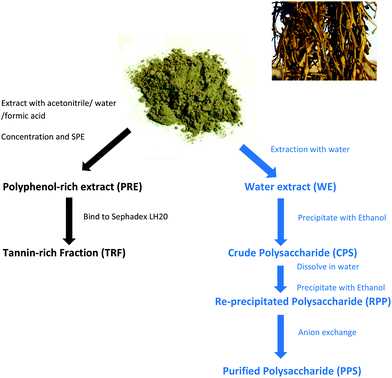
Extracts from the edible seaweed, Ascophyllum nodosum, inhibit lipase activity in vitro: contributions of phenolic and polysaccharide components†
Ceri
Austin
a,
Derek
Stewart
 ab,
J. William
Allwood
ab,
J. William
Allwood
 a and
Gordon J.
McDougall
a and
Gordon J.
McDougall
 *a
*a
aEnvironmental and Biochemical Sciences Group, The James Hutton Institute, Invergowrie, Dundee DD2 5DA, UK. E-mail: Gordon.mcdougall@hutton.ac.uk; Fax: +44 (0)844928 5429; Tel: +44 (0)1382 568782
bSchool of Life Sciences, Heriot-Watt University, Edinburgh, EH14 4AS, Scotland, UK
First published on 8th December 2017
Abstract
A polyphenol-rich extract (PRE) from the edible seaweed, Ascophyllum nodosum, inhibited pancreatic lipase activity in an oil-based turbidimetric assay with an IC50 of 200 μg gallic acid equivalents (GAE) perassay) [∼230 μg DW] whereas the known inhibitor, Orlistat, gave an IC50 at 0.4 μg per assay. A phlorotannin-enriched fraction (TRF) purified from the PRE was more potent with an IC50 = 60 μg GAE per assay (∼65 μg DW). When the assay was started by the addition of lipase, both Orlistat and TRF were much less effective which suggests that pre-incubation of enzyme and inhibitor improved inhibition. Based on phenol content, water extracts from Ascophyllum were more potent lipase inhibitors than PRE (IC50 ∼ 150 μg GAE per assay). However, this was equivalent to ∼580 μg DW and these extracts contained polysaccharides (e.g. alginate content = 110 μg mL−1) which may also contribute to inhibition. Indeed, a polysaccharide-enriched fraction obtained by ethanol precipitation gave an IC50 of 1000 μg DW which was equivalent to 130 μg GAE and 420 μg alginate per assay. Therefore a >3 fold increase in alginate content did not markedly improve inhibition. Re-precipitation increased alginate content and reduced polyphenol content but lipase inhibition was markedly reduced (i.e. IC50 at ∼1100 μg DW per assay, 700 μg alginate and 25 μg GAE). Purifying the polysaccharide fraction by ion exchange removed all phenolics but the IC50 increased to >2500 μg DW, equivalent to >1970 μg alginate per assay. In conclusion, polysaccharides and phlorotannins may inhibit lipase in an additive fashion, with phlorotannins apparently more effective in vitro. However, interactions between these components may be important when food products containing this edible seaweed are consumed.
Introduction
According to the World Health Organisation figures,1 an epidemic of obesity is continuing to threaten the health of Western nations. In the United Kingdom, 1 in 4 adults are categorised as obese and by 2050 obesity is predicted to affect 60% of adult men, 50% of adult women and 25% of children.2 As well as being debilitating in its own right, obesity is a key risk factor for cardiovascular disease, certain cancers, type 2 diabetes and the combination of risk factors often termed Metabolic Syndrome3 and therefore obesity adds a substantial burden to health care systems.4Although the most recognised form of maintaining a healthy weight is to consume a healthy diet and to undertake exercise, adherence to such regimes is low.5 An imbalance between calorie intake and metabolic expenditure is central to many cases of obesity and reductions in intake of energy-dense fats may be useful to reduce weight.6 The reduction of energy intake by inhibiting the action of pancreatic lipase, which splits triacylglycerides (TAG) into absorbable glycerol and fatty acids,7 by drugs such as Orlistat,8 has been employed to treat obesity. Pancreatic lipase plays a crucial role in the hydrolysis of TAG with only 10–30% of hydrolysis occurring before its action in the duodenum.9 Orlistat inhibits pancreatic lipase and can increase ingested fat excreted in the faeces to ∼32% from 5% in controls.10 However, gastrointestinal side effects associated with orlistat treatment can reduce patient compliance11 and finding alternative effective and more natural lipase inhibitors has become an active research area.12
In previous work, we have provided evidence that polyphenol-rich extracts from teas,13,14 green coffee beans and cacao beans,15 and berries16 could inhibit pancreatic lipase in vitro and could potentially influence fat digestion in vivo. Polyphenol-rich extracts from seaweeds (especially the brown edible seaweed, Ascophyllum nodosum) have been shown to have potent inhibitory effects on key enzymes involved in starch digestion17,18 and to offer potential for glycaemic control with high activity residing in the phlorotannin components. Phlorotannins from brown seaweeds have been suggested to have a range of potential bioactivities many of which involve inhibition of key enzymes,19 some of which are involved in digestive processes.20 Recent reports suggest that brown seaweeds, including Ascophyllum extracts, have lipase-inhibitory activity in vitro.21 Brown seaweeds are also a source of soluble polysaccharides, including alginates and fucoidans,22 and alginates have been shown to have lipase inhibitory properties in vitro.23 In this paper, we investigate the potential of Ascophyllum extracts to inhibit pancreatic lipase in vitro and compare polyphenol- and polysaccharide-rich fractions for their potency.
