Finding the connections between a high-school chemistry curriculum and nano-scale science and technology
Ron
Blonder
 * and
Sohair
Sakhnini
* and
Sohair
Sakhnini
Department of Science Teaching, Weizmann Institute of Science, Rehovot, Israel. E-mail: Ron.blonder@weizmann.ac.il
First published on 2nd September 2017
Abstract
The high-school chemistry curriculum is loaded with many important chemical concepts that are taught at the high-school level and it is therefore very difficult to add modern contents to the existing curriculum. However, many studies have underscored the importance of integrating modern chemistry contents such as nanotechnology into a high-school curriculum. When students are exposed to nanotechnology, they perceive chemistry as more relevant to their life, and more modern than the chemistry they usually study at school, and consequently, their continuous motivation to study chemistry and related subjects increases. In the current study we identified topics in the high-school chemistry curriculum in Israel into which the essential nano-scale science and technology (NST) concepts can be integrated. Insertion points for all 8 NST essential concepts were found. We discuss the importance of ways in which chemistry educators can implement the results for updating the chemistry curriculum, thus making it more modern and relevant to the actual chemistry research that is conducted.
Introduction
Nowadays, nano-scale science and technology (NST) is one of the major research fields in chemistry (NAP, 2016). Although the nature of the NST field is interdisciplinary, chemists play essential roles in advancing NST research. Chemists create new nanomaterials and synthesize them. They characterize the new nanomaterials and provide them with unique functionality that enables them to be applied in nanotechnology applications (Jones et al., 2013; Sakhnini and Blonder, 2015). There are many new families of nanomaterials. The most famous are the carbon nanoparticle family, which includes fullerenes, graphene, and different carbon nanotubes. However, inorganic nanoparticles have also been synthesized (e.g., WS2 and MoS2) (Tenne, 2006). The development of advanced microscopes (Blonder et al., 2010) and other advanced techniques (e.g., XPS, electrochemistry techniques) provides a spectrum of characterization methods that provide us with a better understanding of the structure and properties of nanomaterials. And finally, the properties of nanomaterials, which are size dependent, constitute the basis for developing innovative applications in different areas (e.g., medicine, solar energy, and electronics) that have the potential to influence our future life (Sakhnini and Blonder, 2016).Integrating nanotechnology into the school chemistry curriculum would provide students with a realistic view of what modern chemistry actually is. Several studies that evaluated NST educational programs indicated that they influence students’ continuous motivation to learn chemistry in the future. Blonder and Dinur (2011) developed a program about nanotechnology and light-emitting-diodes (LEDs) for middle-school students, which was taught in the STEM hours. They found that students perceived chemistry as a modern science and that the percentage of students who chose to study chemistry increased. Blonder and Sakhnini (2012) developed a different program for middle-school students; it focused on three NST essential concepts (Blonder and Sakhnini, 2015): size and scale, size-dependent properties, and innovations and applications of nanotechnology. They found that a variety of teaching methods were needed for students to comprehend these concepts and that the program positively influenced students’ motivation to choose advanced courses in chemistry and science in high school. Moosavifazel et al. (2014) describe a similar influence by a laboratory program in nanotechnology that was integrated into school science programs in order to increase students’ interest in science. Additional support for the motivational influence of the NST program was obtained from Hutchinson et al. (2011), who found that students appreciated NST learning materials only when the materials were innovative and involved hands-on activities. Other studies were conducted in informal educational settings such as inviting students to participate in a nanotechnology conference (Blonder and Sakhnini, 2015), summer activities (Flynn et al., 2007; Taylor and Jones, 2009), or visits to science museums (Murriello et al., 2009; Gilbert and Lin, 2012; Laherto, 2012).
Indeed, many programs have been developed around the world for introducing NST into science education (Jones et al., 2013; Bryan and Giordano, 2015; Bryan et al., 2015). In 2015, Sakhnini and Blonder published a three-stage Delphi study that was conducted to identify the essential NST concepts that should be taught at the high-school level. Via the Delphi methodology, two expert communities (NST researchers and science teachers) were able to reach a consensus regarding eight NST essential concepts. The concepts that were identified were as follows: (1) size-dependent properties, (2) innovations and applications of nanotechnology, (3) size and scale, (4) characterization methods, (5) functionality, (6) classification of nanomaterials, (7) fabrication approaches of nanomaterials, and (8) the making of nanotechnology. And several studies were published to examine programs that were developed to exemplify teaching part of the NST essential concepts, e.g., size and scale (Blonder and Sakhnini, 2012) and the making of nanotechnology (Blonder and Sakhnini, 2015).
