, ESI† show (a) time-dependent UV-Vis spectra for the reduction of 4-NP, (b) the pseudo-first order plot of (−ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) At/A0) versus reaction time and (c) the apparent rate constant (k) for 4 consecutive cycles, for 2 mg and 3 mg of catalysts, respectively. The time of completion of the reaction, apparent rate constant, R2 values and activity parameter κ (rate constant per unit mass of Cu loading) of the reactions using different amounts of catalyst are shown in Table 2.
At/A0) versus reaction time and (c) the apparent rate constant (k) for 4 consecutive cycles, for 2 mg and 3 mg of catalysts, respectively. The time of completion of the reaction, apparent rate constant, R2 values and activity parameter κ (rate constant per unit mass of Cu loading) of the reactions using different amounts of catalyst are shown in Table 2.
| Serial no. | Amount of catalysts (mg) | Time (min) | k (s−1) | R 2 | κ (s−1 g−1) |
|---|---|---|---|---|---|
| 1 | 1 | 5.0 | 5.60 × 10−3 | 0.996 | 44.09 |
| 2 | 2 | 2.5 | 12.61 × 10−3 | 0.995 | 49.60 |
| 3 | 3 | 4.0 | 7.76 × 10−3 | 0.970 | 20.36 |
The apparent rate constants are found to be 5.60 × 10−3, 12.61 × 10−3 and 7.76 × 10−3 s−1 for 1, 2 and 3 mg of catalyst, respectively. The apparent rate constant for pseudo-first order reaction can be changed with the amount of catalyst36,37 as well as the concentration of NaBH4. Here, the concentration of NaBH4 solution remains constant. Therefore, the apparent rate constant could vary with the catalyst concentration. The highest apparent rate constant i.e., 12.61 × 10−3 s−1 for 2 mg of catalyst is due to the increased mass transfer of reactants within the catalyst particles.38 For further increase in catalyst concentration, i.e. for 3 mg of catalyst, the mass transfer is restricted to some extent with the longer diffusional path through the tortuous pores causing a decrease in the apparent rate constant. The R2 values are closer to unity in each case which could be in good agreement with it being a pseudo-first order reaction. It is reported that the apparent rate constant (k) and the activity parameters (κ) are dependent on the amount of catalyst used.39,40 The activity parameter (κ) is calculated from the apparent rate constant (k) per g of Cu present in CuHS-1R (2.01 mmol Cu per 1 g of CuHS-1R, determined by ICP-MS analysis). The catalytic property of the CuNPs confined in hollow silicalite-1 was compared to the reported literature (Table S1, ESI†) based on the activity parameters (κ). The present value was found to be higher than previously reported values of Cu and other metal nanoparticles.
Furthermore, the catalytic activity of the samples S-1, CuS-1 and CuHS-1 was also studied. For pure silicalite-1 (S-1), the absorption peak of p-nitrophenolate ions at 400 nm remained almost unchanged even after 30 min of absorption suggesting no catalytic activity for the reduction of 4-NP (Fig. S17, ESI†). Fig. S18 and S19, ESI† illustrate (a) the time-dependent UV-Vis spectra for the reduction of 4-NP, and (b) pseudo-first order plot of (−ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) At/A0) versus reaction time for each of the samples CuS-1 and CuHS-1, respectively. The apparent rate constants (k) were calculated as 2.3 × 10−3 s−1 and 4.1 × 10−3 s−1 for 1 mg of CuS-1 and CuHS-1, respectively. However, k for 1 mg of CuHS-1R was found to be 5.6 × 10−3 s−1. This demonstrates that with increasing mesoporosity (mesoporous surface area with respect to microporous surface area) in silicalite-1 (Table 1), the diffusion path length is reduced rendering the catalytic activity in the order of CuS-1 < CuHS-1 < CuHS-1R. Here, CuNPs confined in hollow silicalite-1 (CuHS-1R) exhibit higher catalytic efficiency due to the presence of hollow architecture with increased mesoporous surface area with respect to microporous surface area. It is worth noting that hierarchical porosity has a significant contribution toward the catalytic efficiency of 4-NP. For comparison, the BET surface area and catalytic activity of CuNPs confined in a commercial 4A molecular sieve (Merck) was studied. The BET surface area of the CuNPs confined in a commercial 4A molecular sieve was found to be 30 m2 g−1 (isotherm shown in Fig. S20, ESI†) having only external pores, which is lower than that of CuNPs confined in