Experimental
Seaweed extraction
Ascophyllum nodosum (Linnaeus) Le Jolis seaweed was supplied by Hebridean Seaweeds Ltd (Stornoway, Isle of Lewis, The Outer Hebrides, Scotland) as air dried powder. It was ground to pass a 1 mm sieve (ZM 200 Mill, Retsch Ltd, Castleford, UK).The seaweed powder was extracted as before.18 For extraction, multiple replicates of 25 g were mixed with 250 mL extraction solution, mixed well then incubated at room temperature (∼20 °C) for 1 h in a rotatory shaking incubator (120 rpm). The replicate extracts were combined and filtered through muslin then filter paper (Whatman 114).
Two extractions were carried out, one with ultrapure water (UPW) containing 0.1% formic acid (FA) and the other in 50% aqueous acetonitrile containing 0.2% FA. The water extract yielded 26.2 ± 0.2 mg DW per mL with a TPC = 668.9 ± 1.1 μg mL−1 (n = 6) and the acetonitrile extract yielded 20.3 ± 0.2 mg DW per mL with a TPC of 1594.6 ± 1.2 μg mL−1 (n = 6). The aqueous acetonitrile extract was used to prepare the polyphenol-rich extract (PRE; Scheme 1). The acetonitrile was removed by rotary evaporation and the sample was diluted with an equal volume of 0.1% (v/v) FA prior to solid phase extraction (SPE).17 Briefly, the SPE units (Strata C18-E, GIGA units, 10 g capacity; Phenomenex Ltd, Macclesfield, UK) were primed using 80% acetonitrile/20% UPW containing 0.1% FA and then equilibrated in 0.1% FA in UPW. The seaweed extract (up to 100 mL in 25 mL batches) was applied and the unbound fraction was collected. The unit was washed with 3 × 25 mL of 0.1% FA solution and the bound material (PRE) was eluted using 2 × 25 mL of 80% acetonitrile containing 0.1% FA. PRE was enriched in polyphenols but was devoid of minerals, vitamins and other components that can interfere with the Folin reaction.24 The aqueous acetonitrile extract contained carotenoids including fucoxanthin25 but these were retained on the SPE units. The UPW extract did not contain fucoxanthin (results not shown). The phenol content was measured using a modified Folin–Ciocalteu method,26 estimated and expressed as gallic acid equivalents (GAE) from a standard curve. Samples were dried in aliquots at constant phenol content using a SpeedVac.
Preparation of polysaccharide samples
The water extract was used to prepare the crude polysaccharide sample (CPS). The extract was precipitated by addition of 2 volumes of ice-cold ethanol. The samples were mixed well and left overnight at 5 °C to allow complete precipitation to occur. The precipitated material was collected by centrifugation at 2500g, 15 min at 5 °C. The pellets were re-suspended in UPW, frozen and freeze dried.A portion of the crude polysaccharide sample re-suspended in UPW was re-precipitated with ethanol using the same procedure and the pellets [Re-precipitated Polysaccharide Sample (RPS)] freeze-dried. Another portion of the crude polysaccharide sample was purified by anion exchange. The sample (500 mg) was dissolved in 10 mL of 25 mM Tris pH 8.0. After vortex mixing, the sample was applied to a centrifugal anion exchange unit with quaternary ammonium ligands (Vivapure® Q Maxi H units, Sartorius UK Ltd, Epsom, UK) which had been equilibrated in the same buffer. The crude polysaccharide was applied to the Q-unit and left to permeate into the unit membrane for 5 min at room temperature. The unit was then centrifuged at 500g for 5 min at 5 °C. The eluate was collected as the unbound sample. The unit was then washed two-times by centrifugation with 10 mL of the same buffer to obtain 2 wash samples. The bound material was eluted using 2 × 20 ml of 25 mM Tris pH 8.0 containing 500 mM NaCl to obtain two bound fractions.
Polysaccharide content of fractions was assessed using the phenol sulphuric acid method.27 The bound fractions were combined and precipitated with 2 volumes of ethanol (as above), then polysaccharide sample was re-suspended in UPW, and the anion exchange purified polysaccharide sample (PPS) was freeze dried.
Alginate content
Alginate content was measured using the Alcian blue dye-binding method.28 If required, dried samples were dissolved in UPW. In short, triplicate 100 μL samples were added to 800 μl of 0.5 M acetic acid and then mixed. 100 μL of Alcian Blue [0.5% (w/v) in UPW; Sigma Chemical Co. Ltd product A5268, Dorset, UK] solution was added and remixed. The samples were left overnight at room temperature and then centrifuged in a microfuge (2 min, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500g at room temperature) and the amount of dye removed from solution was measured at 610 nm. The samples were compared against standard curves of sodium alginate (Sigma product 32318) prepared the same day.
500g at room temperature) and the amount of dye removed from solution was measured at 610 nm. The samples were compared against standard curves of sodium alginate (Sigma product 32318) prepared the same day.