However, integrating these concepts into the current high-school chemistry curriculum is not a simple task. The chemistry curriculum already suffers from many problems, as described by Gilbert (2006), of which two are relevant to the current research. The first problem is the overloaded curriculum. Gilbert stresses that “As a consequence of the ever-accelerating accumulation of scientific knowledge, curricula have become over-loaded with content. The consequences of high content loads have been that curricula are too often aggregations of isolated facts detached from their scientific origin” (Gilbert, 2006, p. 958). The second problem is the lack of relevance of the chemistry curriculum. Most of the students perceived chemistry as detached from real life and therefore irrelevant. In the current research we would like to suggest a method to make the chemistry curriculum more relevant and up-to-date without adding new topics to the overloaded existing curricula. Gilbert (2006) suggested using a context-based chemistry education approach in order to overcome the challenges in chemistry education. Here we suggest a different approach for dealing with these challenges. We chose the NST field as an organizing theme that accompanies the high-school chemistry curriculum, since previous studies showed that nanotechnology positively influences students’ interest in and motivation for learning science and chemistry. Visiting and re-visiting the NST concepts through the high-school chemistry program will connect the different parts of the chemistry curriculum and provide an answer to the aggregations of isolated facts that characterize the chemistry curriculum.
Research goal and question
Therefore, the following research goal was set: to find the insertion points of the essential NST concepts within the chemistry curriculum.The formulated research question to achieve this goal was: what are the insertion points for the essential NST concepts in existing high-school chemistry curricula in Israel?
Methodology
In order to provide a valid answer to the research question and to identify the insertion points of the eight NST essential concepts in the high-school chemistry curriculum, we based our research on experienced high-school teachers. However, knowing just the curriculum is not enough in the current study. The teachers who participated in the study also need to deeply understand the eight NST essential concepts. Two different groups of teachers participated in two stages of the study: the identification stage (n = 11) and the validation stage (n = 20). The procedures and the participants for each are described next.The identification stage
This discussion was audio-recorded and transcribed. Then, the researchers sorted the insertion points suggested by the teachers according to the topics in the Israeli chemistry curriculum.
The validation stage
The validation stage was conducted one year after the identification stage. It is aimed to support or deny the insertion points that were suggested in the identification stage and to verify whether the identification process reached a saturation point.The researchers examined the suggested insertion points and classified them into two groups: (1) insertion points that were already identified in the previous identification stage (for validation), and (2) new suggested insertion points (that were not found in the previous stage) to enlarge the possible places in the curriculum in which each NST essential concept could be integrated.
Results
The study was conducted in Israel and used the Israeli high-school chemistry curriculum as a representative example of a high-school chemistry curriculum. Nevertheless, we present the structure of the curriculum to support international readers in implementing the presented results in different national curricula. The Israeli chemistry curriculum consists of ten mandatory topics and four optional curricular modules.The first mandatory part is the basic concepts in chemistry. This part includes the concepts of the state of matter (at the macro and micro levels), melting and boiling points, the chemistry symbols, the type of materials, mixtures, and solutions, chemical reactions and scientific inquiry skills. The second topic is atomic structure, which includes atom particles, isotopes, radioactivity, the periodic table, the electron arrangement, orbitals, the first ionization energy, ions, and the atomic radius. The topic structure and bonding includes the Coulomb law covalent bond, electronegativity, bond energy and chemical bond lengths, molecules, representation forms and the spatial structure of molecules, isomers, functional groups, molecular materials, intermolecular bonds, hydrogen bonds, and van der Waals (vdW) bonds, ionic, atomic and metal structures, as well as properties of materials according to their bonding type. The fourth topic is stoichiometry, which includes the mole, solutions and dilutions, Avogadro's number, the gaseous state pressure–temperature relationship, the temperature scale (Celsius and Kelvin), and the gas molar volume. The topic oxidation–reduction, which comes next, includes definitions, oxidation–reduction reactions, corrosion, oxidation degree levels, and antioxidants. The sixth topic is acids, bases, and the pH scale. Chemistry of food, the seventh topic of the curriculum, is related to the major food groups, fatty acids, amino acids, saccharides, and triglycerides (Herscoviz et al., 2007). The eighth mandatory topic is energy and thermodynamics. It includes basic concepts of the topic: internal, potential energy, kinetic energy, and enthalpy. The ninth topic is kinetics, which deals with reaction rates, equilibrium, changes in the equilibrium system conditions (changes in pressure, temperature, concentration, and the addition of a catalyzer), entropy, and spontaneous reactions. The last topic, inquiry laboratories, includes the inquiry process and experiments at different levels of inquiry (Hofstein et al., 2006; Blonder et al., 2008a; Katchevich et al., 2013).
