Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Copper-catalyzed aminoalkynylation of alkenes with hypervalent iodine reagents†
Kun
Shen
and
Qiu
Wang
 *
*
Department of Chemistry, Duke University, Durham, NC 27708–0346, USA. E-mail: qiu.wang@duke.edu
First published on 16th October 2017
Abstract
A copper-catalyzed aminoalkynylation of alkenes is achieved with ethynylbenziodoxolone (EBX) reagents under mild conditions with only 1 mol% copper catalyst. This transformation allows for rapid construction of diverse important azahetereocycles and installation of valuable alkyne groups in one step. The developed method features remarkable substrate scope for both terminal and internal alkenes as well as different alkynyl groups, presenting great potential for broad applications in synthesis, bioconjugation, and molecular imaging.
Introduction
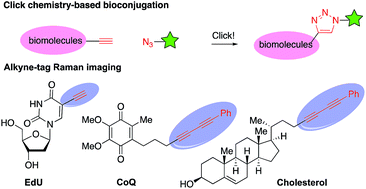
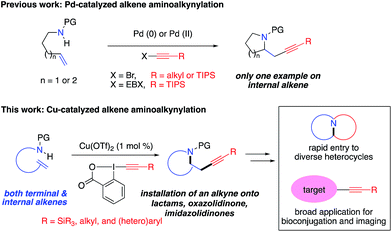
The carbon–carbon triple bond is one of the most valuable functional groups in organic chemistry. It features versatile reactivity as a synthetic intermediate in organic synthesis and has broad applications as a functional tag in biochemistry and materials sciences (Fig. 1).1 For example, alkynes have been widely used in “alkyne–azide click chemistry” for bioconjugation.2 Recently, alkynes have also been demonstrated as a Raman imaging tag because they show distinct, strong Raman scattering at ∼2150 cm−1, in a cellular silent region (1800–2800 cm−1) where most endogenous molecules show no Raman scattering.3 Therefore, developing efficient methods for installation of an alkyne group onto organic molecules is important and has attracted intense interests.Alkene alkynylation represents an attractive and rapid approach to install alkyne groups onto target molecules from readily available alkenes. With azaheterocycles as the most valuable skeletons frequently found in biologically active natural products and pharmaceuticals,4 alkene aminoalkynylation is particularly valued for construction of azaheterocycles and installation of an alkyne group in a single step. Significant advances in this area have been reported by the Waser group (Scheme 1), including Pd-catalyzed alkene aminoalkynylation, as well as oxy- and carbo-alkynylation transformations using ethynylbenziodoxolones (EBX)5 or aliphatic bromoacetylenes.6,7 Despite these progress, only one example have been demonstrated on internal alkenes so far, impeding its utility to construct more complex, diverse azaheterocycles. Furthermore, reaction conditions need to be tailored for different alkyne precursors in previous methods, limiting the scope of alkyne groups for broad application in organic synthesis and chemical biology. Motivated by our interest in developing new, efficient methods to access important azaheterocycles8 and inspired by recent success in alkene functionalization with hypervalent iodine reagents,9 we here report our development of a copper-catalyzed selective aminoalkynylation reaction of both terminal and internal alkenes (Scheme 1). This transformation readily occurs with 1 mol% of copper catalyst under mild conditions and enables rapid access to a wide range of alkyne-labelled fused, and bridged azaheterocyles, which are commonly found in pharmaceutically relevant architectures.4 The aminoalkynylation method will enable broad applications of using alkynes as a handle for rapid entry to diverse azaheterocycles and as a functional tag in bioconjugation and Raman imaging.