Fractionation of tannin-like components using fractionation on Sephadex LH-20
Binding to Sephadex LH-20 in aqueous ethanol and elution with solutions of aqueous ethanol and acetone is an established selection technique for tannin-like polyphenol components (http://www.users.miamioh.edu/hagermae). Briefly, dried PRE samples were dissolved in 50% aqueous ethanol and added to a slurry of Sephadex LH-20 (Sigma product LH20100) in the same solvent. After end-over-end mixing for 5 min, the tubes were allowed to stand at room temperature for 5 min then centrifuged at 2500g for 5 min at 5 °C.17 The supernatant was removed as the unbound sample. The slurry was re-suspended in a suitable volume of 50% aqueous ethanol, mixed well and left to equilibrate at room temperature for 5 min. After centrifugation, the supernatant was removed as the wash sample. A suitable volume of 70% acetone in UPW was added, mixed well and allowed to stand for a further 5 min. After centrifugation, the supernatant was removed as the tannin-rich fraction (TRF). The TRF was dried in a SpeedVac at known amounts of phenols.Lipase assay
The lipase assay was based on a previous method of Vogel & Zieve29 as adapted by Wilcox et al.23 This turbidimetric method measures the reduction in turbidity that accompanies the breakdown of triacylglycerides to free fatty acids catalysed by lipase. Olive oil was passed through an alumina SPE unit (Isolute AL-N units; Biotage Ltd, Uppsala, Sweden) to remove free fatty acids.22 The olive oil free from fatty acids was made up as a 10% (v/v) stock in acetone which was diluted 1 in 10 with acetone to achieve a 1% olive oil working solution. The stock solution was stored at 5 °C for up to four weeks. For the assay, the olive oil substrate solution was prepared by adding 4 ml of the 1% olive oil solution to a heated solution (70 °C) of 100 ml 50 mM Tris buffer (pH 8.3) containing 0.35% (w/v) sodium deoxycholate (Sigma product D6750). This solution was maintained at 70 °C and homogenised for 10 min. Once the froth had settled, the substrate solution was stable for the rest of the day at room temperature. The enzyme solution contained 1.29 mg ml−1 lipase (lipase from porcine pancreas type II, Sigma product L3126) and 18 μg ml−1 colipase (Sigma product C3028) in UPW. Orlistat (Sigma product O4139) was dissolved in UPW and used as a positive inhibition control. Samples of dried seaweed extracts were dissolved in UPW and added to the reaction at known amounts of phenols (μg GAE per assay) or dry weight (μg DW per assay).The control assay contained 100 μL of lipase solution and 100 μL of UPW and the blank contained 200 μL of UPW in triplicate Eppendorf tubes (1.5 mL). After pre-incubation at 37 °C for 15 min, the reaction was started by addition of the substrate solution (800 μL) to all tubes. The initial control assays were maintained at 37 °C and triplicate tubes were read every 5 min at 405 nm over 35 min. The control reaction was linear for up to 10 min under these conditions and 10 min was used for all studies for assessment of inhibition. For inhibitor studies, the extracts were included in the 100 μL UPW volume. % Activity values were calculated as the difference between the average A405 values at t = O and t = 10 min for each sample compared against the control reaction (set at 100% activity) after taking into account the absorbance of individual blanks for each sample. For the order of addition experiments, the inhibitor and substrate solutions were pre-incubated and the reaction was started by the addition of the enzyme solution.
Liquid chromatography-mass spectrometry (LC-MS)
Samples (containing 20 μg GAE) were analysed on a LCQ Fleet Ion Trap mass spectrometer (Thermo Scientific Ltd, Hemel Hempstead, UK) attached to an HPLC system consisting of an Accella 600 quaternary pump and Acella photodiode array PDA detector (PDAD) and autosampler. The PDAD scanned three channels at 280, 365, and 520 nm. Samples were eluted on a gradient of 5% acetonitrile (0.1% FA) to 40% acetonitrile (0.1% FA) on a Synergi Hydro C18 2.0 mm × 150 mm column (Phenomenex Ltd, Macclesfield, UK) over 30 min at a flow rate of 200 μL min−1. The mass spectrometer used an electrospray ionization interface and the samples were analysed in negative-ion mode with two scan events: full-scan analysis followed by data-dependent MS/MS of the most intense ions (normalised collision energies of 45% arbitrary units) in wideband activation mode. The capillary temperature was set at 250 °C, with sheath gas at 60 psi and auxiliary gas at 15 psi. However, phlorotannins do not separate well under reverse phase-HPLC conditions (e.g. Steevensz et al.30) and the main UV peaks contained a number of unresolved components. Therefore the mass spectra obtained across the separation zone (from 5 to 30 min) were presented to illustrate the diversity of phenolic structures present.Results and discussion
