Open Access Article
Open Access ArticleStructure and spin state of nonheme FeIVO complexes depending on temperature: predictive insights from DFT calculations and experiments†
Na Young
Lee
a,
Debasish
Mandal‡
 b,
Seong Hee
Bae
a,
Mi Sook
Seo
a,
Yong-Min
Lee
b,
Seong Hee
Bae
a,
Mi Sook
Seo
a,
Yong-Min
Lee
 a,
Sason
Shaik
a,
Sason
Shaik
 b,
Kyung-Bin
Cho
b,
Kyung-Bin
Cho
 *a and
Wonwoo
Nam
*a and
Wonwoo
Nam
 *a
*a
aDepartment of Chemistry and Nano Science, Ewha Womans University, Seoul 03760, Korea. E-mail: workforkyung@ewha.ac.kr; wwnam@ewha.ac.kr
bInstitute of Chemistry, The Lise Meitner-Minerva Center for Computational Quantum Chemistry, The Hebrew University of Jerusalem, 91904 Jerusalem, Israel
First published on 30th May 2017
Abstract
The spin states (S = 1 and S = 2) of nonheme FeIVO intermediates are believed to play an important role in determining their chemical properties in enzymatic and biomimetic reactions. However, it is almost impossible to investigate the spin state effect of nonheme FeIVO species experimentally, since FeIVO models having the S = 1 and S = 2 spin states at the same time neither exist nor can be synthesized. However, recent synthesis of an FeIVO complex with an S = 1 spin state (triplet), [(Me3NTB)FeIVO]2+ (1), and a structurally similar FeIVO complex but with an S = 2 spin state (quintet), [(TQA)FeIVO]2+ (2), has allowed us to compare their reactivities at 233 K. In the present study, we show that structural variants control the spin-state selectivity and reactivity of nonheme FeIVO complexes. While 1 and 2 were proposed to be in an octahedral geometry based on DFT calculations and spectroscopic characterization done at 4 K, further DFT calculations show that these species may well assume a trigonal bipyramidal structure by losing one coordinated solvent ligand at 233 K. Thus, the present study demonstrates that the structure and spin state of nonheme FeIVO complexes can be different at different temperatures; therefore, the structural and/or spin state information obtained at 4 K should be carefully used at a higher temperature (e.g., 233 K). In addition to 1 and 2, [(TPA)FeIVO]2+ (3) with an S = 1 spin state, whose spin state was determined spectroscopically and theoretically at 233 K, is included in this study to compare the chemical properties of S = 1 and S = 2 FeIVO complexes. The present results add another dimension to the discussion of the reactivites of nonheme FeIVO species, in which the structural preference and spin state of nonheme FeIVO species can vary depending on temperature.
Introduction
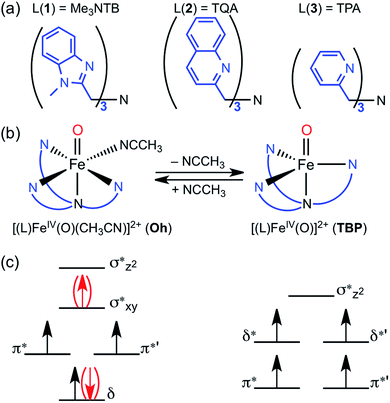
Mononuclear nonheme iron enzymes, such as taurine:α-ketoglutarate dioxygenase (TauD),1 prolyl-4-hydroxylase,2 halogenase CytC3,3 and halogenase SyrB2,4 utilize high-spin (S = 2, quintet) FeIVO intermediates in their enzymatic oxygenation reactions.5 In contrast, most of mononuclear nonheme FeIVO complexes synthesized in biomimetic model studies possess an intermediate-spin (S = 1, triplet) FeIVO moiety.6 Since the spin states (S = 1 and S = 2) of nonheme FeIVO intermediates are believed to play an important role in determining their chemical properties in enzymatic and biomimetic reactions, the spin state effect of nonheme FeIVO species has attracted much attention in the communities of bioinorganic and biological chemistry.1–7The high-spin configuration of nonheme FeIVO species is easily achieved when the FeIVO moiety is in a trigonal bipyramidal (TBP) geometry, which leads to the Fe d-orbitals being arranged in two groups of two energetically degenerated orbitals, with a small energy difference between the two groups (Fig. 1c).8 The preference of the quintet mode for TBP FeIVO species has been explained by the same principles governing Hund's rule; the extra energy required to populate the two higher energy d-orbitals is compensated by the lack of exchange interaction between the four α d-electrons.9
In the case of an octahedral (Oh) geometry, the upper d-orbital energies are high, and consequently the ground state configuration is an intermediate-spin (S = 1, triplet) in most cases (Fig. 1c). Even so, during an oxygenation reaction, an electron from the abstracted hydrogen atom of the substrate reduces the iron(IV) ion, and the resulting exchange enhancement of this fifth electron allows the quintet transition state (TS) of the reaction to be lower in energy than its triplet counterpart.8–10 Thus, for quite some time, it has been theoretically postulated that even triplet species would in fact perform the reaction through quintet surface (i.e., two-state reactivity, TSR),11 unless more energy is required for spin changes due to low spin–orbit coupling.
