Open Access Article
Open Access ArticleRacemic hemiacetals as oxygen-centered pronucleophiles triggering cascade 1,4-addition/Michael reaction through dynamic kinetic resolution under iminium catalysis. Development and mechanistic insights†
Ane
Orue
a,
Uxue
Uria
a,
David
Roca-López
b,
Ignacio
Delso
c,
Efraím
Reyes
a,
Luisa
Carrillo
 a,
Pedro
Merino
*b and
Jose L.
Vicario
a,
Pedro
Merino
*b and
Jose L.
Vicario
 *a
*a
aDepartment of Organic Chemistry II, University of the Basque Country (UPV/EHU), P.O. Box 644, 48080 Bilbao, Spain
bDepartamento de Síntesis y Estructura de Biomoléculas, Instituto de Síntesis Química y Catálisis Homogénea (ISQCH), Universidad de Zaragoza, CSIC, Spain
cServicio de Resonancia Magnética Nuclear, Centro de Química y Materiales de Aragón (CEQMA), Universidad de Zaragoza, CSIC, Spain
First published on 30th January 2017
Abstract
2-Hydroxydihydropyran-5-ones behave as excellent polyfunctional reagents able to react with enals through oxa-Michael/Michael process cascade under the combination of iminium and enamine catalysis. These racemic hemiacetalic compounds are used as unconventional O-pronucleophiles in the initial oxa-Michael reaction, also leading to the formation of a single stereoisomer under a dynamic kinetic resolution (DKR) process. Importantly, by using β-aryl or β-alkyl substituted α,β-unsaturated substrates as initial Michael acceptors either kinetically or thermodynamically controlled diastereoisomers were formed with high stereoselection through the careful selection of the reaction conditions. Finally, a complete experimental and computational study confirmed the initially proposed DKR process during the catalytic oxa-Michael/Michael cascade reaction and also explained the kinetic/thermodynamic pathway operating in each case.
Introduction
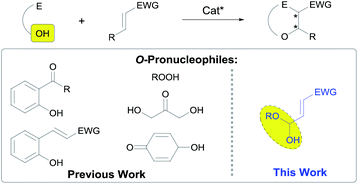
The asymmetric conjugate addition of heteroatom-centered nucleophiles to α,β-unsaturated carbonyl compounds is one of the leading strategies for the enantio- and/or diastereoselective preparation of β-functionalized carbonyl compounds.1 Moreover, the mechanistic profile associated to the conjugate addition reaction makes this transformation an excellent platform to develop cascade reactions.2 This opens the possibility for the construction of complex molecular architectures from simple starting materials. With respect to stereocontrol, enantioselective catalysis, and more specifically, the proven ability of chiral secondary amines3 to promote cascade reactions using enals as Michael acceptors, through the combination of the nowadays well established iminium and enamine activation manifolds is a good example of the high level of sophistication achieved by this chemistry.4In this sense, many examples of enantioselective cascade processes triggered by hetero-Michael reactions exist in the literature,5 although reactions initiated by the conjugate addition of oxygen-centered nucleophiles remain scarce.6 In fact, this type of reactions still represent one of the most challenging cases of conjugate addition of heteroatom-centered nucleophiles under organocatalytic activation because, in addition to reversibility issues, the typical oxygen-centered pronucleophiles, such as alcohols or carboxylic acids, typically show very poor reactivity towards Michael acceptors and normally undergo competitive 1,2-addition. Consequently, the reported examples of organocatalytic and enantioselective conjugate addition of oxygen pronucleophiles are limited to the use of oximes,7 phenols,8 enols,9 alkoxyboronates,10 hydroxylamines,11 or peroxides12 under iminium or H-bonding activation.13 Organocatalytic cascade processes initiated by conjugate addition of O-nucleophiles have been mainly focused on the epoxidation of α,β-unsaturated carbonyls with peroxides14 and some limited examples of conjugate addition followed by aldol,15 Mannich,16 Henry,17 α-amination18 and α-fluorination19 have also been published. However, with respect to 1,4-addition/Michael cascade processes, all cases reported are strictly limited to the use of aliphatic alcohols or phenols as oxygen-centered pronucleophiles (Scheme 1).20
Consequently, we have focused our attention in the use of hemiacetals as potential pronucleophiles in cascade reactions initiated by 1,4-addition. Hemiacetals play a critical role in the chemistry of living cells and this type of compounds is also known to play the role of O-pronucleophiles in biochemical pathways of the secondary metabolism. In particular, the intramolecular conjugate addition of hemiacetals is typically associated with the stereodefined formation of acetal moieties in the total synthesis of complex natural products with relevant biological activities.21 Remarkably, there is only one single example (a diastereoselective approach) that involves the use of a enantioenriched hemiacetal as Michael donor, performing with moderate stereocontrol.22 On the other hand, the intramolecular conjugate addition of in situ generated hemiacetals using functionalized Michael acceptors that incorporate an internal alcohol moiety to assist the reaction has also been reported in both diastereo-23 and enantioselective24 fashion, although the latter relies on the stereocontrolled formation of the chiral hemiacetal moiety under catalyst control, that subsequently undergoes the intramolecular O-1,4-addition reaction. Finally, and quite recently, the catalytic and enantioselective intramolecular conjugate addition of peroxyhemiacetals to dienones has also been described.25 Despite these advances, the conjugate addition of hemiacetal entities to electron-poor alkenes in an intermolecular fashion to initiate a cascade reaction is unprecedented in the chemical literature.
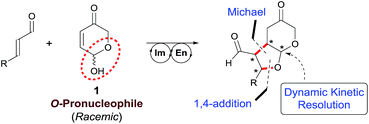
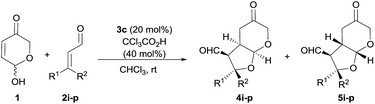
In particular, we wish to present herein a detailed study regarding the ability of hydroxypyranone 1 as an active chiral racemic O-pronucleophile that undergoes diastereo- and enantioselective cascade 1,4-addition/intramolecular Michael reaction with α,β-unsaturated aldehydes in the presence of a chiral secondary amine catalyst, leading to the formation of bicyclic furo[2,3-b]pyranes in a single step (Scheme 2). As far as we are aware of, this is the first example in which a hemiacetal shows its ability to participate as oxygen-based Michael donor in a stereoselective conjugate addition reaction under the combination of iminium and enamine activation manifolds. Moreover, this transformation occurs with the concomitant generation of up to four stereogenic centers in stereocontrolled way using a racemic starting material, in which a dynamic kinetic resolution (DKR) has also taken place to furnish the final adducts as highly enantio- and diastereoenriched compounds.