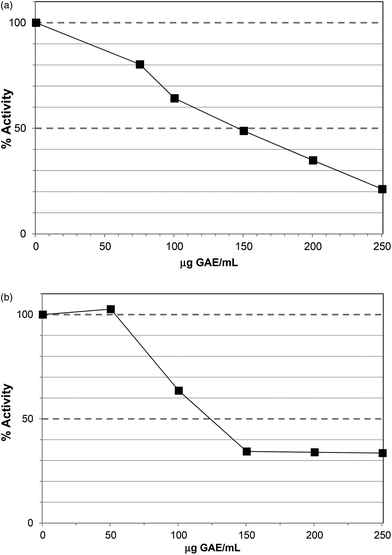
The Polyphenol-Rich Extract (PRE) from Ascophyllum showed inhibition of lipase activity with an IC50 value ∼200 μg GAE per assay (Fig. 1). The PRE was rich in phenolic components so the IC50 value was obtained at 230 μg DW per assay. The unbound material from the PRE procedure showed no lipase inhibition at 500 μg DW per assay. Lower levels of PRE (<115 μg DW or 100 μg GAE per assay) showed activation of lipase activity. Apparent stimulation of lipase activity was noted for PRE from certain berries16 and may occur because low levels of polyphenols stabilise lipase, co-lipase, or influence micelle structure. However, these previous assays were carried out using the colorimetric substrate, p-nitrophenol laurate, and also did not contain co-lipase or bile acids, so are less physiologically-relevant. Indeed, major differences have been noted in the susceptibility of lipase to inhibition in colorimetric versus turbimetric-based assays.22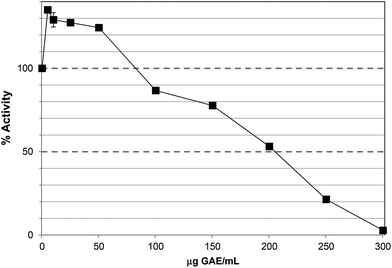 | ||
| Fig. 1 Inhibition of lipase by polyphenol-rich extract (PRE). Each point is the average of three assays carried out on separate days ± SE. | ||
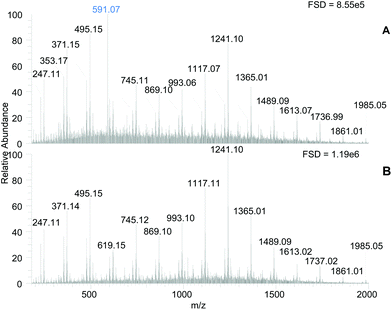
The MS spectra of the PRE and Tannin-rich fraction (TRF) samples contained a set of signals consistent with phlorotannin structures (Fig. 2, panel A & B). Two series of m/z signals that differed by 124 amu were present, which have been previously ascribed to phlorotannin structures in this laboratory17,18 and elsewhere.31 The series at m/z 371, 495, 619, 745, 869, 993, 1117, 1241 etc., may result from C–C linked addition of phloroglucinol (i.e. +124 amu) to a core C–O–C linked dimer of phloroglucinol (m/z 247) and the other series may arise from addition of phloroglucinol units to a core molecule that is 18 amu smaller (i.e. m/z 229). The phlorotannin-like components were enriched in the TRF. According to the full scale deflections (Fig. 2), the tannin-rich fraction had a ∼1.4 fold increased signal intensity over the PRE and the TRF was generally enhanced in putative phlorotannin components especially those with higher m/z values (e.g. 1117, 1241 & 1365). However, these different molecular species probably differ in their ionisation efficiency so simple comparisons of component intensity may not be valid.
Beyond the enhancement in the phlorotannin signals, the main difference between the samples was the abundance of a m/z 591 signal in the PRE (highlighted in Fig. 2A) which was removed in the TRF. This component remained in the unbound fraction during the Sephadex LH-20 procedure and was found previously in our laboratory to contribute to α-amylase inhibition.18 However, the unbound fraction produced during TRF production showed no lipase inhibition at 500 μg DW per assay (results not shown).
The TRF inhibited lipase activity with an IC50 ∼60 μg GAE per assay, equivalent to 65 μg DW per assay (Fig. 3), approximately 3-fold more potent than PRE, which largely agrees with the apparent degree of enrichment in phlorotannins. Unlike PRE, TRF did not stimulate lipase activity at lower concentrations, albeit that the lowest concentration of TRF assayed was 10 μg GAE per assay. Phlorotannins have been previously associated with lipase inhibition. The ethyl acetate extract of the brown seaweed, Eisenia bicyclis, inhibited pancreatic lipase in vitro with an IC50 value of 32.3 ± 2.3 μg DW per assay.32 The IC50 value for TRF was ∼65 μg DW per assay, which is in this range. However, this study used a colorimetric substrate (p-nitrophenol butyrate) so the assays are not directly comparable. Nevertheless, individual phlorotannins purified from this ethyl acetate fraction showed inhibitory effects; 7-phloroeckol was most potent with an IC50 value of 12.7 μM compared to Orlistat at 0.7 μM. Fucofuroeckol-A and eckol were less effective with IC50 values of 37 and 76 μM respectively.32,33
 | ||