 | ||
| Scheme 1 Alkene difunctionalization for installation of an alkyne group onto important azaheterocycles. | ||
Results and discussion
Reaction optimization
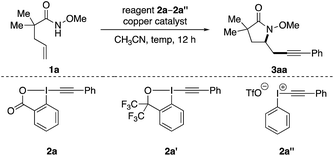
Our investigation of this alkene aminoalkynylation reaction began with unsaturated amide 1a (Table 1), a model substrate that was demonstrated to undergo effectively copper-catalyzed aminocyclization in our previous studies.8c,9h,10 We chose ethynylbenziodoxolones (EBX) as the alkynyl precursors, as they have been successfully used in different alkynylation reactions with nucleophiles,11 C–H bonds,12 carbon radicals,13 and olefins.5 Encouragingly, aminoalkynylation product 3aa was formed in 69% yield in the presence of Cu(OTf)2 in CH3CN at 80 °C (entry 1). Among the various copper salts examined, cationic copper species generally gave higher yields than neutral copper species, with Cu(OTf)2 most effective (entries 1–7). Without a copper catalyst, only trace amounts of 3aa was observed, indicating the important role of a copper catalyst in this reaction (entry 8). Decreasing the catalyst loading was beneficial for the formation of 3aa (entries 9 and 10) and elevating the temperature improved the efficiency of reactions (entries 10–12). In comparison to 2a, other types of alkynyl-based hypervalent iodine reagents such as 2a′ and 2a′′ resulted in much lower yields (entries 13 and 14). Thus entry 11 was chosen as standard conditions for subsequent studies of this aminoalkynylation reaction.14| Entry | Catalyst (mol%) | 2 | T (°C) | Yieldb |
|---|---|---|---|---|
| a Reactions conditions: 1 (0.2 mmol, 1 equiv.), 2 (0.24 mmol, 1.2 equiv.), copper catalyst, CH3CN (1 mL) unless otherwise noted. b Yields were determined by 1H NMR with CH2Br2 as an internal standard. | ||||
| 1 | Cu(OTf)2 (10) | 2a | 80 | 69% |
| 2 | Cu(OAc)2 (10) | 2a | 80 | 33% |
| 3 | Cu(acac)2 (10) | 2a | 80 | 34% |
| 4 | CuCl2 (10) | 2a | 80 | 26% |
| 5 | CuOTf 1/2PhCH3 (10) | 2a | 80 | 63% |
| 6 | Cu(CH3CN)4PF6 (10) | 2a | 80 | 69% |
| 7 | Cu(CH3CN)4BF4 (10) | 2a | 80 | 67% |
| 8 | — | 2a | 80 | 15% |
| 9 | Cu(OTf)2 (5) | 2a | 80 | 73% |
| 10 | Cu(OTf)2 (1) | 2a | 80 | 78% |
| 11 | Cu(OTf) 2 (1) | 2a | 100 | 84% |
| 12 | Cu(OTf)2 (1) | 2a | 60 | 37% |
| 13 | Cu(OTf)2 (1) | 2a′ | 100 | 57% |
| 14 | Cu(OTf)2 (1) | 2a′′ | 100 | <5% |
Reaction scope
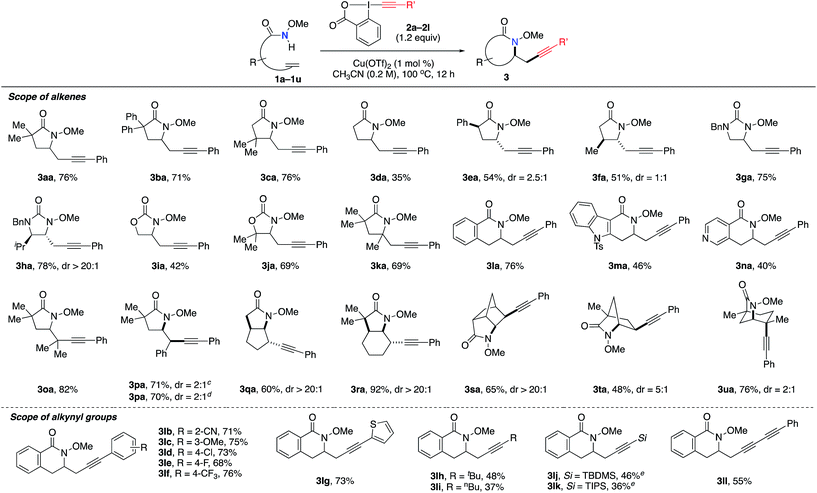
We examined the generality and efficiency of the aminoalkynylation reaction on different unsaturated amide derivatives (Table 2). Monosubstituted alkenes 1a–f bearing various substitutes on the alkenyl chain all underwent smooth 5-exo cyclization and gave γ-lactam products 3aa–fa. The reactions of substrates bearing substitutes on the backbone were more efficient, likely due to the favorable Thorpe–Ingold effect in the cyclization step. Unsaturated ureas 1g–h and carbamates 1i–j were effective in the aminoalkynylation reactions and provided desired imidazolidinones 3ga–ha and oxazolidinones 3ia–ja. 1,1-Disubstituted alkene 1k led to γ-lactam 3ka bearing a quaternary carbon center. δ-Lactam products 3ma–na were also readily formed, with heteroarenes such as indoles and pyridines well tolerated in the reactions. Note that the diastereoselectivity of this reaction may be influenced by the substitution on the backbone, as observed in the formation of 3ea, 3fa and 3ha. Remarkably, this aminoalkynylation reaction was applicable to internal alkenes, which are known to be challenging in metal-catalyzed alkene difunctionalization due to competing β-H elimination. The reaction of tri-substituted alkene 1o gave aminoalkynylation product 3oa bearing a quaternary carbon in 82% yield. The reactions of (E)- and (Z)-1p both led to the formation of product 3pa in the same ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diasteroselectivity, suggesting that (E)- and (Z)-1p may share the same intermediate leading to the product 3pa. The reactions of cyclic internal alkenes well delivered fused-ring products 3qa and 3ra bridged-ring products 3sa–ua. Overall, this aminoalkynylation reaction proved effective on a broad scope of olefin substrates that encompass diverse substitutions on both alkenes and backbones.