The optional curricular modules are polymers, physical chemistry, biochemistry, and environmental chemistry modules. The polymers module deals with macromolecules, polymerization processes, polymer characteristics, thermoplastics, and elastomers. The physical chemistry module concerns the interaction of light and matter and deals with electromagnetic radiation, the electron structure of the atom, the electron excitation of mono atoms or ions, a linear spectrum vs. a continuous spectrum, the Bohr model, the emission spectrum vs. the absorption spectrum, atomic orbitals, light spectrum and colors, light absorption and scattering, the relationship between the molecule's structure and its color, the electronic structure of solids (conductors, semiconductors, and insulators), valence and conduction bands, and doping and LEDs (Dangur et al., 2014; Dori et al., 2014). The biochemistry module relates to cell chemistry, the structure and function of phospholipids, amino acids, proteins and their acid, basic, hydrophilic, and hydrophobic characteristics, protein structures, DNA and RNA structures, the transcription process, the translation process and the genetic code, as well as ribosomes and mutation (Barak and Hussein-Farraj, 2013). The next module, environmental chemistry, deals with the quality of drinking water, water properties, analytical methods used for identifying the concentration of different ions in water, spectroscopy as an analytical tool, purification processes, air quality and the cycle of carbon dioxide, and the greenhouse effect (Mandler et al., 2012, 2014).
The NST essential concepts were suggested to be integrated into different topics of the high-school chemistry curriculum. The results of the insertion points for each concept will be presented in separate tables (Table 1 and 7 tables in Appendices 1–7) that include the suggested insertion points of the concept in the different curricular chemistry topics, quotations, and explanations given by the teachers, explaining the suitability of the integration as they perceive it. Each table will also present the number of teachers from the identification stage who suggested the insertion points of each concept and the insertion point of each concept that was added at the validation stage. A discussion of the insertion points of a related concept will accompany the tables. The concept insertion points are presented in Tables 1–8 (Appendices). Before each results section, which deals with one of the NST essential concepts, the definition of the concept (based on Sakhnini and Blonder, 2015) is presented. For a more detailed description and an explanation of the eight NST essential concepts, please see Sakhnini and Blonder (2015). Table 2 summarizes the number of teachers from the identification stage who suggested the insertion points of each concept and the insertion point of each concept that was added at the validation stage.
| Curriculum subjecta | Teacher's explanation |
|---|---|
| a Number of teachers who suggested the concept. b Insertion points that were confirmed at the validation stage. c Insertion points that were added at the validation stage. | |
| Basic concepts 2b | “When teaching about melting and boiling points of materials, we ask the students if these temperatures are an intrinsic property of materials (as we used to teach so far). Then, we can teach about those factors that affect changes in the melting and boiling points for a specific material, like pressure changes and the size of nanoparticles of the same element”. |
| Atomic structure 4b | “When we teach that every element has a constant melting and boiling point, we can tell the students about the case of nanoparticles. Here, properties that were thought to be constant for each element are suddenly size dependent”. |
| Structure and bonding 8b |
“When teaching about atomic materials, we can expand our teaching about nanotubes, the meaning of the frequency of defects in a material in comparison to its size, and how that affects its strength”.
“In molecular materials, students learn about van der Waals (vdW) bonds, the electronic cloud size, the surface area of a molecule, and the polarity of a molecule. Regarding these factors, the students learn that the bigger the electronic cloud is, the stronger the vdW bonds are. Therefore, the concept size-dependent properties is suitable in this topic”. “In metallic materials-alloys we create defects that affect the material's properties. The number of defects and the quantity of the metal in the alloy affect its properties such as its strength in nanomaterials; there are hardly any defects”. “Regarding allotropic forms of carbon–graphene, graphene can be a small sized mono layer structure, which makes it a pure, defect-free material. These characteristics provide it with its strength, toughness, and high electrical conductivity”. |
| Chemistry of food 4b | “cis-Fatty acids have a less exposed surface and therefore create weaker vdW bonds and have a lower melting point. This is a great place to teach the importance of surface area in small particles”. |
| Kinetics 6b |
“When in grade 12 we teach about catalysis, which occurs on the surface of the catalyzer, we emphasize that the surface area is important. We can integrate the SA/V concept and discuss the advantage of using nanostructures for catalysis”.