Actual experimental proof of this two-spin state theory is in fact difficult to obtain, as TSs are not commonly observable. One is therefore limited to measure the spin states at the reactant or product states. Despite attempts to address this question by such studies,12 this provided limited information regarding this issue, and especially so about the timing of spin change (i.e., before or after the TS). In addition, an energetically lower quintet TS may not be sufficient to guarantee a spin transition. For instance, we have earlier investigated the case of C–H activation by [(N4Py)FeIVO]2+, where the experiments indicate that spin inversion does not occur, hence implying a low spin–orbit coupling.13 One rationalization for this is the large energy difference between the two spin states of the reactant compound (e.g., ΔG > 6 kcal mol−1), which may lead to difficulties in changing the spin state at the beginning of the reaction. Also, in the case of using cyclohexene as a substrate, the small energy difference (e.g., ΔG < 1 kcal mol−1) at the TS suggests that energy gain by the spin flip would be small.
In the opposite end of the spectrum, we showed that while the ground state of [(Me3NTB)FeIVO(CH3CN)]2+ (1Oh; see the ligand structure in Fig. 1a) was experimentally measured to be in the triplet state at 4.2 K; the calculated energy difference to the quintet state was almost non-existent (e.g., ΔG < 0.1 kcal mol−1 at 298 K).14 At the same time, the gain at the TS was substantial (e.g., ΔG > 10 kcal mol−1) when cyclohexane or cyclohexadiene was used as a substrate.14 Hence, these features constitute strong implications that 1Oh can easily switch its spin state and perform C–H activation reactions in a high-spin mode with a large gain in reactivity. In the absence of spin–orbit couplings, this scenario remains just an implication, albeit a strong one. Moreover, we could not exclude a possibility that 1Oh would lose its solvent ligand (e.g., CH3CN) to form a TBP structure ([(Me3NTB)FeIVO]2+, 1TBP) and the reaction was then performed in the quintet state (Fig. 1b) because the TBP structure favours the quintet state by a large margin.
More recently, a quintet ground state complex with an Oh structure, [(TQA)FeIVO(CH3CN)]2+ (2Oh; see the ligand structure in Fig. 1a), was reported;15 the spectroscopic characterization was done at 4.2 K. The TQA ligand uses quinoline groups instead of the 1-methyl-benzimidazole groups in Me3NTB (Fig. 1a). This small ligand modification has generated interesting questions about the differences in the chemical properties of the triplet and quintet FeIVO complexes, and it has been shown very recently that the reactivity rates and patterns of 1Oh and 2Oh were remarkably similar.16 Existing experimental comparisons, however, were limited to the spin states and structures determined at 4 K and the reactivities investigated at 233 K. A quick summary of the experimental results would be as follows: (i) when the spin states of 1Oh and 2Oh were determined spectroscopically at 4 K (i.e., Mössbauer), the preferred spin state of 1Oh was the triplet,14 whereas it was the quintet for 2Oh.15 (ii) The structures were proposed to have a solvent ligand (e.g., CH3CN),14,15 forming a six-coordinated octahedral structure (e.g., 1Oh and 2Oh) at 4 K based on density functional theory (DFT)17 calculations matching Mössbauer data. (iii) In the oxidation of cyclohexene by 1Oh and 2Oh, hydrogen atom transfer (HAT) (i.e., allylic C–H activation) was preferred to oxygen atom transfer (OAT) (i.e., C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C epoxidation), but some OAT products were yielded, indicating that the energy barriers for the HAT and OAT reactions are close to one another at 233 K.16 (iv) The reactivities of 1Oh and 2Oh were found to be comparable in oxidation reactions at 233 K.15,16
C epoxidation), but some OAT products were yielded, indicating that the energy barriers for the HAT and OAT reactions are close to one another at 233 K.16 (iv) The reactivities of 1Oh and 2Oh were found to be comparable in oxidation reactions at 233 K.15,16
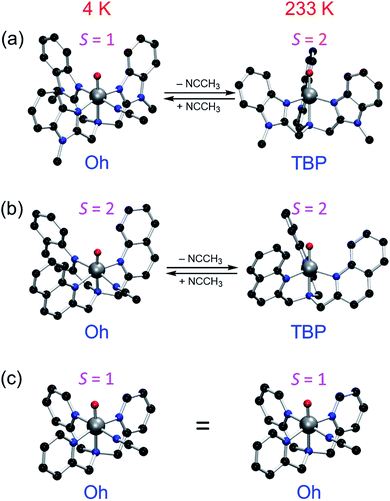
However, as discussed above, the spectroscopic characterization of 1Oh and 2Oh was done at 4 K, whereas all the reactivity studies were performed at 233 K and the experimental results were interpreted with an assumption that the structures and spin states of 1Oh and 2Oh at 233 K were the same as those determined at 4 K.14,15 Moreover, the temperature difference between the spectroscopic characterization and reactivity studies was not considered previously. Therefore, to gain insights into the temperature effect(s) on the structure and spin state of nonheme FeIVO species, we used DFT calculations as well as experiments to elucidate the experimental results obtained at a higher temperature (i.e., 233 K). In the present study, we show that the structural assignment of 1Oh and 2Oh with the spectroscopic data obtained at 4 K do not accurately reflect the change of structure and spin state at a higher temperature (i.e., 233 K) due to the change of the solvent-binding preference at different temperatures (Fig. 1b). Indeed, DFT calculations show that 1Oh and 2Oh lose one coordinated solvent ligand at 233 K, forming a trigonal bipyramidal structure (i.e., 1TBP and 2TBP); unfortunately, the spectroscopic determination of the spin state of the presumed 1TBP and 2TBP species was not successful due to the highly unstable nature of the intermediates in solution at 233 K (e.g., t1/2 < 1 min). Thus, to validate the reliability of the DFT calculations, we have also studied the thermally more stable FeIVO species, [(TPA)FeIVO(CH3CN)]2+ (3Oh), possessing a triplet spin state at both 4 and 233 K.18 The combination of experiment and theoretical calculations lead to new insights into the temperature-structure–spin state relationship that has not been explored previously in nonheme FeIVO model studies.