1 diasteroselectivity, suggesting that (E)- and (Z)-1p may share the same intermediate leading to the product 3pa. The reactions of cyclic internal alkenes well delivered fused-ring products 3qa and 3ra bridged-ring products 3sa–ua. Overall, this aminoalkynylation reaction proved effective on a broad scope of olefin substrates that encompass diverse substitutions on both alkenes and backbones.
Next, the scope of alkynylation reagents 2 was examined in the reactions with alkene 1l (Table 2). As seen in the formation of 3lb–lf, both electron-donating and electron-withdrawing groups on the phenyl group were well tolerated. Neither the electronic nor steric properties of the substitutes on the phenyl group significantly influence the reaction efficiency. The thiophene moiety, an electron-rich heterocycles known for direct alkynylation,12a is compatible in this reaction, affording product 3lg in good yield. Alkynylation reagents bearing alkyl substitutes were also applicable (3lh and 3li), albeit in much lower yields compared to aromatic substitutes. TBS-EBX and TIPS-EBX reagents were also feasible in this transformation (3lj and 3lk). Finally, conjugated dialkyne was readily incorporated into the aminoalkynylation product 3ll. Note that conjugated dialkyne groups are known to offer stronger Raman signal compared to other types of alkynes as potential Raman imagine tags.3b,e
Mechanism study
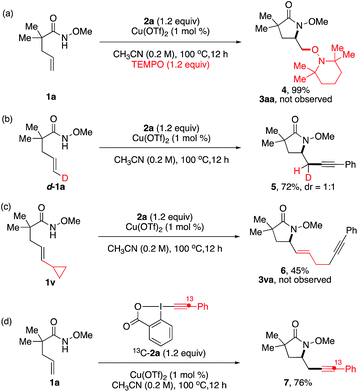
A series of control experiments were performed to obtain mechanistic insights on this aminoalkynylation reaction (Scheme 2). In the presence of TEMPO as a radical scavenger, the reaction of model substrates 1a and 2a provided aminoxygenation product 4 in 99% yield (Scheme 2a). The reaction of the trans-D-substituted alkenyl amide D-1a gave a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
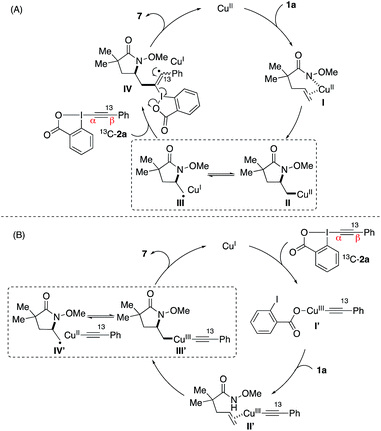
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of D-substituted diastereomers 5 in 72% yield (Scheme 2b). Both results indicate a radical nature of the intermediate after the intramolecular amino-cupration step, which is consistent with the loss of stereochemistry observed in the formation of 3pa from (E)- and (Z)-1p in Table 2. To confirm this speculation, we examined the aminoalkynylation reaction using 1v, an unsaturated amide containing a standard radical clock cyclopropane moiety at the vinyl position (Scheme 2c). The reaction provided ring-opened product 6 in 45% yield with no detection of 3va, confirming the presence of radical alkyl intermediates after the aminocyclization step. Finally, we used 13C labelled Ph–EBX 13C-2a to probe the alkynylation step, revealing the exclusive presence of 13C atom at its original position of 7 (Scheme 2d).