hollow silicalite-1 (CuHS-1R) having hierarchical porosity. As a result, CuNPs confined in a commercial 4A molecular sieve give a lower apparent rate constant (2.3 × 10−3 s−1) for the catalytic reaction (Fig. S21, ESI†) than that of CuHS-1R. The lower apparent rate constant of CuNPs confined in a commercial 4A molecular sieve is due to the absence of hierarchical porosity with lower external surface area. During the catalytic reaction, in the presence of excess BH4− ions, the reduction of 4-NP takes place presumably on the catalyst surface in two steps. In the first step, both donor BH4− ions and accepter 4-NP molecules diffused on the surface of the catalyst through chemisorptions.41 Here, BH4− ions transfer hydrogen to the catalyst (CuHS-1R) surface for the reduction of 4-NP molecules in aqueous conditions.42 In this reaction the catalyst acts as a hydrogen shuttle and simultaneous reduction occurs to 4-AP via a desorption process. The presence of microporosity hinders the catalytic performance. CuO in CuHS-1 could show catalytic performance. However, due to the presence of higher microporosity in CuHS-1, its catalytic performance is lower than that of CuHS-1R. On the other hand, in catalytic reduction of 4-NP in the presence of CuO, the NaBH4 reduces Cu(II) to Cu(0). The BH4− ions and 4-NP are accumulated on the reduced Cu(0) surface, and the reduction of 4-NP to 4-AP takes place on the Cu(0) surface.43 However, for the same catalytic reaction by using Cu(0), the reaction becomes faster than that with CuO because Cu(0) is itself in a reduced state.
At/A0) versus reaction time for each of the samples CuS-1 and CuHS-1, respectively. The apparent rate constants (k) were calculated as 2.3 × 10−3 s−1 and 4.1 × 10−3 s−1 for 1 mg of CuS-1 and CuHS-1, respectively. However, k for 1 mg of CuHS-1R was found to be 5.6 × 10−3 s−1. This demonstrates that with increasing mesoporosity (mesoporous surface area with respect to microporous surface area) in silicalite-1 (Table 1), the diffusion path length is reduced rendering the catalytic activity in the order of CuS-1 < CuHS-1 < CuHS-1R. Here, CuNPs confined in hollow silicalite-1 (CuHS-1R) exhibit higher catalytic efficiency due to the presence of hollow architecture with increased mesoporous surface area with respect to microporous surface area. It is worth noting that hierarchical porosity has a significant contribution toward the catalytic efficiency of 4-NP. For comparison, the BET surface area and catalytic activity of CuNPs confined in a commercial 4A molecular sieve (Merck) was studied. The BET surface area of the CuNPs confined in a commercial 4A molecular sieve was found to be 30 m2 g−1 (isotherm shown in Fig. S20, ESI†) having only external pores, which is lower than that of CuNPs confined in hollow silicalite-1 (CuHS-1R) having hierarchical porosity. As a result, CuNPs confined in a commercial 4A molecular sieve give a lower apparent rate constant (2.3 × 10−3 s−1) for the catalytic reaction (Fig. S21, ESI†) than that of CuHS-1R. The lower apparent rate constant of CuNPs confined in a commercial 4A molecular sieve is due to the absence of hierarchical porosity with lower external surface area. During the catalytic reaction, in the presence of excess BH4− ions, the reduction of 4-NP takes place presumably on the catalyst surface in two steps. In the first step, both donor BH4− ions and accepter 4-NP molecules diffused on the surface of the catalyst through chemisorptions.41 Here, BH4− ions transfer hydrogen to the catalyst (CuHS-1R) surface for the reduction of 4-NP molecules in aqueous conditions.42 In this reaction the catalyst acts as a hydrogen shuttle and simultaneous reduction occurs to 4-AP via a desorption process. The presence of microporosity hinders the catalytic performance. CuO in CuHS-1 could show catalytic performance. However, due to the presence of higher microporosity in CuHS-1, its catalytic performance is lower than that of CuHS-1R. On the other hand, in catalytic reduction of 4-NP in the presence of CuO, the NaBH4 reduces Cu(II) to Cu(0). The BH4− ions and 4-NP are accumulated on the reduced Cu(0) surface, and the reduction of 4-NP to 4-AP takes place on the Cu(0) surface.43 However, for the same catalytic reaction by using Cu(0), the reaction becomes faster than that with CuO because Cu(0) is itself in a reduced state.