| Fig. 3 Inhibition of lipase by the tannin-rich fraction (TRF). Each point is the average of three assays carried out on separate days ± SE. | ||
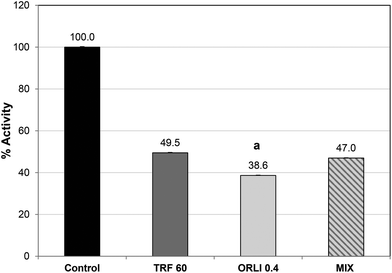
Orlistat caused potent lipase inhibition with an IC50 value of 0.4 μg per assay (see ESI, Fig. S1†), which was similar to previous reports.32 Orlistat reacts with the serine sidechain at the active site and forms an acylated lipase-inhibitor complex which is very stable compared to the normal substrate–lipase intermediate.34 As a result, the lipase cannot bind or cleave lipids and is effectively inactivated. Therefore, it may not be surprising that when TRF and Orlistat were combined at their IC50 values, no additional inhibitory effect was noted over the single components (Fig. 4). In fact, inhibition was significantly greater with Orlistat treatment alone than when Orlistat was applied in combination with TRF. This suggests that TRF components may interfere with the action of Orlistat at the active site perhaps by binding elsewhere and disrupting active site topology. Further studies are required to ascertain if TRF components act at the active site or cause inhibition by binding elsewhere. It is also possible that phlorotannins cause these effects by affecting co-lipase activity or interfering with lipid availability or emulsification.35
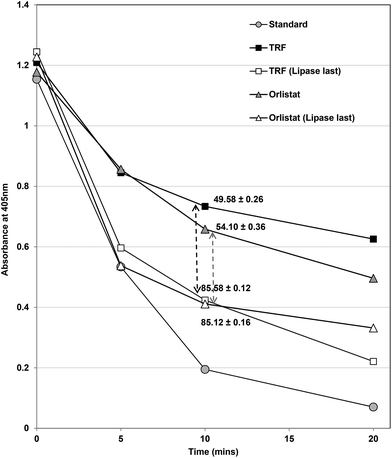
Changing the order of the assay had considerable effects on inhibition of lipase (Fig. 5). In the normal assay, the inhibitors and enzyme were pre-incubated for 15 min before beginning the assay by the addition of substrate. When the substrate and inhibitors were pre-incubated and the assay was started by addition of the enzyme, both TRF and Orlistat (Fig. 5) inhibited lipase less effectively (i.e. to only 15% control compared to 50% at the original IC50 values). This strongly suggests that both Orlistat and TRF components work more effectively when they can form complexes with lipase before substrates are available. Orlistat is recommended to be taken before meals34 and would be present in the gastrointestinal tract before lipids from meals and any lipase inhibitor based on Ascophyllum would probably require the same pre-prandial intake to be most effective.
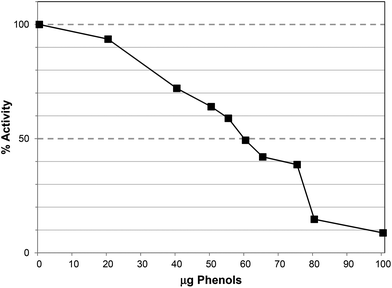
The water extract (WE) of Ascophyllum also caused lipase inhibition with an IC50 at ∼150 μg GAE per assay (Fig. 6a), equivalent to 580 μg DW per assay. The IC50 value was achieved at a lower phenol content than the PRE (200 μg GAE per assay) but the water extract contained other components including water-soluble polysaccharides, such as alginates and fucoidans.22 Indeed, the water extract contained 110 ± 7 μg alginate per assay (Table 1) and it is possible that these polysaccharides and polyphenols exerted additive effects in lipase inhibition as alginates have been shown to inhibit lipase in vitro.23
| Extracts | TRF | PRE | Water | CPS | RPS | PPS | |
|---|---|---|---|---|---|---|---|
| TRF = tannin-rich fraction; PRE = polyphenol-rich extract; water = water extract; CPS = crude polysaccharide sample; RPS = re-precipitated polysaccharide sample; PPS = purified polysaccharide sample. | |||||||
| IC50 obtained at: | Dry weight (μg per assay) | 65 | 230 | 580 | 1000 | 1100 | >2500 |
| Phenol content (μg per assay) | 60 | 200 | 150 | 130 | 25 | 0 | |
| Alginate content (μg per assay) | 0 | 0 | 130 | 420 | 700 | >1970 | |
Ethanol precipitation was used to prepare a crude polysaccharide sample (CPS) and this also inhibited lipase with an IC50 value at ∼130 μg GAE per assay (Fig. 6b), equivalent to 1000 μg DW per assay. The alginate content of this sample was 420 ± 11 μg per assay and therefore more than trebling the relative alginate content did not markedly enhance inhibition. Re-precipitation in ethanol produced the re-precipitated polysaccharide sample (RPS) which was further enriched in alginate (638 ± 18 μg per mg DW) with a low phenol content (22 ± 4 μg GAE per mg DW). Although this fraction also caused lipase inhibition, the IC50 was ∼1100 μg DW per assay (Table 1; ESI, Fig. S2†), equivalent to ∼700 μg alginate and 25 μg GAE. Furthermore, when the polysaccharides applied to anion exchange units and re-assayed for lipase inhibition, the purified polysaccharide sample (PPS) gave an IC50 value >2500 μg DW per assay (see ESI Fig. S3†). This fraction had no detectable TPC but contained 789 ± 12 μg per mg DW alginates. Therefore the IC50 was obtained at >1970 μg alginate per assay.