1 mixture of D-substituted diastereomers 5 in 72% yield (Scheme 2b). Both results indicate a radical nature of the intermediate after the intramolecular amino-cupration step, which is consistent with the loss of stereochemistry observed in the formation of 3pa from (E)- and (Z)-1p in Table 2. To confirm this speculation, we examined the aminoalkynylation reaction using 1v, an unsaturated amide containing a standard radical clock cyclopropane moiety at the vinyl position (Scheme 2c). The reaction provided ring-opened product 6 in 45% yield with no detection of 3va, confirming the presence of radical alkyl intermediates after the aminocyclization step. Finally, we used 13C labelled Ph–EBX 13C-2a to probe the alkynylation step, revealing the exclusive presence of 13C atom at its original position of 7 (Scheme 2d).
Based on these results, two possible reaction pathways are proposed for this copper-catalyzed aminoalkynylation reaction (Scheme 3).15 In pathway A, the reaction may be initiated by the coordination of copper catalyst with alkene 1a followed by the intramolecular aminocupration.10 The resulting alkyl–Cu intermediate II may undergo a reversible C–Cu(II) homolysis to form radical intermediate III, which would subsequently attack the α-position of the alkynyliodonium salt 2a. Finally, the β-elimination of intermediate IV would lead to product 3 and regeneration of the copper catalyst. In an alternative pathway (B), the reaction may be initiated by the copper-catalyzed activation of alkynyliodonium salt 2a. The aminocyclization would occur via copper intermediates I′ and II′ to form copper–alkyl complex III′, which would undergo similar homolysis to form a radical intermediate IV′ and could lead to the formation of product 3. Note that the exclusive presence of 13C atom at its original position in the reaction of EBX reagent 13C-2a (Scheme 2d) indicated the involvement of α-addition in the alkynylation step in either pathway (A) or (B), rather than the β-addition of the alkyne followed by the 1,2-shift of the Ph group.11i,l,16
Synthetic applications
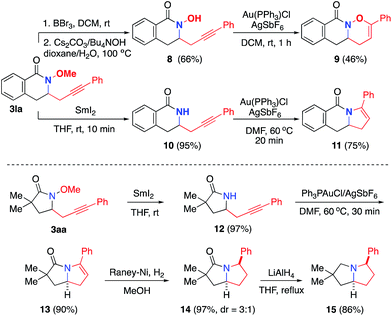
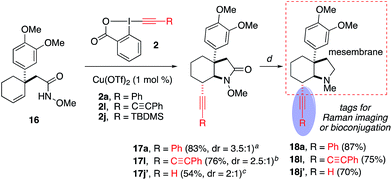
We next explored the synthetic utility of alkynyl group for the construction of various bicyclic heterocycles (Scheme 4).17 First, using 3la, we demonstrate the advantage of the methoxy protecting group of the amide to achieve selective gold-catalyzed oxy- or amino-cyclization onto the alkyne group. For example, upon the removal of methyl group, the resulting hydroxylamide 8 underwent a Au-catalyzed endo oxycyclization to form oxazinane 9. On the other hand, the demethoxylation of 3la provided amide 10, which was transformed into dihydropyrole 11 by an analogous Au-catalyzed aminocyclization. Furthermore, we employed the aminoalkynylation product 3aa to complete the synthesis of a series of important pyrrolizidine derivatives 13–15.18 Finally, this aminoalkynylation method was successfully applied for the preparation of alkyne-labelled derivatives of alkaloid mesembrane (Scheme 5). These readily installed alkynyl groups are expected to serve as valuable labelling tools to facilitate future studies regarding understanding its antidepressant potential and mode of action.19Conclusions
In summary, we have developed a new copper-catalyzed alkene aminoalkynylation reaction that is effective on an extensive scope of alkene substrates and EBX reagents. This method enables simultaneous construction of valuable azahetereocyclic skeletons and the installation an alkynyl group, which will greatly advance broad applications of using alkynes as a handle for rapid entry to complex, diverse heterocycles and as a functional tag in bioconjugation and Raman imaging.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge financial support provided by Duke University and the National Institute of General Medical Sciences of the NIH (GM118786). Q. W. is a fellow of the Alfred P. Sloan Foundation and a Camille Dreyfus Teacher-Scholar. We thank Dr George Dubay (Duke University) for high-resolution mass spectrometry and thank Dr Roger Sommer (NCSU) for X-ray structural analysis.Notes and references
-
F. Diederich, P. J. Stang and R. R. Tykwinski, Acetylene Chemistry: Chemistry, Biology and Material Science, Wiley-VCH Verlag GmbH & Co, Weinheim, Germany, 2005 Search PubMed
.