Results and discussion
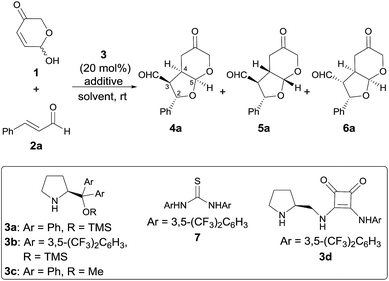
Our studies began by surveying reaction conditions for the projected transformation to proceed with the best yield and stereoselectivity employing (E)-cinnamaldehyde as model substrate (Table 1). We first tested the performance of the well-known O-TMS diphenylprolinol catalyst 3a,26 observing the formation of the expected bicyclic furo[2,3-b]pyranes as a mixture of three diastereoisomers out of the eight possible ones (Table 1, entry 1). An analysis of their stereostructure27 showed that the reaction had proceeded with complete control at the C2 stereocenter, which indicated that the catalyst was able to perfectly control the facial selectivity in the initial 1,4-addition reaction. Compounds 4a and 6a differ on the absolute configuration of the stereochemically labile C3 stereocenter, assuming that the latter was formed after thermodynamic epimerization of the former (a detailed discussion will be provided in the following sections). Finally, the presence of diastereoisomer 5a should be attributed to the participation of the other enantiomer of the racemic starting material 1. It should be noted that an excess of hemiacetal 1 was used in order to achieve full conversion after 18 h but when the reaction was carried out using equimolar amounts of 1 and 2, it proceeded with exactly the same diastereo- and enantiocontrol.28 Moreover, in all cases, the recovered starting material 1 was found to be racemic. Consequently, the observed yield, the high (6a + 4a)/5a ratio and the excellent e.e. obtained for 6a is indicating that a kinetic resolution is operating throughout the overall reaction, which encouraged us to search for improved conditions that could favour the selective formation of major diastereoisomer 6a. Consequently, we next tested the performance of modified catalyst 3b (entry 2), but it was found to be inactive in this transformation, while the use of less bulky O-Me derivative 3c turned into poorer enantiocontrol for the major product 6a (entry 3). The incorporation of Brønsted acids or bases as additives did not end up in any significant improvement (entries 4 and 5). Remarkably, using achiral thiourea 7 as co-catalyst, which had been previously used by us for the activation of Michael acceptors in reactions under dienamine activation,29 led to an important improvement in the yield of the reaction (entry 6). In addition, the formation of 4a could be minimized by stirring the crude reaction mixture with aqueous HCl for 4 h to favour the thermodynamic equilibration of the 4a/6a mixture (entry 7). We also tested bifunctional catalyst 3d, not observing better results (entry 8). Other solvents (entries 9 and 10) and temperatures (entries 11 and 12) were also surveyed, without observing any significant improvement and therefore, it was concluded that the conditions summarized in entry 7 of Table 1 were the most appropriate ones for this transformation.| Entry | 3 | Additive | Solvent | Yieldb (%) |
4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5a 5a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6ac 6ac |
e.e.d (%) |
|---|---|---|---|---|---|---|
| a Reactions were carried out using 0.4 mmol of 1 and 0.2 mmol of 2a, 20 mol% of catalyst 3 and 10 mol% of additive in 0.8 mL of CHCl3 at rt for 18 h, followed by direct purification by flash chromatography. b Combined yield for the mixture of diastereoisomers. c Determined by 1H NMR spectroscopy of crude reaction mixture. d Determined by HPLC after derivatization (see ESI). e n.d.: not determined. f Once the reaction was finished a 4 M HCl solution (2.0 mL) was added to the crude reaction mixture and this was stirred for further 4 h before standard work-up and purification. g Reaction carried out at 4 °C. h Reaction carried out at 45 °C. | ||||||
| 1 | 3a | None | CHCl3 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
91/70/91 |
| 2 | 3b | None | CHCl3 | <5 | — | — |
| 3 | 3c | None | CHCl3 | 43 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
n.d.e/38/50 |
| 4 | 3a | PhCO2H | CHCl3 | 46 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
90/60/95 |
| 5 | 3a | DABCO | CHCl3 | 67 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90/78/90 |
| 6 | 3a | 7 | CHCl3 | 80 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
67/67/95 |
| 7f | 3a | 7 | CHCl3 | 74 | <0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
n.d.e/60/92 |
| 8 | 3d | None | CHCl3 | <5 | — | — |
| 9f | 3a | 7 | Toluene | 62 | <0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
n.d.e/20/94 |
| 10f | 3a | 7 | CH2Cl2 | 43 | <0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
n.d.e/38/90 |
| 11f,g | 3a | 7 | CHCl3 | 45 | <0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
n.d.e/60/95 |
| 12f,h | 3a | 7 | CHCl3 | 75 | <0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
n.d.e/64/93 |
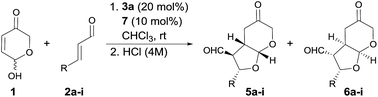
With the optimal conditions in hands, the scope of the reaction was studied with respect to the use of other α,β-unsaturated aldehydes 2a–i. As summarized in Table 2, the reaction proceeded well in all cases in which cinnamaldehyde derivatives incorporating either electron-withdrawing or electron-donating groups at the β-aryl substituent were employed (entries 1–7). In all these cases, adducts 6a–g were isolated with good yields, high diastereoselectivities and as highly enantioenriched materials. β-Heteroaryl substituents were also well tolerated, observing that the reaction performed equally well in terms of chemical yield and stereocontrol (entry 8). On the other hand, when we turned to the use of a β-alkyl substituted enal such as 2-hexenal, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixture of the three diastereoisomers was obtained with an overall yield of 87% (entry 9). Unfortunately, in this case the acidic workup was ineffective for converting diastereoisomer 4i into 6i,30 which also indicated that β-alkyl substituted enals behave differently from cinnamaldehyde derivatives with respect to diastereoselection. Remarkably, the formation of diastereoisomer 5 was negligible in all cases, which indicated that the DKR process was operating with high efficiency. We tried to improve the diastereoselectivity of the reaction between 2i and 1, by surveying other conditions but without any success (see ESI† for details). Remarkably, when catalyst 3c was employed together with trichloroacetic acid as additive, the reaction again turned to be highly diastereo- and enantioselective, in this case favouring the formation of the kinetic product 4i (entry 10). This product showed to be less prone to C3-epimerization compared with the cases in which β-aryl substituted enals were used and all attempts to induce the 4i to 6i isomerization under acidic or basic conditions led to decomposition.
2 mixture of the three diastereoisomers was obtained with an overall yield of 87% (entry 9). Unfortunately, in this case the acidic workup was ineffective for converting diastereoisomer 4i into 6i,30 which also indicated that β-alkyl substituted enals behave differently from cinnamaldehyde derivatives with respect to diastereoselection. Remarkably, the formation of diastereoisomer 5 was negligible in all cases, which indicated that the DKR process was operating with high efficiency. We tried to improve the diastereoselectivity of the reaction between 2i and 1, by surveying other conditions but without any success (see ESI† for details). Remarkably, when catalyst 3c was employed together with trichloroacetic acid as additive, the reaction again turned to be highly diastereo- and enantioselective, in this case favouring the formation of the kinetic product 4i (entry 10). This product showed to be less prone to C3-epimerization compared with the cases in which β-aryl substituted enals were used and all attempts to induce the 4i to 6i isomerization under acidic or basic conditions led to decomposition.
| Entry | R (product) | Yield (%) |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6a 6a |
e.e. 6b (%) |
|---|---|---|---|---|
a Determined by 1H NMR spectroscopy of crude reaction mixture.
b Determined by HPLC (see ESI).
c Reaction also furnished diastereoisomer 4i (4i/5i/6i ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).
d Reaction conditions: catalyst 3c (20 mol%), Cl3CCO2H (40 mol%) in CHCl3 at rt.
e
4i/5i ratio is indicated.
f e.e. of 4i is given. 2).
d Reaction conditions: catalyst 3c (20 mol%), Cl3CCO2H (40 mol%) in CHCl3 at rt.
e
4i/5i ratio is indicated.
f e.e. of 4i is given.
|
||||
| 1 | Ph (6a) | 74 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
95 |
| 2 | 4-(OMe)C6H4 (6b) | 63 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
86 |
| 3 | 4-FC6H4 (6c) | 73 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
92 |
| 4 | 4-(NO2)C6H4 (6d) | 78 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
92 |
| 5 | 4-BrC6H4 (6e) | 87 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
88 |
| 6 | 2-(OMe)C6H4 (6f) | 61 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
94 |
| 7 | 2-(NO2)C6H4 (6g) | 71 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
92 |
| 8 | 2-(Furyl) (6h) | 64 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
97 |
| 9 | n-Pr (6i) | 87 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2c 2c |
84 |
| 10d | n-Pr (4i) | 76 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1e 1e |
93f |
Once we had realized that β-alkyl substituted enals required the use of catalyst 3c, we proceeded to extend those conditions to a variety of substrates (Table 3). As it can be seen in this table, the reaction tolerates well the use of enals containing alkyl chains of different length and size, also observing that the yield of the process became only moderately affected when the length of the chain was considerably increased (entries 1–5). Aldehyde 2n containing a bulkier non-linear alkyl chain also performed well, although enantioselectivity was slightly affected (compound 4n) and a functionalized α,β-unsaturated aldehyde such as 2o also performed well, furnishing 4o in good yield and stereocontrol (entry 7). Furthermore, β,β-disubstituted aldehyde 2p was also reactive under these conditions, forming adduct 4p in good yield, although with moderate diastereo- and enantioselectivity (entry 8). Contrary to the reaction with cinnamaldehyde derivatives shown before, the formation of diastereoisomer 6 was not observed in any of the cases. The absolute configuration of compounds 4l and 6a was unambiguously assigned by X-ray analysis and it was extended to all obtained products 4 and 6 based on mechanistic analogy.