Results and discussion
Our first set of calculations involves large basis set geometry optimizations (B3LYP/Def2-TZVPP)19,20 to obtain accurate geometries and energies on the component structures 1, 2 and 3. Table 1 lists the relative energies for the species in both triplet and quintet spin states. Looking at the electronic energies only (ΔE), the energies show that the triplet is preferred for 1Oh and 3Oh, whereas the quintet is preferred for 2Oh; all are superficially in agreement with the spin states determined spectroscopically at 4 K.14–16,18 Hence, in this particular case, the small difference in the Me3NTB and TQA ligands allows a slight preference of one spin state to change to a slight preference of the other (i.e., triplet for 1Ohversus quintet for 2Oh).| Compound | ΔE | ΔG (4 K) | ΔG (233 K) |
|---|---|---|---|
| a Energies are relative to the separated compounds, i.e., [(L)FeIVO]2+ (quintet) + CH3CN, where L is Me3NTB for 1, TQA for 2 and TPA for 3. Thus, negative energies indicate a preference for the solvent bound structure. | |||
| [1TBP/2TBP/3TBP] + CH3CN | 0.0 | 0.0 | 0.0 |
| 1 Oh (triplet) | −0.6 | −4.7 | 3.5 |
| 1 Oh (quintet) | 2.2 | −4.3 | 2.7 |
| 2 Oh (triplet) | 1.6 | −3.6 | 4.5 |
| 2 Oh (quintet) | 0.9 | −6.5 | 0.2 |
| 3 Oh (triplet) | −7.5 | −11 | −3.6 |
| 3 Oh (quintet) | −1.0 | −3.4 | 3.3 |
However, a closer look into Table 1 reveals further insights. Note that the reference point (0.0 kcal mol−1 in Table 1) is set at the separated components of 1TBP, 2TBP and 3TBP in its quintet state + CH3CN; therefore, these energies express the bond dissociation energies (BDE) of the CH3CN ligand (see the equilibrium between [(L)FeIV(O)(CH3CN)]2+ and [(L)FeIV(O)]2+ in Fig. 1b). To correlate better to experiments, one may look at the free energies (ΔG, which here includes dispersion21) at the experimental temperatures, instead of ΔE. Assuming that ΔG is reliable here (see ESI,† ΔG vs. k2 comparison discussion), an interesting story develops. Mössbauer experiments at 4 K together with DFT calculations have indicated that all the FeIVO compounds are in an octahedral geometry, such as 1Oh, 2Oh and 3Oh (i.e., with one solvent molecule ligated).14,15,18 These results are in agreement with the current results in Table 1, which show that the octahedral complexes are stable at the free energy scale (ΔG) at 4 K. We also note that at 4 K, the experimentally determined spin states are also in agreement with the calculated ΔG (i.e., triplet for 1Oh and 3Oh and quintet for 2Oh). However, at a higher temperature (i.e., 233 K) where the reactivity studies were performed, ΔG values are positive for all except triplet 3Oh (see the column of ΔG at 233 K in Table 1). This indicates a possibility that the solvent ligand is released at some temperature higher than 4 K and the structure changes to a TBP structure in the cases of 1 and 2, where the quintet state is known to be dominant.8 Thus, although the preferred structure and spin state could not be determined spectroscopically at 233 K due to their instability (e.g., t1/2 < 1 min at 233 K), TBP structures should not be disregarded at 233 K. However, different from 1 and 2, species 3 is relatively stable at 233 K, and we were able to determine a magnetic moment of 3.1 μB for 3 at 233 K using Evans 1H NMR method22 and assigned a triplet spin state for 3 (see Methods section, ESI†). This result suggests the structure of 3Oh, since the triplet 3TBP structure + uncoordinated solvent is 11.4 kcal mol−1 higher in energy (Table S1, ESI†). This is in agreement with Table 1, showing that the triplet 3Oh is lower in energy than the quintet 3Oh. Hence, at face value, the relative free energies ΔG seem to be in agreement with experiments at every available and verifiable points in the present system up until this stage, such as the experimental results (i) and (ii) discussed above and experiments done here for 3Oh, even though the differences sometimes are within the error margins of the calculation methods.