-
(a) J. Ando, M. Asanuma, K. Dodo, H. Yamakoshi, S. Kawata, K. Fujita and M. Sodeoka, J. Am. Chem. Soc., 2016, 138, 13901–13910 CrossRef CAS PubMed
; (b) P. Thirumurugan, D. Matosiuk and K. Jozwiak, Chem. Rev., 2013, 113, 4905–4979 CrossRef CAS PubMed
.
-
(a) H. Yamakoshi, K. Dodo, M. Okada, J. Ando, A. Palonpon, K. Fujita, S. Kawata and M. Sodeoka, J. Am. Chem. Soc., 2011, 133, 6102–6105 CrossRef CAS PubMed
; (b) H. Yamakoshi, K. Dodo, A. Palonpon, J. Ando, K. Fujita, S. Kawata and M. Sodeoka, J. Am. Chem. Soc., 2012, 134, 20681–20689 CrossRef CAS PubMed
; (c) Z. Chen, D. W. Paley, L. Wei, A. L. Weisman, R. A. Friesner, C. Nuckolls and W. Min, J. Am. Chem. Soc., 2014, 136, 8027–8033 CrossRef CAS PubMed
; (d) Z. L. Song, Z. Chen, X. Bian, L. Y. Zhou, D. Ding, H. Liang, Y. X. Zou, S. S. Wang, L. Chen, C. Yang, X. B. Zhang and W. Tan, J. Am. Chem. Soc., 2014, 136, 13558–13561 CrossRef CAS PubMed
; (e) L. Wei, F. H. Hu, Y. H. Shen, Z. X. Chen, Y. Yu, C. C. Lin, M. C. Wang and W. Min, Nat. Methods, 2014, 11, 410–412 CrossRef CAS PubMed
; (f) H. J. Lee, W. Zhang, D. Zhang, Y. Yang, B. Liu, E. L. Barker, K. K. Buhman, L. V. Slipchenko, M. Dai and J. X. Cheng, Sci. Rep., 2015, 5, 7930 CrossRef CAS PubMed
; (g) J. Ando, M. Kinoshita, J. Cui, H. Yamakoshi, K. Dodo, K. Fujita, M. Murata and M. Sodeoka, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 4558–4563 CrossRef CAS PubMed
; (h) J. Ando, A. F. Palonpon, H. Yamakoshi, K. Dodo, S. Kawata, M. Sodeoka and K. Fujita, Biophotonics Japan, 2015, vol. 2015, p. 979207 Search PubMed
; (i) F. H. Hu, M. R. Lamprecht, L. Wei, B. Morrison and W. Min, Sci. Rep., 2016, 6, 39660 CrossRef CAS PubMed
; (j) Y. Li, Z. Wang, X. J. Mu, A. Ma and S. Guo, Biotechnol. Adv., 2017, 35, 168–177 CrossRef CAS PubMed
.
- E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed
.
-
(a) S. Nicolai, S. Erard, D. F. Gonzalez and J. Waser, Org. Lett., 2010, 12, 384–387 CrossRef CAS PubMed
; (b) S. Nicolai, C. Piemontesi and J. Waser, Angew. Chem., Int. Ed., 2011, 50, 4680–4683 CrossRef CAS PubMed
; (c) Y. Li, D. P. Hari, M. V. Vita and J. Waser, Angew. Chem., Int. Ed., 2016, 55, 4436–4454 CrossRef CAS PubMed
; (d) J. Waser, Top. Curr. Chem., 2016, 373, 187–222 CrossRef CAS PubMed
.
-
(a) S. Nicolai and J. Waser, Org. Lett., 2011, 13, 6324–6327 CrossRef CAS PubMed
; (b) S. Nicolai, R. Sedigh-Zadeh and J. Waser, J. Org. Chem., 2013, 78, 3783–3801 CrossRef CAS PubMed
; (c) S. Nicolai, P. Swallow and J. Waser, Tetrahedron, 2015, 71, 5959–5964 CrossRef CAS
.
- Other examples on Pd-catalyzed heteroalkynylation of olefins:
(a) A. Faulkner, J. S. Scott and J. F. Bower, J. Am. Chem. Soc., 2015, 137, 7224–7230 CrossRef CAS PubMed
; (b) H. Shi, C. Tan, W. Zhang, Z. Zhang, R. Long, T. Luo and Z. Yang, Org. Lett., 2015, 17, 2342–2345 CrossRef CAS PubMed
; (c) N. Sun, Y. Li, G. Yin and S. Jiang, Eur. J. Org. Chem., 2013, 13, 2541–2544 CrossRef
.