| Entry | R 1, R2 (product) | Yield (%) |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5a 5a |
e.e. 4b (%) |
|---|---|---|---|---|
| a Determined by 1H NMR spectroscopy of crude reaction mixture. b Determined by HPLC (see ESI). | ||||
| 1 | n-Pr, H (4i) | 76 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
93 |
| 2 | Et, H (4j) | 72 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92 |
| 3 | n-Bu, H (4k) | 69 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
94 |
| 4 | n-C5H11, H (4l) | 73 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
95 |
| 5 | n-C6H13, H (4m) | 61 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
96 |
| 6 | i-Pr, H (4n) | 59 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
84 |
| 7 | (Z)-EtCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(CH2)2, H (4o) CH(CH2)2, H (4o) |
60 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
96 |
| 8 | Me, Me (4p) | 73 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
77 |
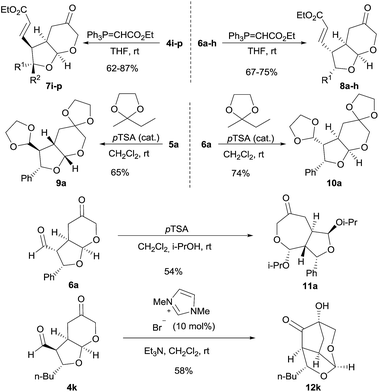
Bicyclic furo[2,3-b]pyranes 4 and 6 showed to be somewhat unstable compounds because of their densely functionalized nature, and therefore, and for better characterization purposes, we accomplished their conversion into a more stable and easy-to-handle derivative after isolation. Attempts to carry out the selective reduction of the formyl group led to extensive decomposition but Wittig olefination took place smoothly leading to the formation of adducts 7i–p and 8a–h which were stable compounds that could be stored for several weeks without decomposition (Scheme 3). In addition, we also decided to explore some possible transformations to be carried out on the obtained bicyclic adducts, in order to illustrate their potential applications as chiral building blocks in organic synthesis. For example, the fully protection of the formyl and the ketone moieties in adduct 6a could be carried out by reaction with 2-ethyl-2-methyl-1,3-dioxolane under Brønsted acid catalysis yielding cleanly compound 10a. This transformation was also carried out on the minor diastereoisomeric byproduct 5a, isolating compound 9a in good yield and for which crystals could be grown that also allowed the unequivocal assignment of its absolute configuration. Alternatively, the reaction of 6a with p-TSA in propan-2-ol led to the formation of compound 11a after an acid-catalyzed transketalization process, which took place with full chemoselectivity considering the presence of two chemically differentiated formyl units in the starting material. On the other hand, intramolecular benzoin condensation under NHC catalysis was also carried out on adduct 4k, leading to compound 12k which was isolated in good yield as a single diastereoisomer. This reaction involved the activation of the formyl moiety by condensation with the carbene catalysts followed by addition of the nucleophilic Breslow intermediate to the ketone moiety, generating a quaternary carbinol with complete diastereocontrol.
Mechanistic considerations
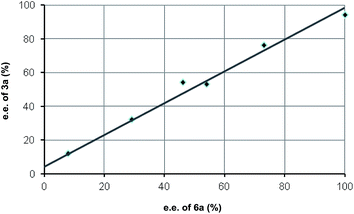
In order to get a good mechanistic understanding on the behavior of the reaction, we decided to carry out a detailed experimental and computational study, mainly directed to understand the origin of the difference in the diastereoselectivity of this cascade process when β-aryl or β-alkyl-substituted Michael acceptors were employed as substrates, and also trying to understand the pathway operating in the resolution process, which is key for the very high stereocontrol. In this sense, and considering the presence of multiple reactive positions in the starting materials in which the amine catalyst can incorporate through either covalent bonding or via H-bonding interactions, we firstly carried out some experiments directed to discard the possible participation of complex reaction intermediates in which two or more molecules of the catalyst could be incorporated. In this sense, the process did not show any significant non-linear behavior when the reaction between 1 and 2a using catalyst 3a with a variable enantiopurity (Fig. 1). This is a clear indication that only one molecule of catalyst is involved in the rate determining step of the overall catalytic cycle.We next moved to study the different mechanistic possibilities with the help of computational methods (for details see ESI†). Firstly, we set a preliminary computational study with an achiral system in order to establish the overall mechanistic picture and the preferred relative configuration of the products. Considering that the reaction consists on a cascade 1,4-addition/Michael process we initially worked under the hypothesis that the first step should be considered as the stereodefining step of the overall process. We started by calculating the uncatalyzed background reaction between 1 and 2a. But the energy barrier of the process was found to be 20.0 kcal mol−1. We also considered the participation of the catalyst by forming an encounter complex EC1 with both aldehyde and alcohol without formation of the iminium salt as suggested by Lattanzi, and co-workers for the epoxidation of enones catalyzed by diarylprolinols.12c After formation of the encounter complex at −25.9 kcal mol−1, the corresponding transition structure was located 3.0 kcal mol−1 below the ground state (for details on these approaches see ESI†).
We next moved to study the reaction between 1 and 2a under covalent activation by pyrrolidine following the well-known combination of iminium and enamine activation manifolds (Scheme 4). We arbitrarily chose the addition of hemiacetal (R)-1 to the Si face of the iminium ion intermediate.31 We also considered the exclusive participation of the (E,E)-s-trans iminium species in agreement with previous reports.32 Isomeric (Z,E)-iminium salts were also considered but they turned out to be more energetic, as expected (see ESI†). The study was initiated with the formation of encounter complex EC2 (located at −22.3 kcal mol−1 with respect to the reactants) that can be considered as the initial point of a catalytic cycle in which both reactants are activated before the formation of the iminium ion. Evolution of EC2 through TS3 afforded hemiaminal IN1 from which IN2 is formed through TS4 (energy barrier of TS4 2.0 kcal mol−1) assisted by the hemiacetal reagent 1. IN2 should actually be regarded as a reactive complex formed by the iminium salt, the alcohol and the hydroxyl anion eliminated from the hemiaminal leading to the formation of the C–O bond through the initial conjugate addition. The corresponding transition structure TS5, leading to intermediate enamine ENa, was located at 13.8 kcal mol−1 with respect to EC2 (−8.5 kcal mol−1 with respect to the reagents). Such energy barrier is lower than alternative uncatalyzed TS1 (20 kcal mol−1) and non-covalent activated TS2 (−3.0 kcal mol−1), confirming that formation of EC2 is the preferred starting point for the catalytic cycle. It should be taken into consideration that even in achiral series, the addition of two enantiomeric alcohols (R)-1 and (S)-1 by one face of the iminium ion renders two diastereomers. Consequently, this step is determinant for the diastereoselectivity of the reaction involving a kinetic resolution of the two alcohols. We determined during this preliminary analysis that TS5-(R), consisting of the attack of (R)-1 by the Si face of the iminium ion, was the preferred one leading to enamine ENa-(S,S) predicting a relative dr of ca. 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with respect to the addition of (S)-1 leading to ENb-(S,R) (for details see ESI†). Further discussion will be based on this preferred route. Once the initial 1,4-addition reaction has taken place, the second step of the cascade consists in an intramolecular Michael reaction between the enamine and the α,β-unsaturated ketone moiety occurring after migration of the coordinated water (ENa to ENb). The intramolecular Michael reaction on enamine intermediate ENb can take place through the two diastereotopic faces of the pyranone scaffold33 and consequently two transition structures can be found, from which TS6a (located at −15.3 kcal mol−1) was calculated to be the most stable one. Remarkably, these calculations also indicated that the initial 1,4-addition step (associated to TS5) resulted to be the rate-determining step of the overall reaction (ΔGTS5 > ΔGTS6a). Finally, regeneration of activated reactants in EC2 from IN3a should take place through intermediates IN4a and IN5. The energy diagram for the catalytic cycle illustrated in Scheme 4 is given in Fig. 2. From this initial study carried out on this achiral model system it can be concluded that the preferred diastereoselectivity for the reaction will lead to the formation of the final product P1a with a relative 2S*, 3S*, 3aR*, 7aS* configuration. The minor diastereomer (2S*, 3S*, 3aS*, 7aR*) should be formed from the opposite enantiomer of the pronucleophile.