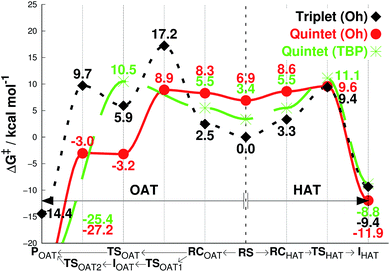
We then investigated the reactivities of 1, 2 and 3 with cyclohexene, allowing us to explore both OAT (i.e., C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C epoxidation) and HAT (i.e., allylic C–H activation).16 Since the usage of large basis set was not practical here, we used the smaller LACVP basis set23–25 for geometry optimizations and frequency calculations, including tunneling, with Def2-TZVPP20 used for single-point energy evaluation only (see Methods section, ESI†). As can be seen in Fig. 2a, the energy difference between quintet 1TBP and 1Oh at the center of graph (3.2 kcal mol−1) indicates that binding of a sixth ligand (i.e., CH3CN) to form an octahedral structure is endothermic with the 1Oh triplet and 1Oh quintet difference being within the round-off limits. These values are slightly different from that of Table 1 (3.5 and 2.7 kcal mol−1 for triplet and quintet, respectively) due to the smaller basis set used in the optimization; nevertheless, they both predict that a TBP structure is preferred at 233 K.
C epoxidation) and HAT (i.e., allylic C–H activation).16 Since the usage of large basis set was not practical here, we used the smaller LACVP basis set23–25 for geometry optimizations and frequency calculations, including tunneling, with Def2-TZVPP20 used for single-point energy evaluation only (see Methods section, ESI†). As can be seen in Fig. 2a, the energy difference between quintet 1TBP and 1Oh at the center of graph (3.2 kcal mol−1) indicates that binding of a sixth ligand (i.e., CH3CN) to form an octahedral structure is endothermic with the 1Oh triplet and 1Oh quintet difference being within the round-off limits. These values are slightly different from that of Table 1 (3.5 and 2.7 kcal mol−1 for triplet and quintet, respectively) due to the smaller basis set used in the optimization; nevertheless, they both predict that a TBP structure is preferred at 233 K.
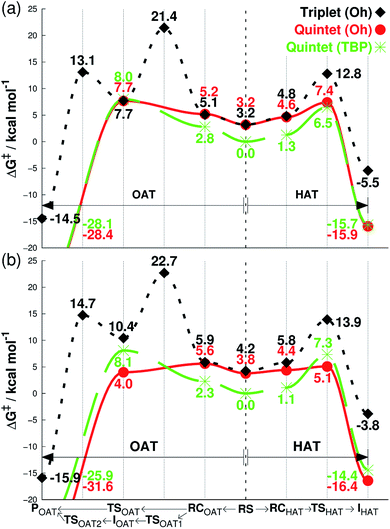 | ||
| Fig. 2 Free energies calculated at 233 K (ΔG, see Methods section for details†), starting from separated reactants in the center of the graph and performing OAT (going left) or HAT (going right) for the oxidation of cyclohexene by FeIVO complexes bearing (a) Me3NTB and (b) TQA ligands. The energy differences in the center between quintet (TBP) and other states correspond to the CH3CN binding energy as the sixth ligand. RS = reactants separated, RC = reactants complexed, TS = transition state, I = intermediate, P = product. | ||
Subsequent complexation with cyclohexene and OAT reaction occurs differently for the two spin states. With triplet 1Oh, OAT occurs in two steps, with one O–C bond formed before the other (Fig. 2a, going from the center to the left). The rate-limiting step is the first O–C bond formation with a barrier of 21.4 kcal mol−1. A substrate radical intermediate bound to 1Oh through the Fe–O–C bond is formed, and a second lower barrier (13.1 kcal mol−1) is crossed when forming the second O–C bond. In comparison, the quintet state features a concerted step where the two O–C bonds are formed at the same time. This occurs over a low barrier, such as 8.0 kcal mol−1 for 1TBP and 7.7 kcal mol−1 for 1Oh (Fig. 2a).
Similarly, the HAT reaction features a high triplet barrier (12.8 kcal mol−1) (Fig. 2a, going from the center to the right), compared to the quintet states (6.5 and 7.4 kcal mol−1 for 1TBP and 1Oh, respectively). This shows that the HAT reaction may actually be mediated by 1TBP rather than 1Oh. However, even if the structure somehow converts to a triplet 1Oh, a spin crossover could transpire to perform this reaction on the (much) more favorable quintet surface of 1Oh. Indeed, we found a minimum energy crossing point (MECP) between the two spin states very close to the reactant complex geometry (Tables S2 and S3, ESI†), showing that a spin cross-over is at least geometrically plausible. In addition, at the substrate radical intermediate stage where the catalyst is an FeIIIOH species, the intermediate complex is clearly energetically lower in its quintet state. Hence, the only thing that would prevent the reaction from utilizing the quintet pathway is an extremely low spin–orbit coupling, something that can be excluded by comparing the reactivity with a bona fide high-spin complex, such as 2Oh (vide infra). This corroborates the results found in the previous study that the reaction proceeds in the quintet surface regardless of being in 1TBP or 1Oh structure.14 In agreement with the experimental result (iii) described above, the calculations show that the lowest energy barrier in Fig. 2a is actually for the HAT reaction by 1TBP and that the lowest epoxidation barrier is slightly higher (i.e., by 1.2 kcal mol−1). Comparing the experimental k2 directly to experiments, the ΔG barrier is somewhat low, but as shown below, this seems to be a systematic trend, not affecting relative energy comparisons (see discussion in Methods section, ESI†).