-
(a) H.-T. Huang, T. C. Lacy, B. Błachut, G. X. Ortiz Jr and Q. Wang, Org. Lett., 2013, 15, 1818–1821 CrossRef CAS PubMed
; (b) G. X. Ortiz Jr, B. Kang and Q. Wang, J. Org. Chem., 2014, 79, 571–581 CrossRef PubMed
; (c) K. Shen and Q. Wang, Chem. Sci., 2015, 6, 4279–4283 RSC
; (d) B. N. Hemric, K. Shen and Q. Wang, J. Am. Chem. Soc., 2016, 138, 5813–5816 CrossRef CAS PubMed
.
- Selected recent examples:
(a) D. J. Wardrop, E. G. Bowen, R. E. Forslund, A. D. Sussman and S. L. Weerasekera, J. Am. Chem. Soc., 2010, 132, 1188–1189 CrossRef CAS PubMed
; (b) R. Zhu and S. L. Buchwald, J. Am. Chem. Soc., 2012, 134, 12462–12465 CrossRef CAS PubMed
; (c) W. Kong, M. Casimiro, E. Merino and C. Nevado, J. Am. Chem. Soc., 2013, 135, 14480–14483 CrossRef CAS PubMed
; (d) F. Wang, X. Qi, Z. Liang, P. Chen and G. Liu, Angew. Chem., Int. Ed., 2014, 53, 1881–1886 CrossRef CAS PubMed
; (e) W. Yuan and K. J. Szabo, Angew. Chem., Int. Ed., 2015, 54, 8533–8537 CrossRef CAS PubMed
; (f) R. Zhu and S. L. Buchwald, J. Am. Chem. Soc., 2015, 137, 8069–8077 CrossRef CAS PubMed
; (g) D. Holt and M. J. Gaunt, Angew. Chem., Int. Ed., 2015, 54, 7857–7861 CrossRef CAS PubMed
; (h) K. Shen and Q. Wang, Org. Chem. Front., 2016, 3, 222–226 RSC
; (i) F. Wang, D. Wang, X. Wan, L. Wu, P. Chen and G. Liu, J. Am. Chem. Soc., 2016, 138, 15547–15550 CrossRef CAS PubMed
; (j) K. Shen and Q. Wang, J. Am. Chem. Soc., 2017, 139, 13110–13116 CrossRef CAS PubMed
.
- For the leading research on copper-catalyzed aminofunctionalization of olefins, see:
(a) E. S. Sherman, S. R. Chemler, T. B. Tan and O. Gerlits, Org. Lett., 2004, 6, 1573–1575 CrossRef CAS PubMed
; (b) T. P. Zabawa, D. Kasi and S. R. Chemler, J. Am. Chem. Soc., 2005, 127, 11250–11251 CrossRef CAS PubMed
; (c) E. S. Sherman, P. H. Fuller and D. Kasi, J. Org. Chem., 2007, 72, 3896–3905 CrossRef CAS PubMed
; (d) P. H. Fuller, J.-W. Kim and S. R. Chemler, J. Am. Chem. Soc., 2008, 130, 17638–17639 CrossRef CAS PubMed
; (e) F. C. Sequeira, B. W. Turnpenny and S. R. Chemler, Angew. Chem., Int. Ed., 2010, 49, 6365–6368 CrossRef CAS PubMed
; (f) T. W. Liwosz and S. R. Chemler, J. Am. Chem. Soc., 2012, 134, 2020–2023 CrossRef CAS PubMed
; (g) M. T. Bovino and S. R. Chemler, Angew. Chem., Int. Ed., 2012, 51, 3923–3927 CrossRef CAS PubMed
; (h) B. W. Turnpenny and S. R. Chemler, Chem. Sci., 2014, 5, 1786–1793 RSC
; (i) Z. M. Khoder, C. E. Wong and S. R. Chemler, ACS Catal., 2017, 7, 4775–4779 CrossRef CAS
.