1 with respect to the addition of (S)-1 leading to ENb-(S,R) (for details see ESI†). Further discussion will be based on this preferred route. Once the initial 1,4-addition reaction has taken place, the second step of the cascade consists in an intramolecular Michael reaction between the enamine and the α,β-unsaturated ketone moiety occurring after migration of the coordinated water (ENa to ENb). The intramolecular Michael reaction on enamine intermediate ENb can take place through the two diastereotopic faces of the pyranone scaffold33 and consequently two transition structures can be found, from which TS6a (located at −15.3 kcal mol−1) was calculated to be the most stable one. Remarkably, these calculations also indicated that the initial 1,4-addition step (associated to TS5) resulted to be the rate-determining step of the overall reaction (ΔGTS5 > ΔGTS6a). Finally, regeneration of activated reactants in EC2 from IN3a should take place through intermediates IN4a and IN5. The energy diagram for the catalytic cycle illustrated in Scheme 4 is given in Fig. 2. From this initial study carried out on this achiral model system it can be concluded that the preferred diastereoselectivity for the reaction will lead to the formation of the final product P1a with a relative 2S*, 3S*, 3aR*, 7aS* configuration. The minor diastereomer (2S*, 3S*, 3aS*, 7aR*) should be formed from the opposite enantiomer of the pronucleophile.
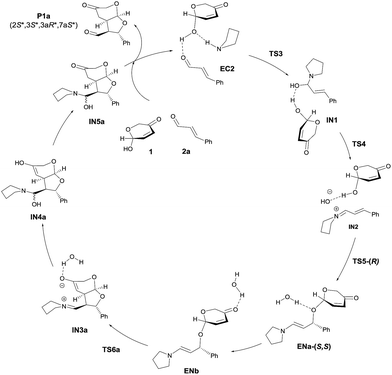 | ||
| Scheme 4 Calculated catalytic cycle for the model reaction between 1 and 2a catalyzed by pyrrolidine (achiral model). | ||
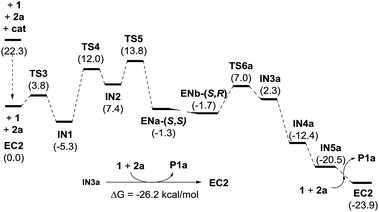 | ||
| Fig. 2 Energy diagram of the catalytic cycle illustrated in Scheme 4. | ||
For the analysis of the selectivity of the reaction and prediction of the absolute configuration of the different products formed, the whole real system was considered, involving the participation of chiral catalyst 3a. The study was restricted to the key rate-limiting step found for the achiral model system, i.e. the 1,4-addition of hemiacetal 1 to the iminium ion derived from 2a, since the size of the model is in the limit of computational resources.34 Catalyst 3a is known to operate according to a steric model26,35 in reactions proceeding through both enamine and iminium intermediates, and particularly when these intermediates are involved in Michael-type reactions.36 As a consequence, only the attack through the less hindered faces of these intermediates (iminium ion or enamine) have been considered in agreement with previous calculations for other reactions.26 We also considered the (E)-iminium ion (with respect to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond) as the most reactive isomer according to previous reports by Seebach and co-workers.35 Therefore and for the complete study, we considered as possible variable structural parameters, the participation of the catalyst through north and south conformations at the pyrrolidine ring, two different diastereoisomers (Z and E) of the C
N bond) as the most reactive isomer according to previous reports by Seebach and co-workers.35 Therefore and for the complete study, we considered as possible variable structural parameters, the participation of the catalyst through north and south conformations at the pyrrolidine ring, two different diastereoisomers (Z and E) of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of the iminium ion intermediate (resulting into the reaction with the Michael donor 1 through a different facial selectivity) and the participation of the two enantiomeric hemiacetal reagents 1 (in order to evaluate kinetic resolution). Thus, a total of 8 TS were located and characterized (see ESI† for details). Boltzmann analysis for these transition structures predicted a 91
C bond of the iminium ion intermediate (resulting into the reaction with the Michael donor 1 through a different facial selectivity) and the participation of the two enantiomeric hemiacetal reagents 1 (in order to evaluate kinetic resolution). Thus, a total of 8 TS were located and characterized (see ESI† for details). Boltzmann analysis for these transition structures predicted a 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ratio of products 4
9 ratio of products 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
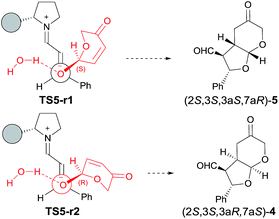
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, in good agreement with experimental observations (considering that product 4 subsequently epimerizes onto 6, see below). The two preferred transition structures TS5-r1 and TS5-r2 leading to 5 and 4, respectively, are shown in Fig. 3 (for geometries see ESI†).
5, in good agreement with experimental observations (considering that product 4 subsequently epimerizes onto 6, see below). The two preferred transition structures TS5-r1 and TS5-r2 leading to 5 and 4, respectively, are shown in Fig. 3 (for geometries see ESI†).
The difference in energy of 1.6 kcal mol−1 observed between TS5-r1 and TS5-r2 can be explained on the basis of a combination of electronic effects and steric interactions. In fact, 1,4-addition is facilitated by the loss of the proton of the OH group of the hemiacetal to form a water molecule upon combination with the hydroxyl anion. NCI analysis (see ESI†) shows that, in the transition state, the negative charge is located between the water oxygen atom and the attacking oxygen of 1 (actually, most of the negative charge is located at the oxygen atom of 1; which should be interpreted as the driving force for the nucleophilic attack). The partial negative charge located at the incipient water molecule should be close to the iminium nitrogen atom which has a partial positive charge. This electrostatic interaction forces the orientation of the attack of the hydroxyl group, which should release the proton to form water. According to this model calculations predict a kinetic resolution in favour of the attack of (R)-1 to give compound 4 (which might epimerize, see below) as the major isomer.
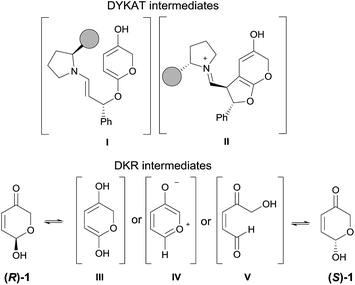
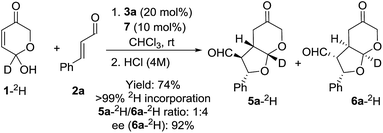
The mechanistic picture presented up to this moment involves the occurrence of a DKR process through the fast racemization of the starting hemiacetal reagent 1 before it gets involved in the cascade 1,4-addition/Michael reaction.37 However, a potential DYKAT cannot be discarded as the epimerization of the 7a position could also take place after the 1,4-addition process (through the potential formation of a dienol intermediate under the intermediacy of the Brønsted acid co-catalyst 7, see DYKAT intermediates I and II in Scheme 5). In order to rule out this possibility, the resolution process of the starting hydroxypyranone 1 was studied experimentally. For this purpose, we carried out the reaction between cinnamaldehyde (2a) and deuterated hydroxypyranone 1 under optimized conditions, observing the formation of products 5a-2H and 6a-2H in good yield and with an excellent enantiocontrol (Scheme 6).