4. Conclusions
In summary, metallic CuNPs (10–40 nm) have been successfully confined in a hollow silicalite-1 zeolite having hierarchical porosity. The confinement process has been done by a selective “desilication–recrystallization” method using TPAOH as a templating as well as desilicating agent. Cu nanoparticles in the silicalite-1 particles were confirmed by XRD, XPS and Raman spectroscopy. This is further corroborated by HRTEM and SAED images. The CuNPs confined in hollow silicalite-1 particles showed excellent catalytic activities for the reduction of 4-NP to 4-AP with an apparent rate constant value of 5.6 × 10−3 s−1. Compared with literature reports, CuNPs confined in hollow silicalite-1 exhibit enhanced catalytic efficiency because of the hierarchical porosity of hollow silicalite-1. The present work offers a general strategy for the synthesis of various metal doped zeolites towards other catalytic activities by tuning the porosity and the size of Cu and/or other transition metal and noble metal confined hollow zeolites.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the Director of the Institute for his kind permission to publish this paper. RD is thankful to UGC, India for her fellowship.References
- A. Roucoux, J. Schulz and H. Patin, Chem. Rev., 2002, 102, 3757 CrossRef CAS PubMed.
- F. Tang, L. Li and D. Chen, Adv. Mater., 2012, 24, 1504 CrossRef CAS PubMed.
- D. Tahir and S. Tougaard, J. Phys.: Condens. Matter, 2012, 24, 175002 CrossRef PubMed.
- N. Ren, Y. H. Yang, Y. H. Zhang, Q. R. Wang and Y. Tang, J. Catal., 2007, 246, 215 CrossRef CAS.
- S. Ghosh, R. Das, I. H. Chowdhury, P. Bhanja and M. K. Naskar, RSC Adv., 2015, 5, 101519 RSC.
- Y. F. Zhu, J. L. Shi, W. H. Shen, X. P. Dong, J. W. Feng, M. L. Ruan and Y. S. Li, Angew. Chem., Int. Ed., 2005, 44, 5083 CrossRef CAS PubMed.
- J. F. Chen, H. M. Ding, J. X. Wang and L. Shao, Biomaterials, 2004, 25, 723 CrossRef CAS PubMed.
- J. Kim, S. Lee, K. Cho, K. Na, C. Lee and R. Ryoo, ACS Catal., 2014, 4, 3919 CrossRef CAS.
- C. Zhao, S. Kasakov, J. He and J. A. Lercher, J. Catal., 2012, 296, 12 CrossRef CAS.
- C. Dai, S. Zhang, A. Zhang, C. Song, C. Shi and X. Guo, J. Mater. Chem. A, 2015, 3, 16461 CAS.
- D. Fodor, T. Ishikawa, F. Krumeich and J. A. van Bokhoven, Adv. Mater., 2015, 27, 1919 CrossRef CAS PubMed.
- S. Li, A. Tuel, D. Laprune, F. Meunier and D. Farrusseng, Chem. Mater., 2015, 27, 276 CrossRef CAS.
- K. H. Rhodes, S. A. Davis, F. Caruso, B. J. Zhang and S. Mann, Chem. Mater., 2000, 12, 2832 CrossRef CAS.
- V. Valtchev and S. Mintova, Microporous Mesoporous Mater., 2001, 43, 41 CrossRef CAS.
- C. S. Mei, Z. C. Liu, P. Y. Wen, Z. K. Xie, W. M. Hua and Z. Gao, J. Mater. Chem., 2008, 18, 3496 RSC.
- C. Dai, A. Zhang, L. Li, K. Hou, F. Ding, J. Li, D. Mu, C. Song, M. Liu and X. Guo, Chem. Mater., 2013, 25, 4197 CrossRef CAS.
- D. Fodor, L. Pacosova, F. Krumeich and J. A. van Bokhoven, Chem. Commun., 2014, 50, 76 RSC.
- R. Das, S. Ghosh, I. H. Chowdhury and M. K. Naskar, New J. Chem., 2016, 40, 50 RSC.
- S. Li, L. Burel, C. Aquino, A. Tuel, F. Morfin, J.-L. Rousset and D. Farrusseng, Chem. Commun., 2013, 49, 8507 RSC.