Therefore purifying the crude polysaccharide by re-precipitating with ethanol or anion exchange greatly reduced lipase inhibition whilst reducing phenolic content and increasing alginate content (see Table 1 for summary). This suggests that alginates may be able to inhibit lipase but in a less effective manner than the phlorotannins but there may be some additive effects between alginates and the tannins. Sodium alginate (SIGMA, low molecular weight from brown seaweeds; Product 32318) did not inhibit lipase at 1500 μg DW per assay (results not shown). This fits with figures in Wilcox et al.23 who reported lipase inhibition by a range of alginates with different structures and molecular weights, and found that alginates with higher levels of guluronic acid were more effective. However, the maximal inhibition noted was ∼70% and IC50 values for most alginates were in the 2–4 mg per assay range using the same olive oil turbimetric assay. Fucoidan (Sigma product F8190) also showed no effect at 1500 μg DW per assay (results not shown). This strongly suggests that these polysaccharides play a minor role in the inhibition caused by the water extract, but they may act additively with the polyphenols, specifically the phlorotannins. Indeed, that polysaccharide and polyphenol components may act additively in lipase inhibition generally fits with the results noted for Ascophyllum extracts as prepared by Chater et al.21 They found that whole aqueous seaweed homogenates of Ascophyllum were more effective lipase inhibitors than alcohol extracts (i.e. IC50 values of 748 μg DW per assay vs. 1932 μg DW per assay respectively). One would assume that the water extract could contain polysaccharide and polyphenol components but the ethanol extract would not contain polysaccharides but would be enriched in polyphenol components. Extracts prepared from Fucus vesiculosus were much more potent lipase inhibitors.21 Again, whole aqueous seaweed homogenates were more effective inhibitors than alcohol extracts but, in this case, the difference was not so great (i.e. IC50 values of 119 μg DW per assay vs. 159 μg DW per assay respectively). Although the TPC levels were not given, it is possible that the Fucus extracts were more potent as they contained higher levels of phlorotannins as Fucus is known to yield high total phenol contents (up to 23% in alcohol extracts36) and to have higher TPC levels than Ascophyllum.37 Overall, this data supports the idea that polysaccharide and polyphenol components can provide additive effects on lipase inhibition.
The polysaccharide fraction from Ascophyllum contains alginates but has been reported to contain fucoidan-like polysaccharides.22 Indeed, initial compositional work found substantial amounts of fucose in acid hydrolysates of the crude ethanol precipitate, most probably arising from fucoidans (results not shown). Chater et al.21 suggested a role for fucoidan in lipase inhibition but, although fucoidans from Fucus vesiculous can inhibit α-glucosidase and α-amylase,38 no evidence for lipase inhibition by fucoidans has been reported. In fact, fucoidan has been shown to increase lipid digestion under certain conditions,39 perhaps through effects on oil droplet emulsification. In this study, no lipase inhibition was noted at 1500 μg per assay pure fucoidan. Indeed, although fucoidan has been shown to influence lipid metabolism and obesity in animal models, the suggested range of mechanisms did not include lipase inhibition.40–42
However, alginates and fucoidans are soluble dietary fibres and may exert other beneficial effects in vivo including effects related to enhanced viscosity of gut contents, altered gastric emptying and reduced calorie intake through enhanced satiety (Fig. 6).43
Conclusions
The effectiveness of the Ascophyllum water extract against lipase activity strongly suggests that any food product containing Ascophyllum or enriched “clean food label” Ascophyllum extracts or supplements could provide components that would enter the gut and could influence lipid digestion in situ. Indeed, the mixture of polysaccharides and phlorotannins in these extracts offers the potential for additive inhibitory effects and/or independent beneficial physiological effects. The simple water extract produced in this study contained ∼670 μg phenols per mL so a four-fold dilution in foods and digestive fluids [in line with that estimated to occur in vivo44] would maintain levels above the IC50 value for lipase inhibition at 150 μg GAE per mL. Alternatively, bespoke extracts could readily be designed that had higher levels of phlorotannins, polysaccharides or both components. Additionally, we have confirmed that phlorotannins are released from Ascophyllum tissues during simulated gastrointestinal digestion in vitro (results not shown) and total phenol content increased after in vitro digestion in other studies,21 so direct consumption of Ascophyllum tissues could be another means to supply these compounds to the small intestine. However, phlorotannins are naturally astringent and may bind other proteins in the gastrointestinal tract (e.g. mucins or food proteins) which could lessen their effect on digestive enzymes. Nevertheless, phlorotannins and polysaccharides appear to associate during extraction and preparation and it is possible that this interaction, allied to high initial levels of phlorotannins, could lead to protection and their greater availability in the gut.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We acknowledge underpinning funding from the Scottish Government's Rural and Environment Science and Analytical Services (RESAS) Division. We thank Hebridean Seaweeds Ltd for the supply of dried seaweed.References
- Anon, Obesity and Overweight – Facts, World Health Organisation Report 2003. Available at http://www.who.int/dietphysicalactivity/media/en/gsfs_obesity.pdf.
- Anon, Obesity in Scotland: An epidemiology briefing. Scottish Public Health. Observatory Publication 2007 - http://www.scotpho.org.uk/home/Publications/scotphoreports/pub_obesityinscotland.asp.
- M. Lean, J. Lara and J. O'Hill, ABC of obesity: Strategies for preventing obesity, Br. Med. J., 2006, 333, 959–962 CrossRef PubMed.