The fact that the deuterium atom present at the starting material had been preserved in the same position across the overall cascade reaction confirmed our initial hypothesis that a DKR process was operating and that the possible intermediates that could operate through a hypothetical DYKAT process (DYKAT intermediates I and II) were not formed in the reaction. The racemization of the starting material could eventually occur through the participation of dienol intermediate III, through oxidopyrylium ylide IV or, alternatively, via ring-opening of the hemiacetal moiety through intermediate V. The experiment using deuterated substrate 1 shown in Scheme 6 directly rules out the possible participation of intermediate III, but it does not provide any evidence to support the participation of either IV or V. In order to discern between IV and V as the intermediates involved in the racemization process we carried out another computational study through the several channels involving those intermediates and under the intermediacy of the Brønsted acid. For that purpose, this process was initially modelled by using formic acid as promoter of the reaction.
From the several paths studied (see ESI† for details) there is a clear preference for the open-chain intermediate V which is located at 3.5 kcal mol−1 with respect to compound 1. Intermediate IV is located at 20 kcal mol−1. The corresponding barriers leading to V and IV were found to be 11.9 and 21.3 kcal mol−1, respectively. We also calculated a more realistic transition structure leading to intermediate V promoted by either water or thiourea 7 for which energy barriers of 21.1 and 28.8 kcal mol−1 were found (see ESI†). Consequently, this computational study shows that the calculated barrier for the racemization of 1 is 11.9 kcal mol−1, which is lower than the following rate-determining step (13.8 kcal mol−1) associated to the oxa-Michael/Michael cascade sequence, thus being consistent with a DKR process. The proposed racemization of 1 through an open-chain intermediate is in good agreement with highly accurate recent studies on mutarrotation of D-glucose in water as a solvent.38
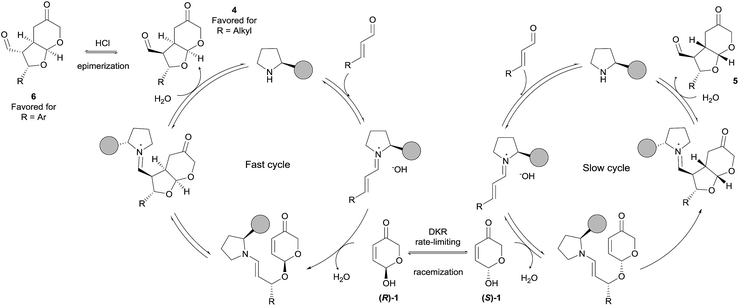
Taking into account all experimental and computational investigations, we propose a catalytic cycle (Scheme 7) based on the known iminium/enamine activation manifold starting with the conjugate addition of the hemiacetal moiety of pyranone 1 with the intermediate iminium ion that installs the first stereocenter and in which one of the enantiomers of 1 is reacting faster than the other. This type of 1,4-additions of O-nucleophiles with enals under iminium activation has been proposed to be reversible processes,39 which means that in our case it must be followed by a fast intramolecular Michael reaction that generates the other two stereocenters. Once the product has been released and catalyst regenerated, preferential formation of diastereoisomer 4 should occur. Finally, calculations predict the formation of isomer 4 (2S, 3S, 3aR, 7aS) as the major one, and compound 5 (2S, 3S, 3aS, 7aR) as the minor one. However, when β-aryl substituted enals were employed as substrates mixtures of 5 and 6 were experimentally obtained, the latter having a (2S, 3R, 3aS, 7aR) configuration, and being this isomer obtained almost exclusively after in situ acidic treatment (see Table 2). This behaviour, together with the data obtained by calculations suggests that, once isomer 4 is formed, it undergoes fast epimerization at the α-position to the formyl substituent. Experimentally, epimerization of 4 into 6 has been observed under acidic conditions for those substrates containing aryl substituents (R = Ph). On the other hand, for adducts incorporating alkyl substituents at that position (R = Et), epimerization was observed in rather less extent after treatment with hydrochloric acid, and no epimerization at all was detected when the reaction was carried out in the presence of trichloroacetic acid.40
In order to understand this atypical behaviour, an exhaustive conformational analysis showed that epimerization of 4 into 6 is thermodynamically favoured independently of the nature (aromatic vs. aliphatic) of the substituent. This 4 to 6 epimerization process was subsequently studied in detail using DFT calculations in order to determine the energy barrier and to analyze the kinetics of the process (see ESI†). The preferred pathway corresponds to the formation of (E)-enols and energy barriers showed that epimerization of adducts containing an aryl substituent (R = Ph) is favoured by 1.5 kcal mol−1 with respect to those containing an alkyl group at the same position (R = Et). Accordingly, epimerization of 4a is kinetically favoured and it should be expected that epimerization of 4a to 6a takes place faster than that of 4j to 6j.
A complete epimerization could not be achieved in the reaction of aliphatic enals because they trend to decompose under treatment with aqueous HCl. Indeed, by using 1 M HCl (pKa < 1) a slower epimerization was observed for 4j (see Table 2, entry 9) with respect to 4a, which epimerized completely. Other acids like benzoic acid (pKa = 4.19) and trichloroacetic acid (pKa = 0.77) were not acidic enough to promote epimerization at an appreciable rate. In fact, modelization using formic acid (pKa = 3.75) provided energy barriers of ca. 22 kcal mol−1 which are in the limit for a spontaneous process at room temperature (see ESI†).41
Conclusions
The enantio- and diastereoselective synthesis of furopyranes has been developed through a transformation that operates under a dynamic kinetic resolution process of involving a hemiacetal reagent as unconventional oxygen-centered Michael donor that undergoes oxa-Michael/Michael cascade reaction with enals in the presence of a chiral secondary amine catalyst. This transformation constitutes a rare example of cascade iminium/enamine reaction with an unconventional oxygen pronucleophile that involves the stereocontrolled formation of one single enantiomer over 16 possible stereoisomers. Experimental studies together with computational methods demonstrate that the initial racemization of the hemiacetal occurs through an open-chain pathway before the catalytic cycle takes place. A final epimerization in the stereocenter incorporating the formyl group also takes place when the thermodynamically more stable compound can be isolated without decomposition.Acknowledgements
This research was supported by the Spanish MINECO (FEDER-CTQ2014-52107-P, FEDER-CTQ2013-44367-C2-1-P and FEDER-CTQ2016-76155-R), the Basque Government (Grupos IT908-16), UPV/EHU (UFI QOSYC 11/22) and Government of Aragón (Grupos Consolidados, E.10). D. R.-L. thanks MEC for a pre-doctoral FPU fellowship. The authors thankfully acknowledge the resources from the supercomputers "Memento" and “Cierzo”, technical expertise and assistance provided by BIFI-ZCAM (Universidad de Zaragoza, Spain).Notes and references
- (a) E. Reyes, U. Uria, J. L. Vicario and L. Carrillo, Org. React., 2016, 90, 1 Search PubMed; (b) Catalytic Asymmetric Conjugate Reactions, ed. A. Cordova, Wiley-VCH, Weinheim, 2010 Search PubMed; (c) Comprehensive Asymmetric Catalysis, ed. E. N. Jacobsen, A. Pfaltz and Y. Yamamoto, Springer-Verlag, Berlin, vol. 3, 1999 Search PubMed.