For the substrate part, an important point found in this study is the number of equivalent C–H bonds on cyclohexene. While there may seem to be four equivalent C–H bonds in cyclohexene (two each in 2 and 5 positions, as removing any of these would result in equivalent cyclohexenyl radical), the two C–H bonds attached to the same carbon are not equivalent. This can be understood by the structure of cyclohexene, a half-chair conformation, where the two C–H bonds are not at equal distance to the 3- or 4-position carbons. Calculating the vertical single-point energy by removing one of these C–H bonds at time, at the cyclohexene geometry, will show that there is a vertical BDE difference between these two C–H bonds. This vertical energy difference has the potential to change the energy barriers of an HAT reaction.26 We found in this and a parallel study27 that the energy barrier difference in the HAT reaction when using these two C–H bonds is around 2 kcal mol−1 (see Tables S2 and S4, ESI†). Hence, the number of equivalent C–H bonds in cyclohexene is not 4 but 2, and it is potentially important to choose the “right” C–H bonds for calculations. We use the lower value of these two alternative reaction barriers henceforth. The substrate radical intermediate energy, however, is largely unaffected by the choice of C–H bonds, as the geometry optimization of the cyclohexenyl radical will allow the reorganization energy to compensate for the different vertical BDE.
As can be seen in Fig. 2b, the reaction energy profiles for OAT and HAT by 2TBP and 2Oh with cyclohexene are almost identical to those of 1TBP and 1Oh within a maximum absolute deviation of 3.7 kcal mol−1 for any of the individual values. Within this small discrepancy, however, we can still find some potentially important differences. For the OAT reaction, the lowest TS is the quintet 2Oh structure at 4.0 kcal mol−1. However, as the reactant complex lies energetically higher (a consequence of adding single-point entropy and dispersion effects; see Table S5, ESI†), the rate-limiting reaction barrier can be considered to be 5.6 kcal mol−1. The overall lowest energy barrier is therefore the HAT barrier performed by 2Oh at 5.1 kcal mol−1. The reacting species here is thus the octahedral structure, unlike in the case with the Me3NTB ligand (vide supra). At the same time, the experimental result (iii) described above is still corroborated. However, the close OAT and HAT barriers suggest that any small changes in the experimental setup, for instance deuteration of the substrate, can and does change the outcome of the reactions.16 Comparing the lowest barriers from Fig. 2a and b (6.5 versus 5.1 kcal mol−1), it can be seen that the energy barriers are similar, confirming the experimental result (iv) described above.
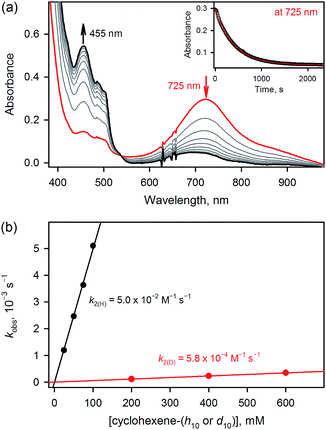
The results for species 3 are somewhat different. Our calculations correctly predict an intermediate-spin reactant state for 3Oh (Table 1), showing that the consistent high-spin preference of 1 and 2 is not a generic artifact of the calculation methods. In fact, 3TBP has in this system higher OAT and HAT barriers than the quintet 3Oh (Fig. 3). Even the triplet 3Oh is very competitive with the quintet state when it comes to HAT reaction, having a 0.2 kcal mol−1 lower barrier than the quintet 3Oh. It is therefore somewhat doubtful that it is energetically all that beneficial for 3Oh to do a spin flip for the reaction, as the gain would be just 0.5 kcal mol−1 at TS, in which case should lead to an OAT reaction rather than HAT. To shed light on this issue, we performed the oxidation reaction of cyclohexene-h10 and cyclohexene-d10 by 3 under an Ar atmosphere, as we have done for the oxidation of cyclohexene and cyclohexene-d10 by 1 and 2.16 As the experiments show that the majority products are from HAT reactions in the oxidation of cyclohexene-h10 (Table 2) with a very large kinetic isotope effect (KIE) value of ∼80 (see Table 3 and Fig. 4), a preliminary assessment is that this reaction goes on in the triplet surface due to low spin–orbit coupling as well as low energy gains, and in line with the very high KIE, which typify the S = 1 pathway.28 Any spin state flip could hence occur after the rate-limiting barrier step. This is similar to what was postulated in the case of the FeIVO complex bearing N4Py ligand.13 In addition, it is of interest to note that the reactivity patterns of the S = 1 and S = 2 FeIVO complexes in the cyclohexene-h10 oxidation were similar, such as nonheme FeIVO species prefer the C–H bond activation over the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C epoxidation irrespective of their S = 1 or S = 2 spin state.16 More interestingly, the reactivity of 3Oh is shown here to be more than 340 times slower than that of 1TBP and 2TBP in the oxidation of cyclohexene-h10 (Table 3). One possible explanation for this reactivity difference is the different spin states of those FeIVO species (i.e., S = 1 for 3Ohversus S = 2 for 1TBP and 2TBP); however, other factors, such as the supporting ligand and the structure of FeIVO complexes, may modulate the reactivity of nonheme FeIVO species in oxidation reactions.29
C epoxidation irrespective of their S = 1 or S = 2 spin state.16 More interestingly, the reactivity of 3Oh is shown here to be more than 340 times slower than that of 1TBP and 2TBP in the oxidation of cyclohexene-h10 (Table 3). One possible explanation for this reactivity difference is the different spin states of those FeIVO species (i.e., S = 1 for 3Ohversus S = 2 for 1TBP and 2TBP); however, other factors, such as the supporting ligand and the structure of FeIVO complexes, may modulate the reactivity of nonheme FeIVO species in oxidation reactions.29