“When teaching reactivity, we teach that graphene has a monolayer structure; therefore, all of its carbon atoms are organized on its surface area and its SA/V is very high”. “It is suitable to teach this concept when teaching the catalytic converter, its structure, and function. We discuss the catalytic converter when teaching about air pollution and the methods used to reduce air pollution from vehicles”. |
| Inquiry laboratories 2b | “We can relate to the concept in any experiments involving dissolving materials. Students realize how the SA/V of a crystallized cube of sugar will influence its dissolving rate”. |
| Optional curricular modules | |
| Polymers 1b | “When teaching about fibers, the straighter the polymer chain is, the larger its surface area is. This will increase the number of bonds within the polymer chains, and as a result, it increases the melting point Tm”. |
| Physical chemistryc | “When we discuss the color of conjugated molecules we show that the energy gap depends on the length of the conjugation. This is an example of a size-dependent property: the length of the conjugation determines the energy gap of the electron's excitation, namely, it determines the color of the molecule”. |
| Biochemistryc | “When discussing the packaging of DNA around proteins in the chromosome, we can demonstrate the size-dependent property of the SA/V ratio”. |
| Curriculum subject | 8 NST essential conceptsa | |||||||
|---|---|---|---|---|---|---|---|---|
| 1. Size-dependent properties | 2. Innovations and applications of nanotechnology | 3. Size and scale | 4. Characterization methods | 5. Functionality | 6. Classification of nanomaterials | 7. Fabrication approaches of nanomaterials | 8. The making of nanotechnology | |
| a Number of teachers who suggested the concept. b Insertion points that were confirmed at the validation stage. c Insertion points that were added at the validation stage. | ||||||||
| Mandatory curricular subjects | ||||||||
| 1. Basic concepts | 2b | 2b | 3b | 3b | 2b | 3b | 3b | 3b |
| 2. Atomic structure | 4b | 4b | 11b | 2b | 4b | 11b | 4b | 4b |
| 3. Structure and bonding | 8b | 7b | 7b | 8b | 10b | 10b | 4b | 3b |
| 4. Stoichiometry | 0 | 0 | 8b | 0 | 0 | 3b | 0 | 1 |
| 5. Oxidation–reduction | 0 | 3b | 0 | 0 | 3b | 0 | 1 | 1b |
| 6. Acids and bases | 0 | 2b | 0 | 0 | 2 | 0 | 0 | 0 |
| 7. Chemistry of food | 4b | 2b | 3b | 0 | 7b | 0 | 3b | 3b |
| 8. Kinetics | 6b | 2b | 0 | 3b | 0 | 0 | 0 | 0 |
| 9. Energy | 0 | 0 | 0 | 0 | 0 | 0 | 4b | 0 |
| 10. Inquiry laboratories | 2b | 0 | 0 | 2 | 4b | 3b | 3b | 4b |
| No. insertion point (validate) | 6 (6) | 7 (7) | 5 (5) | 5 (4) | 7 (6) | 5 (5) | 7 (6) | 7 (6) |
| Optional curricular subjects | ||||||||
| 11. Polymers | 1b | 7b | 5b | 2b | 6b | 0 | 5b | 4b |
| 12. Physical chemistry | 0c | 6b | 2b | 3b | 7b | 7b | 2b | 2 |
| 13. Biochemistry | 0c | 3b | 0c | 2 | 6b | 3b | 3b | 3b |
| 14. Environmental chemistry | 0 | 0 | 2b | 0 | 3b | 0 | 0 | 0 |
| No. insertion point (validate) | 1 (3) | 3 (3) | 3 (4) | 3 (2) | 4 (4) | 2 (2) | 3 (3) | 3 (2) |
1. Size-dependent properties
The essential concept, size-dependent properties, was defined as the properties of materials that change as a function of the material's size. This effect does not exist in the macroscopic world. This concept has four sub-concepts: the surface area-to-volume (SA/V) ratio, quantum properties, optical properties, and defects.†The NST essential concept, size-dependent properties, was suggested to be inserted into different places in the high-school chemistry curriculum as is presented in Table 1.
As presented in Table 1, teachers suggested several insertion points for the essential NST concept size-dependent properties in different topics of the high-school chemistry curriculum. They suggested integrating the concept into six of the ten chemistry curricular topics (basic concepts, the periodic table, structure and bonding, the chemistry of food, kinetics, and inquiry laboratories) and in three of the four optional curricular modules (polymers, physical chemistry, and biochemistry) of the Israeli high-school chemistry curriculum.