- For some selected reviews, see: (a) Y. Hayashi, Chem. Sci., 2016, 7, 866 RSC; (b) J. Muzart, Tetrahedron, 2013, 69, 6735 CrossRef CAS; (c) H. Pellissier, Chem. Rev., 2013, 113, 442 CrossRef CAS PubMed; (d) C. J. Ball and M. C. Willis, Eur. J. Org. Chem., 2013, 425 CrossRef CAS; (e) E. Ricca, B. Brucher and J. H. Schrittwieser, Adv. Synth. Catal., 2011, 353, 2239 CrossRef CAS; (f) E. A. Anderson, Org. Biomol. Chem., 2011, 9, 3997 RSC; (g) T. Vlaar, E. Ruijter and R. V. A. Orru, Adv. Synth. Catal., 2011, 353, 809 CrossRef CAS; (h) K. C. Nicolaou and J. S. Chen, Chem. Soc. Rev., 2009, 38, 2993 RSC.
- For some selected reviews specifically focused on aminocatalysis, see: (a) B. M. Paz, H. Jiang and K. A. Jørgensen, Chem.–Eur. J., 2015, 21, 1846 CrossRef PubMed; (b) M. C. Holland and R. Gilmour, Angew. Chem., Int. Ed., 2015, 54, 3862 CrossRef CAS PubMed; (c) P. Melchiorre, Angew. Chem., Int. Ed., 2012, 51, 9748 CrossRef CAS PubMed; (d) P. Melchiorre, M. Marigo, A. Carlone and G. Bartoli, Angew. Chem., Int. Ed., 2008, 47, 6138 CrossRef CAS PubMed; (e) B. List, Chem. Commun., 2006, 819 RSC.
- For some reviews, see: (a) C. Volla, I. Atodiresei and M. Rueping, Chem. Rev., 2014, 114, 2390 CrossRef CAS PubMed; (b) Y.-C. Chen and H.-L. Cui, Organocatalytic Cascade Reactions in Science of Synthesis, Asymmetric Organocatalysis, ed. B. List and K. Maruoka, Thieme: Stuttgart, 2012, vol. 2, p. 787 Search PubMed; (c) D. Bonne, T. Constantieux, Y. Coquerel and J. Rodriguez, Org. Biomol. Chem., 2012, 10, 3969 RSC; (d) H. Pellissier, Adv. Synth. Catal., 2012, 354, 237 CrossRef CAS; (e) A. Grossmann and D. Enders, Angew. Chem., Int. Ed., 2012, 51, 314 CrossRef CAS PubMed; (f) B. Westermann, M. Ayaz and S. S. van Berkel, Angew. Chem., Int. Ed., 2010, 49, 846 CrossRef CAS PubMed; (g) C. Grondal, M. Jeanty and D. Enders, Nat. Chem., 2010, 2, 167 CrossRef CAS PubMed.
- For reviews, see: (a) Z. Galestokova and R. Sebesta, Eur. J. Org. Chem., 2012, 6688 CrossRef CAS; (b) M. Ruiz, P. Lopez-Alvarado, G. Giorgi and J. C. Menendez, Chem. Soc. Rev., 2011, 40, 3445 RSC; (c) D. Bonne, Y. Coquerel, T. Constantieux and J. Rodriguez, Tetrahedron: Asymmetry, 2010, 21, 1085 CrossRef CAS; (d) H. Lebel, J. F. Marcoux, C. Molinaro and A. B. Charette, Chem. Rev., 2003, 103, 977 CrossRef CAS PubMed; (e) D. Caine, Tetrahedron, 2001, 57, 2643 CrossRef CAS.
- (a) C. F. Nising and S. Brase, Chem. Soc. Rev., 2012, 41, 988 RSC; (b) C. F. Nising and S. Brase, Chem. Soc. Rev., 2008, 37, 1218 RSC.
- (a) S. Bertelsen, P. Diner, R. L. Johansen and K. A. Jørgensen, J. Am. Chem. Soc., 2007, 129, 1536 CrossRef CAS PubMed; (b) A. Pohjakallio, P. M. Pihko and U. M. Laitinen, Chem.–Eur. J., 2010, 16, 11325 CrossRef CAS PubMed; (c) F.-L. Liu, J.-R. Chen, B. Feng, X.-Q. Hu, L.-H. Ye, L.-Q. Lu and W.-J. Xiao, Org. Biomol. Chem., 2014, 12, 1057 RSC.
- (a) Y. Kobayashi, R. Kuramoto and Y. Takemoto, Beilstein J. Org. Chem., 2015, 11, 2654 CrossRef CAS PubMed; (b) Y.-L. Zhang and Y.-Q. Wang, Tetrahedron Lett., 2014, 55, 3255 CrossRef CAS; (c) P. H. Poulsen, K. S. Feu, B. M. Paz, F. Jensen and K. A. Jørgensen, Angew. Chem., Int. Ed., 2015, 54, 8203 CrossRef CAS PubMed; for the use of heteroaromatic alcohols see: (d) A. K. Ghosh, X. Cheng and B. Zhou, Org. Lett., 2012, 14, 5046 CrossRef CAS PubMed; (e) D. Enders, A. Grossmann, B. Gieraths, M. Duezdemir and C. Merkens, Org. Lett., 2012, 14, 4254 CrossRef CAS PubMed; (f) E. Sekino, T. Kumamoto, T. Tanaka, T. Ikeda and T. Ishikawa, J. Org. Chem., 2004, 69, 2760 CrossRef CAS PubMed.
- B. Parhi, J. Gurjar, S. Pramanik, A. Midya and P. Ghorai, J. Org. Chem., 2016, 81, 4654 CrossRef CAS PubMed.
- (a) B. Ravindra, S. Maity, B. G. Das and P. Ghorai, J. Org. Chem., 2015, 80, 7008 CrossRef CAS PubMed; (b) T. Azuma, A. Murata, Y. Kobayashi, T. Inokuma and Y. Takemoto, Org. Lett., 2014, 16, 4256 CrossRef CAS PubMed.
- (a) Y. Kobayashi, Y. Taniguchi, N. Hayama, T. Inokuma and Y. Takemoto, Angew. Chem., Int. Ed., 2013, 52, 11114 CrossRef CAS PubMed; (b) R. Noël, V. Gembus, V. Levacher and J.-F. Briere, Org. Biomol. Chem., 2014, 12, 1245 RSC; (c) Q.-Y. Dou, Y.-Q. Tu, Y. Zhang, J.-M. Tian, F.-M. Zhang and S.-H. Wang, Adv. Synth. Catal., 2016, 358, 874 CrossRef CAS.
- (a) C. M. Reisinger, X. Wang and B. List, Angew. Chem., Int. Ed., 2008, 47, 8112 CrossRef CAS PubMed; (b) X. Lu, Y. Liu, B. Sun, B. Cindric and L. Deng, J. Am. Chem. Soc., 2008, 130, 8134 CrossRef CAS PubMed. See also: (c) A. Russo and A. Lattanzi, Adv. Synth. Catal., 2008, 350, 1991 CrossRef CAS.
- Some examples regarding the use of aliphatic alcohols as O-pronucleophiles in intramolecular versions: (a) Y. Fukata, R. Miyaji, T. Okamura, K. Asano and S. Matsubara, Synthesis, 2013, 45, 1627 CrossRef CAS; (b) Y. Lu, G. Zou and G. Zhao, ACS Catal., 2013, 3, 1356 CrossRef CAS; (c) W. Wu, X. Li, H. Huang, X. Yuan, J. Lu, K. Zhu and J. Ye, Angew. Chem., Int. Ed., 2013, 52, 1743 CrossRef CAS PubMed; (d) M. O. Ratnikov, L. E. Farkas and M. P. Doyle, J. Org. Chem., 2012, 77, 10294 CrossRef CAS PubMed; for an earlier example of attempted amine-catalyzed conjugate addition of aliphatic alcohols to enals furnishing low levels of enantioselection, see: (e) D. Díez, M. G. Nuñez, A. Benéitez, R. F. Moro, I. S. Marcos, P. Basabe, H. Broughton and J. G. Urones, Synlett, 2009, 390 CrossRef; (f) T. Kano, Y. Tanaka and K. Maruoka, Tetrahedron, 2007, 63, 8658 CrossRef CAS.