| Complex | Substrate | Product yield (%) | ||
|---|---|---|---|---|
| –oxide | –ol | –one | ||
| a Reactions were run with intermediates (1.0 mM) and substrates (100 mM) under an Ar atmosphere in CH3CN at 233 K. ‘N.D.’ denotes ‘not detected’. b Product yields for 1 and 2 were taken from ref. 16. | ||||
| 1 | Cyclohexene-h10 | N.D. | 27(3) | 11(3) |
| Cyclohexene-d10 | 34(3) | 25(3) | 4(2) | |
| 2 | Cyclohexene-h10 | N.D. | 23(3) | 14(3) |
| Cyclohexene-d10 | 30(4) | 17(4) | 8(2) | |
| 3 | Cyclohexene-h10 | N.D. | 31(3) | 10(3) |
| Cyclohexene-d10 | 19(3) | 20(3) | 13(2) | |
Further, we have shown earlier that nonheme MIIIOH species (M = Cr, Mn, Fe and Ru)30 prefer a dissociative reaction pathway rather than a rebound or desaturation reaction pathway after an H-atom abstraction by MIVO species takes place.31 We therefore explored the rebound vs. dissociation pathways in the current cyclohexene HAT reactions, using the quintet TBP structure of [(Me3NTB)FeIIIOH]2+ (1TBP-OH) and the quintet Oh structure of [(TQA)FeIIIOH]2+ (2Oh-OH) and [(TPA)FeIIIOH(CH3CN)]2+ (3Oh-OH). Calculations show very low dissociation energies for the cyclohexene radical (Table 4), as found in the other nonheme MIIIOH cases.30 However, different from those MIIIOH cases, it is not immediately clear whether dissociation is favored in the current FeIIIOH cases. Here, the triplet values should be irrelevant as the substrate radical intermediate stage has a clear quintet ground state. In the case of 1TBP-OH, dissociation is then indeed favored over rebound by 2.1 kcal mol−1. For 2Oh-OH and 3Oh-OH, the differences are smaller (within the round off limits in case of 2Oh). Since the rebound barriers are so low in these cases, it is possible that dissociation reaction may not dominate completely. It is therefore an open question which way the reaction will go; it would depend on the precise reaction conditions favoring one way or another, as we have discussed in our recent review article.31
The current results need to be discussed in the context of existing error margins. How to obtain correct solvation entropy is an ongoing discussion;32 however, the method used here work well for our systems (see also results of an alternative method32e,33 in Table S6, ESI,† leading to the same conclusions). Our conclusion that both 1 and 2 perform their reactions in the quintet state should be solid enough due to the large energy differences in the rate-limiting barrier to the triplet value. There is some uncertainty in this issue in the case of 3 due to the closeness of the triplet and quintet TSs, but either path will be slower than the cases of 1 and 2. Nevertheless, the face values of ΔG for the calculations of 1, 2 and 3 are all in agreement with the experimental results (i)–(iv) as well as the experimental results obtained in this study. Similarly, the calculated TBP or Oh preference for the compounds at 4 K and 233 K is sometimes within the presumed error margins, but it does correctly predict the Oh structure of 1, 2 and 3 at 4 K and 3 at 233 K. Therefore, the TBP structure preference for 1 and 2 at 233 K should warrant serious considerations (Fig. 5).
Conclusions
We have shown that (1) DFT calculations correctly reproduce the experimental data (i)–(iv) reported previously, thus validating the methodology; (2) DFT calculations predict that the structure of 1 and 2 in solution at 233 K is TBP with a quintet spin state, due to a loss of a coordinating solvent ligand; (3) reactions of 1 and 2 therefore probably occur in the quintet spin state, explaining the similar reactivity rates and patterns for the reactions of 1 and 2;15,16 (4) DFT calculations predict that the structure of 3 is Oh with a triplet spin even at 233 K; (5) experiments performed with 3 are in agreement with the DFT calculations for the spin state, excluding a possibility that the computational results are due to inherent quintet spin bias in the methods; (6) experimental reactivities of 1 and 2 with the quintet spin state are much greater than that of 3 with the triplet spin state in oxidation reactions, proposing a possibility that the different reactivities result from the different spin states of FeIVO species; and (7) in the oxidation of cyclohexene-h10 by the S = 2 FeIVO complexes (i.e., 1 and 2) and the S = 1 FeIVO complex (i.e., 3), they all show the preference of the allylic C–H activation rather than the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C epoxidation.16 Finally, the present study highlights the possible influence of solvent and temperature to the structures and spin states of nonheme metal–oxo complexes synthesized in biomimetic model studies. This work is therefore predictive and at the same time explanatory for the previous reports on the nonheme S = 1 and S = 2 FeIVO complexes.14–16
C epoxidation.16 Finally, the present study highlights the possible influence of solvent and temperature to the structures and spin states of nonheme metal–oxo complexes synthesized in biomimetic model studies. This work is therefore predictive and at the same time explanatory for the previous reports on the nonheme S = 1 and S = 2 FeIVO complexes.14–16
Acknowledgements
The authors acknowledge financial support from the NRF of Korea through the CRI (NRF-2012R1A3A2048842 to W. N.), GRL (NRF-2010-00353 to W. N.) and MSIP (NRF-2013R1A1A2062737 to K.-B. C.). The Israel Science Foundation (ISF) is acknowledged by S. S. (ISF grant 1183/13).Notes and references
- J. C. Price, E. W. Barr, B. Tirupati, J. M. Bollinger Jr and C. Krebs, Biochemistry, 2003, 42, 7497 CrossRef CAS PubMed.