- M. Tovey, Obesity and the public purse: Weighing up the true cost to the taxpayer, IEA Discussion Paper No.80 2017- https://iea.org.uk/wp-content/uploads/2017/01/Obesity-and-the-Public-Purse-PDF.pdf.
- C. Ayyad and T. Andersen, Long-term efficacy of dietary treatment of obesity: a systematic review of studies published between 1931 and 1999, Obes. Rev., 2000, 1, 113–119 CrossRef CAS PubMed.
- C. Mulvihill and R. Quigley, The management of obesity and overweight: an analysis of reviews of diet, physical activity and behavioural approaches, Health Development Agency Evidence Briefing, London, 2003 Search PubMed.
- M. E. Lowe, Molecular mechanisms of rat and human pancreatic triglyceride lipases, J. Nutr., 1997, 127, 549–557 CAS.
- L. Sjöström, A. Rissanen, T. Andersen, M. Boldrin, A. Golay, H. P. Koppeschaar and M. Krempf, Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group, Lancet, 1998, 352, 167–172 CrossRef.
- M. Hamosh and R. O. Scow, Lingual lipase and its role in the digestion of dietary lipid, J. Clin. Invest., 1973, 52, 88–95 CrossRef CAS PubMed.
- J. Zhi, A. T. Melia, R. Guerciolini, J. Chung, J. Kinberg, J. B. Hauptman and I. H. Patel, Retrospective population-based analysis of the dose-response (fecal fat excretion) relationship of Orlistat in normal and obese volunteers, Clin. Pharmacol. Ther., 1994, 56, 82–85 CrossRef CAS PubMed.
- A. Gursoy, M. F. Erdogan, M. O Cin, M. Cesur and N. Baskal, Comparison of orlistat and sibutramine in an obesity management program: efficacy, compliance, and weight regain after noncompliance, Eat. Weight Disord., 2006, 11, e127–e132 CrossRef CAS PubMed.
- R. B. Birari and K. K. Bhutani, Pancreatic lipase inhibitors from natural sources: unexplored potential, Drug Discovery Today, 2007, 19–20, 879–889 CrossRef PubMed.
- A. Gondoin, D. Grussu, D. Stewart and G. J. McDougall, White and green tea polyphenols inhibit pancreatic lipase in vitro, Food Res. Int., 2010, 43, 1537–1544 CrossRef CAS.
- B. S. Roberto, G. A. Macedo, J. A. Macedo, I. M. Martins, V. M. Nakajima, J. W Allwood, D. Stewart and G. J McDougall, Immobilized tannase treatment alters polyphenolic composition in teas and their potential anti-obesity and hypoglycemic activities in vitro, Food Funct., 2016, 7, 3920–3932 CAS.
- S. Almoosawi, G. J. McDougall, L. Fyfe and E. A. S. Al-Dujaili, Investigating the inhibitory activity of green coffee and cacao bean extracts on pancreatic lipase, Nutr. Bull., 2010, 35, 207–212 CrossRef.
- G. J. McDougall, N. N. Kulkarni and D. Stewart, Berry polyphenols inhibit pancreatic lipase actvity in vitro, Food Chem., 2009, 115, 193–199 CrossRef CAS.
- F. Nwosu, J. Morris, V. A. Lund, D. Stewart, H. A. Ross and G. J. McDougall, Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae, Food Chem., 2011, 126, 1006–1012 CrossRef CAS.
- N. Pantidos, A. Boath, V. Lund, S. Conner and G. J. McDougall, Phenolic-rich extracts from the edible seaweed, Ascophyllum nodosum, inhibit alpha-amylase and alpha-glucosidase: Potential anti-hyperglycemic effects, J. Funct. Foods, 2014, 10, 201–209 CrossRef CAS.
- Y.-X. Li, I. Wijesekara, Y. Li and S.-K. Kim, Phlorotannins as bioactive agents from brown algae, Process Biochem., 2011, 46, 2219–2224 CrossRef CAS.
- P. I. Chater, W. D. Wilcox, D. Houghton and J. P Pearson, The role of seaweed bioactives in the control of digestion: implications for obesity treatments, Food Funct., 2015, 6, 3420–3427 CAS.
- P. I. Chater, W. D. Wilcox, P. Cherry, A. Herford, S. Mustar, H. Wheater, I. Brownlee, C. Seal and J. P. Pearson, Inhibitory activity of extracts of Hebridean brown seaweeds on lipase activity, J. Appl. Phycol., 2016, 28, 1303–1310 CrossRef PubMed.
- L.-E. Rioux, S. L. Turgeon and M. Beaulieu, Characterization of polysaccharides extracted from brown seaweeds, Carbohydr. Polym., 2007, 69, 530–537 CrossRef CAS.
- M. D. Wilcox, I. A. Brownlee, J. C. Richardson, P. W. Dettmar and J. P. Pearson, The modulation of pancreatic lipase activity by alginates, Food Chem., 2014, 146, 479–484 CrossRef CAS PubMed.
- S. George, P. Brat, P. Alter and M. J. Amiot, Rapid determination of polyphenols and vitamin C in plant-derived products, J. Agric. Food Chem., 2005, 53, 1370–1373 CrossRef CAS PubMed.