- (a) O. Lifchits, M. Mahlau, C. M. Reisinger, A. Lee, C. Fares, I. Polyak, G. Gopakumar, W. Thiel and B. List, J. Am. Chem. Soc., 2013, 135, 6677 CrossRef CAS PubMed; (b) M. Marigo, J. Franzen, T. B. Poulsen, W. Zhuang and K. A. Jørgensen, J. Am. Chem. Soc., 2005, 127, 6964 CrossRef CAS PubMed; (c) S. Lee and D. W. C. MacMillan, Tetrahedron, 2006, 62, 11413 CrossRef CAS; (d) C. Sparr, W. B. Schweizer, H. M. Senn and R. Gilmour, Angew. Chem., Int. Ed., 2009, 48, 3065 CrossRef CAS PubMed; (e) X. Lu, Y. Liu, B. Sun, B. Cindric and L. Deng, J. Am. Chem. Soc., 2008, 130, 8134 CrossRef CAS PubMed; (f) X. Wang, C. M. Reisinger and B. List, J. Am. Chem. Soc., 2008, 130, 6070 CrossRef CAS PubMed; (g) J. Vesely, I. Ibrahem, G.-L. Zhao, R. Rios and A. Cordova, Angew. Chem., Int. Ed., 2007, 46, 778 CrossRef CAS PubMed; (h) F. Pesciaioli, F. De Vicentiis, P. Galzerano, G. Bencivenni, G. Bartoli, A. Mazzanti and P. Melchiorre, Angew. Chem., Int. Ed., 2008, 47, 8703 CrossRef CAS PubMed; (i) A. Armstrong, C. A. Baxter, S. G. Lamont, A. R. Pape and R. Wincewicz, Org. Lett., 2007, 9, 351 CrossRef CAS PubMed; (j) E. J. Corey and F.-Y. Zhang, Org. Lett., 1999, 1, 1287 CrossRef CAS PubMed.
- (a) J. Zhang, M. J. Ajitha, L. He, K. Liu, B. Dai and K.-W. Huang, Adv. Synth. Catal., 2015, 357, 967 CrossRef CAS; (b) Y.-H. Feng, R.-S. Luo, L. Nie, J. Weng and G. Lu, Tetrahedron: Asymmetry, 2014, 25, 523 CrossRef; (c) J. Aleman, C. Alvarado, V. Marcos, A. Nunez and J. L. Garcia-Ruano, Synthesis, 2011, 1840 CrossRef CAS; (d) C.-L. Liu, X.-S. Zhang, R. Wang and W. Wang, Org. Lett., 2010, 12, 4948 CrossRef CAS PubMed; (e) B. C. Das, S. Mohapatra, P. D. Campbell, S. Nayak, S. M. Mahalingam and T. Evans, Tetrahedron Lett., 2010, 51, 2567 CrossRef CAS PubMed; (f) S.-P. Luo, Z.-B. Li, L.-P. Wang, Y. Guo, A.-B. Xia and D.-Q. Xu, Org. Biomol. Chem., 2009, 7, 4539 RSC; (g) E. Reyes, G. Talavera, J. L. Vicario, D. Badia and L. Carrillo, Angew. Chem., Int. Ed., 2009, 48, 5701 CrossRef CAS PubMed; (h) T. Govender, L. Hojabri, F. M. Moghaddam and P. I. Arvidsson, Tetrahedron: Asymmetry, 2006, 17, 1763 CrossRef CAS; (i) S. Sunden, I. Ibrahem, G.-L. Zhao, A. Eriksson and A. Cordova, Chem.–Eur. J., 2007, 13, 574 CrossRef PubMed.
- (a) J. Aleman, A. Nunez, L. Marzo, V. Marcos, C. Alvarado and J. L. Garcia Ruano, Chem.–Eur. J., 2010, 16, 9453 CrossRef CAS PubMed; (b) A.-B. Xia, D.-Q. Xu, S.-P. Luo, J.-R. Jiang, J. Tang, Y.-F. Wang and Z.-Y. Xu, Chem.–Eur. J., 2010, 16, 801 CrossRef CAS PubMed.
- (a) B. Zheng, W. Hou and Y. Peng, ChemCatChem, 2014, 6, 2527 CrossRef CAS; (b) G. Yin, R. Zhang, L. Li, J. Tian and L. Chen, Eur. J. Org. Chem., 2013, 5431 CrossRef CAS; (c) Z. Zhang, G. Jakab and P. R. Schreiner, Synlett, 2011, 1262 Search PubMed.
- J. Weng, L.-J. Huang, L. Long, L.-Y. Xu and G. Lu, Tetrahedron Lett., 2016, 57, 2554 CrossRef CAS.
- H.-F. Wang, H.-F. Cui, Z. Chai, P. Li, C.-W. Zheng, Y.-Q. Yang and G. Zhao, Chem.–Eur. J., 2009, 15, 13299 CrossRef CAS PubMed.
- (a) P. Saha, A. Biswas, N. Molleti and V. K. Singh, J. Org. Chem., 2015, 80, 11115 CrossRef CAS PubMed; (b) A.-B. Xia, C. Wu, T. Wang, Y.-P. Zhang, X.-H. Du, A.-G. Zhong, D.-Q. Xu and Z.-Y. Xu, Adv. Synth. Catal., 2014, 356, 1753 CrossRef CAS; (c) Y. Huang, C. Zheng, Z. Chai and G. Zhao, Adv. Synth. Catal., 2014, 356, 579 CrossRef CAS; (d) H. Mao, A. Lin, Y. Tang, Y. Shi, H. Hu, Y. Cheng and C. Zhu, Org. Lett., 2013, 15, 4062 CrossRef CAS PubMed; (e) M. T. Corbett and J. S. Johnson, Chem. Sci., 2013, 4, 2828 RSC; (f) P. G. McGarraugh and S. E. Brenner-Moyer, Org. Lett., 2011, 13, 6460 CrossRef CAS PubMed; (g) H. Wang, J. Luo, X. Han and Y. Lu, Adv. Synth. Catal., 2011, 353, 2971 CrossRef CAS; (h) L. Zu, S. Zhang, H. Xie and W. Wang, Org. Lett., 2009, 11, 1627 CrossRef CAS PubMed; (i) F.-L. Zhang, A.-W. Xu, Y.-F. Gong, M.-H. Wei and X.-L. Yang, Chem.–Eur. J., 2009, 15, 6815 CrossRef CAS PubMed.
- For some selected examples, see: (a) K. C. Nicolaou, E. A. Theodorakis, F. P. J. T. Rutjes, J. Tiebes, M. Sato, E. Untersteller and X.-Y. Xiao, J. Am. Chem. Soc., 1995, 117, 1171 CrossRef CAS; (b) R. M. de Figueiredo, R. Fröhlich and M. Christmann, Angew. Chem., Int. Ed., 2007, 46, 2883 CrossRef CAS PubMed; (c) L. K. Geisler, S. Nguyen and C. J. Forsyth, Org. Lett., 2004, 6, 4159 CrossRef CAS PubMed; (d) S. Sommer and H. Waldmann, Chem. Commun., 2005, 5684 RSC; (e) M. T. Crimmins and P. Siliphaivanh, Org. Lett., 2003, 5, 4641 CrossRef CAS PubMed; (f) C. F. Nising, U. K. Ohnemüller and S. Bräse, Angew. Chem., Int. Ed., 2006, 45, 307 CrossRef CAS PubMed; (g) A. Matviitsuk, F. Berndt and R. Mahrwald, Org. Lett., 2014, 16, 5474 CrossRef CAS PubMed; (h) D. A. Evans, P. Nagorny, D. J. Reynolds and K. J. McRae, Angew. Chem., Int. Ed., 2007, 46, 541 CrossRef CAS PubMed; (i) T. A. Dineen and W. R. Roush, Org. Lett., 2004, 6, 2043 CrossRef CAS PubMed; (j) D. T. Hung, J. B. Nerenberg and S. L. Schreiber, J. Am. Chem. Soc., 1996, 118, 11054 CrossRef CAS.