- L. M. Hoffart, E. W. Barr, R. B. Guyer, J. M. Bollinger Jr and C. Krebs, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 14738 CrossRef CAS PubMed.
- D. P. Galonic, E. W. Barr, C. T. Walsh, J. M. Bollinger Jr and C. Krebs, Nat. Chem. Biol., 2007, 3, 113 CrossRef CAS PubMed.
- S. D. Wong, M. Srnec, M. L. Matthews, L. V. Liu, Y. Kwak, K. Park, C. B. Bell III, E. E. Alp, J. Zhao, Y. Yoda, S. Kitao, M. Seto, C. Krebs, J. M. Bollinger Jr and E. I. Solomon, Nature, 2013, 499, 320 CrossRef CAS PubMed.
- C. Krebs, D. Galonić Fujimori, C. T. Walsh and J. M. Bollinger Jr, Acc. Chem. Res., 2007, 40, 484 CrossRef CAS PubMed.
- (a) X. Engelmann, I. Monte-Pérez and K. Ray, Angew. Chem., Int. Ed., 2016, 55, 7632 CrossRef CAS PubMed; (b) M. Puri and L. Que Jr, Acc. Chem. Res., 2015, 48, 2443 CrossRef CAS PubMed; (c) S. A. Cook and A. S. Borovik, Acc. Chem. Res., 2015, 48, 2407 CrossRef CAS PubMed; (d) W. Nam, Y.-M. Lee and S. Fukuzumi, Acc. Chem. Res., 2014, 47, 1146 CrossRef CAS PubMed; (e) A. R. McDonald and L. Que Jr, Coord. Chem. Rev., 2013, 257, 414 CrossRef CAS; (f) S. P. de Visser, J.-U. Rohde, Y.-M. Lee, J. Cho and W. Nam, Coord. Chem. Rev., 2013, 257, 381 CrossRef CAS.
- E. Andris, J. Jašík, L. Gómez, M. Costas and J. Roithová, Angew. Chem., Int. Ed., 2016, 55, 3637 CrossRef CAS PubMed.
- K.-B. Cho, S. Shaik and W. Nam, Chem. Commun., 2010, 46, 4511 RSC.
- D. Usharani, D. Janardanan, C. Li and S. Shaik, Acc. Chem. Res., 2013, 46, 471 CrossRef CAS PubMed.
- S. Shaik, H. Chen and D. Janardanan, Nat. Chem., 2012, 4, 511 CrossRef CAS.
- (a) S. Shaik, D. Danovich, A. Fiedler, D. Schröder and H. Schwarz, Helv. Chim. Acta, 1995, 78, 1393 CrossRef CAS; (b) H. Hirao, D. Kumar, L. Que Jr and S. Shaik, J. Am. Chem. Soc., 2006, 128, 8590 CrossRef CAS PubMed; (c) C. V. Sastri, J. Lee, K. Oh, Y. J. Lee, J. Lee, T. A. Jackson, K. Ray, H. Hirao, W. Shin, J. A. Halfen, J. Kim, L. Que Jr, S. Shaik and W. Nam, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 19181 CrossRef CAS PubMed; (d) H. Hirao, L. Que Jr, W. Nam and S. Shaik, Chem.–Eur. J., 2008, 14, 1740 CrossRef CAS PubMed.
- (a) T. Kojima, Y. Hirai, T. Ishizuka, Y. Shiota, K. Yoshizawa, K. Ikemura, T. Ogura and S. Fukuzumi, Angew. Chem., Int. Ed., 2010, 49, 8449 CrossRef CAS PubMed; (b) D. Schröder and S. Shaik, Angew. Chem., Int. Ed., 2011, 50, 3850 CrossRef PubMed; (c) T. Kojima and S. Fukuzumi, Angew. Chem., Int. Ed., 2011, 50, 3852 CrossRef CAS.
- Y. H. Kwon, B. K. Mai, Y.-M. Lee, S. N. Dhuri, D. Mandal, K.-B. Cho, Y. Kim, S. Shaik and W. Nam, J. Phys. Chem. Lett., 2015, 6, 1472 CrossRef CAS PubMed.
- M. S. Seo, N. H. Kim, K.-B. Cho, J. E. So, S. K. Park, M. Clémancey, R. Garcia-Serres, J.-M. Latour, S. Shaik and W. Nam, Chem. Sci., 2011, 2, 1039 RSC.
- A. N. Biswas, M. Puri, K. K. Meier, W. N. Oloo, G. T. Rohde, E. L. Bominaar, E. Münck and L. Que Jr, J. Am. Chem. Soc., 2015, 137, 2428 CrossRef CAS PubMed.
- S. H. Bae, M. S. Seo, Y.-M. Lee, K.-B. Cho, W.-S. Kim and W. Nam, Angew. Chem., Int. Ed., 2016, 55, 8027 CrossRef CAS PubMed.