-
(a) D. Fernandez Gonzalez, J. P. Brand and J. Waser, Chem.–Eur. J., 2010, 16, 9457–9461 CrossRef CAS PubMed
; (b) R. Frei and J. Waser, J. Am. Chem. Soc., 2013, 135, 9620–9623 CrossRef CAS PubMed
; (c) D. Fernández González, J. P. Brand, R. Mondiere and J. Waser, Adv. Synth. Catal., 2013, 355, 1631–1639 CrossRef
; (d) T. Aubineau and J. Cossy, Chem. Commun., 2013, 49, 3303–3305 RSC
; (e) X. Wu, S. Shirakawa and K. Maruoka, Org. Biomol. Chem., 2014, 12, 5388–5392 RSC
; (f) M. V. Vita, P. Mieville and J. Waser, Org. Lett., 2014, 16, 5768–5771 CrossRef CAS PubMed
; (g) P. Finkbeiner, N. M. Weckenmann and B. J. Nachtsheim, Org. Lett., 2014, 16, 1326–1329 CrossRef CAS PubMed
; (h) C. C. Chen and J. Waser, Chem. Commun., 2014, 50, 12923–12926 RSC
; (i) R. Frei, M. D. Wodrich, D. P. Hari, P. A. Borin, C. Chauvier and J. Waser, J. Am. Chem. Soc., 2014, 136, 16563–16573 CrossRef CAS PubMed
; (j) C. C. Chen and J. Waser, Org. Lett., 2015, 17, 736–739 CrossRef CAS PubMed
; (k) M. Kamlar, I. Cisarova and J. Vesely, Org. Biomol. Chem., 2015, 13, 2884–2889 RSC
; (l) M. D. Wodrich, P. Caramenti and J. Waser, Org. Lett., 2016, 18, 60–63 CrossRef CAS PubMed
.
-
(a) J. P. Brand, C. Chevalley, R. Scopelliti and J. Waser, Chem.–Eur. J., 2012, 18, 5655–5666 CrossRef CAS PubMed
; (b) J. P. Brand and J. Waser, Synthesis, 2012, 44, 1155–1158 CrossRef CAS
; (c) G. L. Tolnai, S. Ganss, J. P. Brand and J. Waser, Org. Lett., 2013, 15, 112–115 CrossRef CAS PubMed
; (d) F. Xie, Z. Qi, S. Yu and X. Li, J. Am. Chem. Soc., 2014, 136, 4780–4787 CrossRef CAS PubMed
; (e) C. Feng and T. P. Loh, Angew. Chem., Int. Ed., 2014, 53, 2722–2726 CrossRef CAS PubMed
; (f) K. D. Collins, F. Lied and F. Glorius, Chem. Commun., 2014, 50, 4459–4461 RSC
; (g) J. Jeong, P. Patel, H. Hwang and S. Chang, Org. Lett., 2014, 16, 4598–4601 CrossRef CAS PubMed
; (h) X. Zhang, Z. Qi, J. Gao and X. Li, Org. Biomol. Chem., 2014, 12, 9329–9332 RSC
; (i) Y. Wu, Y. Yang, B. Zhou and Y. Li, J. Org. Chem., 2015, 80, 1946–1951 CrossRef CAS PubMed
; (j) N. Jin, C. D. Pan, H. L. Zhang, P. Xu, Y. X. Cheng and C. J. Zhu, Adv. Synth. Catal., 2015, 357, 1149–1153 CrossRef CAS
; (k) P. Finkbeiner, U. Kloeckner and B. J. Nachtsheim, Angew. Chem., Int. Ed., 2015, 54, 4949–4952 CrossRef CAS PubMed
; (l) W. Ai, Y. Wu, H. Tang, X. Yang, Y. Yang, Y. Li and B. Zhou, Chem. Commun., 2015, 51, 7871–7874 RSC
; (m) Z. Z. Zhang, B. Liu, C. Y. Wang and B. F. Shi, Org. Lett., 2015, 17, 4094–4097 CrossRef CAS PubMed
; (n) A. C. Shaikh, D. R. Shinde and N. T. Patil, Org. Lett., 2016, 18, 1056–1059 CrossRef CAS PubMed
; (o) G. D. Tang, C. L. Pan and F. Xie, Org. Biomol. Chem., 2016, 14, 2898–2904 RSC
; (p) B. Liu, X. Wang, Z. Ge and R. Li, Org. Biomol. Chem., 2016, 14, 2944–2949 RSC
; (q) Y. Li, F. Xie and X. Li, J. Org. Chem., 2016, 81, 715–722 CrossRef CAS PubMed
.