- C. Xong, A. Ovens, W. Pilling, J. W. Ward and D. J. Dixon, Org. Lett., 2008, 10, 565 CrossRef PubMed.
- For some examples, see: (a) D. A. Evans and J. A. Gauchet-Prunet, J. Org. Chem., 1993, 58, 2446 CrossRef CAS; (b) P. A. Evans, A. Grisin and M. J. Lawler, J. Am. Chem. Soc., 2012, 134, 2856 CrossRef CAS PubMed; (c) A. Grisin, S. Oliver, M. D. Ganton, J. Bacsa and P. A. Evans, Chem. Commun., 2015, 51, 15681 RSC; (d) L. Becerra-Figueroa, E. Brun, M. Mathieson, L. J. Farrugia, C. Wilson, J. Prunet and D. Gamba-Sanchez, Org. Biomol. Chem., 2017, 15, 301 RSC.
- (a) T. Okamura, K. Asano and S. Matsubara, Chem. Commun., 2012, 48, 5076 RSC; (b) A. Matsumoto, K. Asano and S. Matsubara, Chem. Commun., 2015, 51, 11693 RSC; (c) N. Yoneda, A. Hotta, K. Asano and S. Matsubara, Org. Lett., 2014, 16, 6264 CrossRef CAS PubMed.
- (a) D. M. Rubush, M. A. Morges, B. J. Rose, D. H. Thamm and T. Rovis, J. Am. Chem. Soc., 2012, 134, 13554 CrossRef CAS PubMed; (b) S. Maity, B. Parhi and P. Ghorai, Angew. Chem., Int. Ed., 2016, 55, 7723 CrossRef CAS PubMed.
- First reports: (a) M. Marigo, T. C. Wabnitz, D. Fielenbach and K. A. Jorgensen, Angew. Chem., Int. Ed., 2005, 44, 794 CrossRef CAS PubMed; (b) H. Gotoh, T. Hayashi and M. Shoji, Angew. Chem., Int. Ed., 2005, 44, 4212 CrossRef PubMed. For some reviews: (c) B. S. Donslund, T. K. Johansen, P. H. Poulsen, K. S. Halskov and K. A. Jørgensen, Angew. Chem., Int. Ed., 2015, 54, 13860 CrossRef CAS PubMed; (d) S. Meninno and A. Lattanzi, Chem. Commun., 2013, 49, 3821 RSC; (e) K. L. Jensen, G. Dickmeiss, H. Jiang, L. Albrecht and K. A. Jørgensen, Acc. Chem. Res., 2012, 45, 248 CrossRef CAS PubMed; (f) A. Mielgo and C. Palomo, Chem.–Asian J., 2008, 3, 922 CrossRef CAS PubMed.
- Determined by X-ray analysis of several adducts prepared when extending the reaction to other enals see ESI.†.
- Yields were typically 10–15% lower when the reaction was quenched at the same time using 1 and 2 in a 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio.
1 ratio. - (a) E. Reyes, G. Talavera, J. L. Vicario, D. Badia and L. Carrillo, Angew. Chem., Int. Ed., 2009, 48, 5701 CrossRef CAS PubMed; (b) N. Z. Burns, M. R. Witten and E. N. Jacobsen, J. Am. Chem. Soc., 2011, 133, 14578 CrossRef CAS PubMed; (c) M. R. Witten and E. N. Jacobsen, Angew. Chem., Int. Ed., 2014, 53, 5912 CrossRef CAS PubMed.
- Stirring a purified sample of compound 4i in different acidic media (aqueous or water-free) led to decomposition of the product, only detecting the presence of traces of the desired diastereoisomer 6i after few minutes.
- Water was selected as model structure of the Brønsted acid cocatalyst (either trichloroacetic acid or thiourea 7) because it is a more computationally tractable surrogate that can be can used in a neutral form conserving both donor and acceptor H-bond capabilities. Introduction of a carboxylate anion would cause unbalance of charges that would introduce significant errors in calculations. For the reactions using thiourea 7 as additive, this has no effect on the stereoselectivity.
- (a) D. Seebach, U. Groselj, D. M. Badine, W. B. Schweizer and A. K. Beck, Helv. Chim. Acta, 2008, 91, 1999 CrossRef CAS; (b) U. Groselj, D. Seebach, D. M. Badine, W. B. Schweizer, A. K. Beck, I. Krossing, P. Klose, Y. Hayashi and T. Uchimaru, Helv. Chim. Acta, 2009, 92, 1225 CrossRef CAS.
- Technically, the two faces of the enamine should also be considered but this is not possible due to steric hindrance.
- The fully optimized structures for the real system using cc-pVTZ basis set resulted in 1808 basis functions. For a discussion on current advances and limits in computational methods, see: A. Armstrong, R. A. Boto, P. Dingwall, J. Contreras-Garcia, M. J. Harvey, N. J. Masona and H. S. Rzepa, Chem. Sci., 2014, 5, 2057 RSC.
- Y. Hayashi, D. Okamura, T. Yamazaki, Y. Ameda, H. Gotoh, S. Tsuzuki, T. Uchimaru and D. Seebach, Chem.–Eur. J., 2014, 20, 17077 CrossRef CAS PubMed.
- (a) S. Bertelsen, M. Marigo, S. Brandes, P. Diner and K. A. Jørgensen, J. Am. Chem. Soc., 2006, 128, 12973 CrossRef CAS PubMed; (b) S. Reboredo, E. Reyes, J. L. Vicario, D. Badia, L. Carrillo, A. Cozar and F. Cossio, Chem.–Eur. J., 2012, 18, 7179 CrossRef CAS PubMed . See also ref. 26.
- For examples regarding the use of dihydropyranone acetals undergoing DKR processes, see: (a) A. Orue, E. Reyes, J. L. Vicario, L. Carrillo and U. Uria, Org. Lett., 2012, 14, 3740 CrossRef CAS PubMed; (b) H.-Y. Wang, K. Yang, S. R. Bennett, S. Guo and W. Tang, Angew. Chem., Int. Ed., 2015, 54, 8756 CrossRef CAS PubMed.
- (a) A. M. Silva, E. C. da Silva and C. O. da Silva, Carbohydr. Res., 2006, 341, 1029 CrossRef CAS PubMed , and references cited therein; (b) S. Morpugo, A. Grandi, C. Zazza and M. Bossa, THEOCHEM, 2005, 729, 71 CrossRef; (c) X. Qian, J. Phys. Chem. B, 2013, 117, 11460 CrossRef CAS PubMed; for a definitive computational demonstration by using molecular simulations based on the combination of DFT methodology with the molecular dynamics formalism, see: (d) W. Plazinski, A. Plazinska and M. Drach, Phys. Chem. Chem. Phys., 2015, 17, 21622 RSC.
- P. G. McGarraugh, R. C. Johnston, A. Martinez-Muñoz, P. H.-Y. Cheong and S. E. Brenner-Moyer, Chem.–Eur. J., 2012, 18, 10742 CrossRef CAS PubMed , see also ref. 20f.
- Adducts with bulkier alkyl substituents at this position decomposed upon treatment with aqueous HCl.
- A process with an energy barrier of 20 kcal mol−1 has an interconversion rate (k) of 1.3 × 10−2 s−1 at 25 °C, i.e. a half-life τ1/2 of 53 seconds. (half-life τ1/2 is the time it takes 50% of the molecules to cross the barrier).
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and characterization of all new compounds, including copies of NMR spectra and HPLC chromatograms traces, computational details and Cartesian coordinates of all stationary points. CCDC 1525188 (4l), 1525189 (6a) and 1525190 (9a). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc00009j |
| This journal is © The Royal Society of Chemistry 2017 |