- D. G. Stengel and M. J. Dring, Seasonal variation in the pigment content and photosynthesis of different thallus regions of Ascophyllum nodosum (Fucales, Phaeophyta) in relation to position in the canopy, Phycologia, 1998, 37, 259–268 CrossRef.
- N. Deighton, R. Brennan, C. Finn and H. V. Davies, Antioxidant properties of domesticated and wild Rubus species, J. Sci. Food Agric., 2000, 80, 1307–1313 CrossRef CAS.
- M. Dubois, K. A. Gilles, J. K. Hamilton, P. A. T. Rebers and F. Smith, Colorimetric method for determination of sugars and related substances, Anal. Chem., 1956, 28, 350–356 CrossRef CAS.
- P. Schiener, K. Black, M. Stanley and D. Green, The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta, J. Appl. Phycol., 2015, 27, 363–373 CrossRef CAS.
- W. C. Vogel and L. Zieve, A rapid and sensitive turbidimetric method for serum lipase based upon differences between the lipases of normal and pancreatitis serum, Clin. Chem., 1963, 9, 168–181 CAS.
- A. J. Steevensz, S. L. Mackinnon, R. Hankinson, C. Craft, S. Connan, D. B. Stengel and J. E. Melanson, Profiling phlorotannins in brown macroalgae by liquid chromatography-high resolution mass spectrometry, Phytochem. Anal., 2012, 23, 547–553 CrossRef CAS PubMed.
- M. S. Tierney, T. J. Smyth, D. K. Rai, A. Soler-Vila, A. K. Croft and N. Brunton, Enrichment of polyphenol contents and antioxidant activities of Irish brown macroalgae using food-friendly techniques based on polarity and molecular size, Food Chem., 2013, 139, 753–761 CrossRef CAS PubMed.
- S. H. Eom, M. S. Lee, E. W. Lee, Y. M. Kim and T. H. Kim, Pancreatic lipase inhibitory activity of phlorotannins isolated from Eisenia bicyclis, Phytother. Res., 2013, 27, 148–151 CrossRef CAS PubMed.
- K. B. W. R. Kim, J. Y. Jung, J. Y. Cho and D. H. Ahn, Lipase inhibitory activity of ethyl acetate fraction from Ecklonia cava extracts, Biotechnol. Bioprocess Eng., 2012, 17, 739–745 CrossRef CAS.
- R. Guerciolini, Mode of action of orlistat, Int. J. Obes. Relat. Metab. Disord., 1997, 21, S12–S23 CAS.
- Y. Shishikura, S. Khokhar and B. S. Murray, Effects of tea polyphenols on emulsification of olive oil in a small intestine model system, J. Agric. Food Chem., 2009, 54, 1906–1913 CrossRef PubMed.
- Q. Zhang, J. Zhang, J. Shen, A. Silva, D. Dennis and C. Barrow, A simple 96-well microplate method for estimation of total polyphenol content in seaweeds, J. Appl. Phycol., 2006, 18, 445–450 CrossRef CAS.
- T. Wang, R. Jonsdottir and G. Olafsdottir, Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds, Food Chem., 2009, 116, 240–248 CrossRef CAS.
- Y. G. Chang and D. J. McClements, Influence of emulsifier type on the in vitro digestion of fish oil-in-water emulsions in the presence of an anionic marine polysaccharide (fucoidan): caseinate, whey protein, lecithin, or Tween 80, Food Hydrocolloids, 2016, 61, 92–101 CrossRef CAS.
- K. T. Kim, L.-E. Rioux and S. L. Turgeon, Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum, Phytochemistry, 2014, 98, 27–33 CrossRef CAS PubMed.
- M. J. Kim, J. Jeon and J. S. Lee, Fucoidan prevents high-fat diet-induced obesity in animals by suppression of fat accumulation, Phytother. Res., 2014, 28, 137–143 CrossRef CAS PubMed.
- T. Yokota, K. Nomura, M. Nagashima and N. Kamimura, Fucoidan alleviates high-fat diet-induced dyslipidemia and atherosclerosis in ApoE(Shl) mice deficient in apolipoprotein E expression, J. Nutr. Biochem., 2016, 14, 67–74 Search PubMed.
- X. Hu, N. Tao, X. Wang, J. Xiao and M. Wang, Marine-derived bioactive compounds with anti-obesity effect: A review, J. Funct. Foods, 2016, 21, 372–387 CrossRef CAS.
- I. A. Brownlee, A. Allen, J. P. Pearson, P. W. Dettmar, M. E. Havler, M. R. Atherton and E. Onsøyen, Alginate as a source of dietary fiber, Crit. Rev. Food Sci. Nutr., 2005, 45, 497–510 CrossRef CAS PubMed.
- M. Alminger, A.-M. Aura, T. Bohn, C. Dufour, S. N. El, A. Gomes, S. Karakaya, M. C. Martinez-Cuesta, G. J. McDougall, T. Requena and C. N. Santos, In vitro models for studying secondary plant metabolite digestion and bioaccessibility, Compr. Rev. Food Sci. Food Saf., 2014, 13, 413–436 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7fo01690e |
| This journal is © The Royal Society of Chemistry 2018 |