-
(a) S. Wang, L. N. Guo, H. Wang and X. H. Duan, Org. Lett., 2015, 17, 4798–4801 CrossRef CAS PubMed
; (b) H. Tan, H. Li, W. Ji and L. Wang, Angew. Chem., Int. Ed., 2015, 54, 8374–8377 CrossRef CAS PubMed
; (c) X. Liu, Z. Wang, X. Cheng and C. Li, J. Am. Chem. Soc., 2012, 134, 14330–14333 CrossRef CAS PubMed
; (d) H. Huang, G. Zhang, L. Gong, S. Zhang and Y. Chen, J. Am. Chem. Soc., 2014, 136, 2280–2283 CrossRef CAS PubMed
; (e) Q. Q. Zhou, W. Guo, W. Ding, X. Wu, X. Chen, L. Q. Lu and W. J. Xiao, Angew. Chem., Int. Ed., 2015, 54, 11196–11199 CrossRef CAS PubMed
; (f) K. Jia, F. Zhang, H. Huang and Y. Chen, J. Am. Chem. Soc., 2016, 138, 1514–1517 CrossRef CAS PubMed
; (g) R.-Y. Zhang, L.-Y. Xi, L. Zhang, S. Liang, S.-Y. Chen and X.-Q. Yu, RSC Adv., 2014, 4, 54349–54353 RSC
; (h) X. Liu, L. Yu, M. Luo, J. Zhu and W. Wei, Chem.–Eur. J., 2015, 21, 8745–8749 CrossRef CAS PubMed
; (i) H. Wang, L. N. Guo, S. Wang and X. H. Duan, Org. Lett., 2015, 17, 3054–3057 CrossRef CAS PubMed
; (j) R.-Y. Zhang, L.-Y. Xi, L. Zhang, S.-Y. Chen and X.-Q. Yu, Tetrahedron, 2015, 71, 6176–6182 CrossRef CAS
; (k) X. H. Ouyang, R. J. Song, C. Y. Wang, Y. Yang and J. H. Li, Chem. Commun., 2015, 51, 14497–14500 RSC
; (l) P. F. Wang, Y. S. Feng, Z. F. Cheng, Q. M. Wu, G. Y. Wang, L. L. Liu, J. J. Dai, J. Xu and H. J. Xu, J. Org. Chem., 2015, 80, 9314–9320 CrossRef CAS PubMed
; (m) H. C. Huang, G. J. Zhang and Y. Y. Chen, Angew. Chem., Int. Ed., 2015, 54, 7872–7876 CrossRef CAS PubMed
; (n) C.-Y. Wang, R.-J. Song, Y.-X. Xie and J.-H. Li, Synthesis, 2016, 48, 223–230 CAS
; (o) F. Le Vaillant, T. Courant and J. Waser, Angew. Chem., Int. Ed., 2015, 54, 11200–11204 CrossRef CAS PubMed
.
- See the ESI† for more studies on amides bearing different protecting groups and reaction optimizations using different catalysts, ligands, additives, temperatures, and hypervalent iodine reagents.
- (a) The formation of aminoalkynylation product (15%) in the absence of copper catalyst (Table 1, entry 8) indicates that hypervalent iodine reagents could promote a catalyst-free radical pathway for aminocyclization and alkynylation, which may also contribute to the formation of desired products under copper-catalyzed conditions; (b) Note that the hypervalent iodine reagent is not necessary for the copper-catalyzed aminocyclization step. See more details in the ESI† for the control experiment in the absence of hypervalent iodine reagents.
- F. Le Vaillant, M. D. Wodrich and J. Waser, Chem. Sci., 2017, 8, 1790–1800 RSC
.
- Selected recent review:
(a) R. Dorel and A. M. Echavarren, Chem. Rev., 2015, 115, 9028–9072 CrossRef CAS PubMed
; (b) Z. Zheng, Z. Wang, Y. Wang and L. Zhang, Chem. Soc. Rev., 2016, 45, 4448–4458 RSC
.
- We also demonstrated that 3aa readily underwent selective removal of the methyl group and subsequent Au-catalyzed endo oxycyclization to form dihydro oxazine. See more details in the ESI.†.
-
(a) M. Mori, S. Kuroda, C. S. Zhang and Y. Sato, J. Org. Chem., 1997, 62, 3263–3270 CrossRef CAS PubMed
; (b) M. K. Das, S. De, S. Bisai and A. Bisai, Org. Biomol. Chem., 2015, 13, 3585–3588 RSC
; (c) K. Konoura, H. Fujii, S. Imaide, H. Gouda, S. Hirayama, S. Hirono and H. Nagase, Bioorg. Med. Chem., 2015, 23, 439–448 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization data and experimental procedures. CCDC 1567005–1567007. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc03420b |
| This journal is © The Royal Society of Chemistry 2017 |