- W. Kohn and L. J. Sham, Phys. Rev., 1965, 140, A1133 CrossRef.
- M. H. Lim, J.-U. Rohde, A. Stubna, M. R. Bukowski, M. Costas, R. Y. N. Ho, E. Münck, W. Nam and L. Que Jr, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 3665 CrossRef CAS PubMed.
- (a) A. D. Becke, Phys. Rev. A: At., Mol., Opt. Phys., 1988, 38, 3098 CrossRef CAS; (b) A. D. Becke, J. Chem. Phys., 1993, 98, 1372 CrossRef CAS; (c) A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS; (d) C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297 RSC.
- S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456 CrossRef CAS PubMed.
- (a) D. F. Evans, J. Chem. Soc., 1959, 2003 RSC; (b) D. F. Evans and D. A. Jakubovic, J. Chem. Soc., Dalton Trans., 1988, 2927 RSC.
- (a) P. J. Hay and W. R. Wadt, J. Chem. Phys., 1985, 82, 270 CrossRef CAS; (b) P. J. Hay and W. R. Wadt, J. Chem. Phys., 1985, 82, 299 CrossRef CAS.
- A. D. Bochevarov, E. Harder, T. F. Hughes, J. R. Greenwood, D. A. Braden, D. M. Philipp, D. Rinaldo, M. D. Halls, J. Zhang and R. A. Friesner, Int. J. Quantum Chem., 2013, 113, 2110 CrossRef CAS.
- (a) W. J. Hehre, R. Ditchfield and J. A. Pople, J. Chem. Phys., 1972, 56, 2257 CrossRef CAS; (b) M. M. Francl, W. J. Pietro, W. J. Hehre, J. S. Binkley, M. S. Gordon, D. J. DeFrees and J. A. Pople, J. Chem. Phys., 1982, 77, 3654 CrossRef CAS.
- W. Lai, C. Li, H. Chen and S. Shaik, Angew. Chem., Int. Ed., 2012, 51, 5556 CrossRef CAS PubMed.
- R. Gupta, X.-X. Li, K.-B. Cho, M. Guo, Y.-M. Lee, Y. Wang, S. Fukuzumi and W. Nam, J. Phys. Chem. Lett., 2017, 8, 1557 CrossRef CAS PubMed.
- D. Mandal, R. Ramanan, D. Usharani, D. Janardanan, B. Wang and S. Shaik, J. Am. Chem. Soc., 2015, 137, 722 CrossRef CAS PubMed.
- (a) G. Xue, R. De Hont, E. Münck and L. Que Jr, Nat. Chem., 2010, 2, 400 CrossRef CAS PubMed; (b) D. J. Xiao, E. D. Bloch, J. A. Mason, W. L. Queen, M. R. Hudson, N. Planas, J. Borycz, A. L. Dzubak, P. Verma, K. Lee, F. Bonino, V. Crocellà, J. Yano, S. Bordiga, D. G. Truhlar, L. Gagliardi, C. M. Brown and J. R. Long, Nat. Chem., 2014, 6, 590 CrossRef CAS PubMed.
- (a) K.-B. Cho, H. Kang, J. Woo, Y. J. Park, M. S. Seo, J. Cho and W. Nam, Inorg. Chem., 2014, 53, 645 CrossRef CAS PubMed; (b) K.-B. Cho, S. Shaik and W. Nam, J. Phys. Chem. Lett., 2012, 3, 2851 CrossRef CAS; (c) K.-B. Cho, X. Wu, Y.-M. Lee, Y. H. Kwon, S. Shaik and W. Nam, J. Am. Chem. Soc., 2012, 134, 20222 CrossRef CAS PubMed; (d) S. N. Dhuri, K.-B. Cho, Y.-M. Lee, S. Y. Shin, J. H. Kim, D. Mandal, S. Shaik and W. Nam, J. Am. Chem. Soc., 2015, 137, 8623 CrossRef CAS PubMed.
- K.-B. Cho, H. Hirao, S. Shaik and W. Nam, Chem. Soc. Rev., 2016, 45, 1197 RSC.
- (a) J. Ho, A. Klamt and M. L. Coote, J. Phys. Chem. A, 2010, 114, 13442 CrossRef CAS PubMed; (b) C. J. Cramer and D. G. Truhlar, Acc. Chem. Res., 2008, 41, 760 CrossRef CAS PubMed; (c) A. Klamt, B. Mennucci, J. Tomasi, V. Barone, C. Curutchet, M. Orozco and F. J. Luque, Acc. Chem. Res., 2009, 42, 489 CrossRef CAS PubMed; (d) R. C. Harris and B. M. Pettitt, J. Chem. Theory Comput., 2015, 11, 4593 CrossRef CAS PubMed; (e) R. F. Ribeiro, A. V. Marenich, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. B, 2011, 115, 14556 CrossRef CAS PubMed.
- B. B. Averkiev and D. G. Truhlar, Catal. Sci. Technol., 2011, 1, 1526 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Methods section, Tables S1–S8, all the data for DFT calculations (Tables S9–S53 for energies, Mulliken spin density distribution, and selected geometries), and coordinates. See DOI: 10.1039/c7sc01738c |
| ‡ Present address: CSIR-Indian Institute of Toxicological Research, Lucknow 226005, India. |
| This journal is © The Royal Society of Chemistry 2017 |