- S. Li, T. Boucheron, A. Tuel, D. Farrusseng and F. Meunier, Chem. Commun., 2014, 50, 1824 RSC.
- C. Dai, A. Zhang, M. Liu, X. Guo and C. Song, Adv. Funct. Mater., 2015, 25, 7479 CrossRef.
- M. M. J. Treacy and J. B. Higgins, Collection of Simulated XRD Powder Patterns for Zeolite, Elsevier, Amsterdam, 2001 Search PubMed.
- X. Song, Y. Chen, M. Rong, Z. Xie, T. Zhao, Y. Wang, X. Chen and O. S. Wolfbeis, Angew. Chem., Int. Ed., 2016, 55, 3936 CrossRef CAS PubMed.
- Y. Yang, H. Tu, A. Zhang, D. Du and Y. Lin, J. Mater. Chem., 2012, 22, 4977 RSC.
- M. A. Dar, Q. Ahsanulhaq, Y. S. Kim, J. M. Sohn, W. B. Kim and H. S. Shin, Appl. Surf. Sci., 2009, 255, 6279 CrossRef CAS.
- S. Velu, K. Suzuki, C. S. Gopinath, H. Yoshida and T. Hattori, Phys. Chem. Chem. Phys., 2002, 4, 1990 RSC.
- D. P. Volanti, D. Keysonb, L. S. Cavalcante, A. Z. Simões, M. R. Joya, E. Longo, J. A. Varela, P. S. Pizani and A. G. Souza, J. Alloys Compd., 2008, 459, 537 CrossRef CAS.
- T. Iida, M. Sato, C. Numako, A. Nakahira, S. Kohara, T. Okuboa and T. Wakihara, J. Mater. Chem. A, 2015, 3, 6215 CAS.
- S. Wu, Z. Yin, Q. He, G. Lu, X. Zhoua and H. Zhang, J. Mater. Chem., 2011, 21, 3467 RSC.
- Y. Maoa, J. Hea, X. Suna, W. Li, X. Lua, J. Gana, Z. Liua, L. Gongb, J. Chenb, P. Liua and Y. Tonga, Electrochim. Acta, 2012, 62, 1 CrossRef.
- D. W. Rutherford, C. T. Chiou and D. D. Eberl, Clays Clay Miner., 1997, 45, 534 CAS.
- A.-H. Lu, W.-C. Li, W. Schmidt and F. Schuth, Microporous Mesoporous Mater., 2005, 80, 117 CrossRef CAS.
- S. M. El-Sheikh, A. A. Ismail and J. F. Al-Sharab, New J. Chem., 2013, 37, 2399 RSC.
- Z. Jin, M. Xiao, Z. Bao, P. Wang and J. Wang, Angew. Chem., Int. Ed., 2012, 51, 6406 CrossRef CAS PubMed.
- Z. Dong, X. Le, Y. Liu, C. Dong and J. Ma, J. Mater. Chem. A, 2014, 2, 18775 CAS.
- R. G. Mortimer, Physical Chemistry, Elsevier Academic Press, USA, 3rd edn, 2008, pp. 504–506 Search PubMed.
- J. C. Kuriacose and J. Rajaram, Chemistry in Engineering and Technology, Tata McGraw Hill Education Pvt. Ltd, New Delhi, 1984, vol. 1, pp. 562–601 Search PubMed.
- S. Katoh, J. Horiuchi and F. Yoshida, Biochemical Engineering, Wiley-VCH Verlag GmbH & Co., Germany, 2015, p. 103 Search PubMed.
- C. Kastner and A. F. Thunemann, Langmuir, 2016, 32, 7383 CrossRef PubMed.
- P. Deka, R. C. Deka and P. Bharali, New J. Chem., 2014, 38, 1789 RSC.
- Z. Jiang, J. Xie, D. Jiang, X. Wei and M. Chen, CrystEngComm, 2013, 15, 560 RSC.
- R. Kaur, C. Giordano, M. Gradzielski and S. Mehta, Chem. – Asian J., 2014, 9, 189 CrossRef CAS PubMed.
- J. Pal, C. Mondal, A. K. Sasmal, M. Ganguly, Y. Negishi and T. Pal, ACS Appl. Mater. Interfaces, 2014, 6, 9173 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7nj04005a |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2018 |