The first suggested insertion point of the concept was in the “basic concepts” topic of the curriculum. Teachers suggested integrating the sub-concept, the SA/V ratio, when they discuss states of matter and its physical properties (e.g., melting and boiling points), factors that affect these properties and the changes in them. According to the teachers, by teaching this concept, students learn that melting and boiling points are not an intrinsic property of the material as they were taught so far, and they will better understand the mechanism underlying the melting process. The next insertion point was suggested when teaching the periodic table and the element properties that are conceived as constant (e.g., the melting and boiling point). These properties start to be size dependent and are not constant when you go down to the nanoscale. The third insertion point was related to the sub-concept defects when teaching the topic of “structure and bonding”. Teachers suggested inserting the concept when teaching atomic, molecular and metallic materials (alloys). Students learn how the frequency of defects in a material, in comparison to its size, affects the material's properties and characteristics (e.g., strength, polarity, vdW bonds, toughness, and electrical conductivity). The next insertion point suggested was related to the “chemistry of food” topic. Teachers suggested teaching the importance of the surface area in small particles, and its influence in determining the melting point of the matter by taking into consideration its molecules’ spatial structure such as cis-fatty acids, which have less exposed surfaces in contrast to trans-fatty acids and therefore create weaker vdW bonds and have a lower melting point. The kinetics topic is another insertion point that the teachers suggested. In this topic the teachers related to the sub-concept SA/V when discussing the catalytic processes that occur on the surface of the catalyzer and when discussing reactivity and the role of the SA/V ratio in increasing the effectiveness of the catalyzer. The last suggested insertion point was the “inquiry laboratories” topic. Teachers suggested relating to the concept in many experiments involving dissolving (e.g., Al catalyzer), in which students can learn how the SA/V of a crystallized cube of a solid will influence the dissolving rate.
The sub-concept SA/V ratio was also suggested to be integrated into several optional curricular modules. In the polymers module it was suggested to be taught when teaching the connection between the polymer chain's straightness and the increase in its surface area, which leads to an increase in the melting point as a result of the increase in the number of bonds within the polymer chains. In the physical chemistry module it was suggested to be integrated when teaching about the length of the conjugated molecules, which determines the energy gap of the electron excitation, namely, it determines the color of the molecule. This insertion point was added at the validation stage. The suggested insertion point of the sub-concept SA/V was also added to the biochemistry optional curricular module in the validation stage, when teaching about the packaging of DNA around proteins in the chromosome, where one can demonstrate the size-dependent property of the SA/V ratio.
2. Innovations and applications of nanotechnology
The essential concept “Innovations and applications of nanotechnology” was defined as the potential applications and innovations of nanotechnology including the following four sub-concepts: Current and future applications (Innovative implementations of nanoscience and nanomaterials into current and future technologies and products for everyday use), mimicking nature, which is based on single molecules or collections of them for many tasks such as energy harvesting and transfer, motion, cleaning surfaces, and replication, the risks and benefits of nanotechnology to our health and environment and its socio-scientific issues, and the last sub-concept, tailoring nano-materials to the application, as a means of constructing complicated systems to meet the needs of a certain application.The NST essential concept, applications and innovations in nanotechnology, was suggested to be inserted into different places in the high-school chemistry curriculum, as presented in Table 2. The teachers explained that starting with the first lesson in chemistry they try to demonstrate the applicative nature of chemistry to their students, as reflected in the following quote: “In the first lesson in chemistry, students are exposed to its definition. Therefore, we can relate to the notion that chemistry is a continuously developing science that produces new materials with new properties that fit a desired application. We can always relate to this idea as we progress through the curriculum contents”. Teachers’ explanations for the other suggested insertion points of this NST essential concept are presented in Appendix 2.
Teachers suggested integrating the NST concept applications and innovations in nanotechnology into seven of the ten chemistry curricular topics (basic concepts, the periodic table, structure and bonding, oxidation–reduction, acids and bases, the chemistry of food, and kinetics) and in three of the four optional curricular modules (polymers, physical chemistry, and biochemistry) of the Israeli high-school chemistry curriculum.
The students will first encounter the concept when they learn the basic concepts of the chemistry curriculum, in the first chemistry lesson where students are exposed to the definition of chemistry, and about chemistry's contribution to their everyday life. Teachers believe that it is important to relate to the notion that chemistry is a continuously developing science that produces new materials with new properties that fit desired applications. They also claim that this concept should always be integrated, all the time, as they progress in the chemistry curriculum contents and in the teaching process because of the concept's ability to increase students’ motivation by connecting them to everyday life problems and the solutions these applications have to offer. In addition, learning the basic concepts encourages students to discuss socio-scientific ethical issues that will lead them to develop meta-cognitive skills, and it makes them learn differently, which will lead to meaningful learning. Some teachers suggested integrating the concept while teaching research skills by reading articles dealing with nanotechnology applications to practice the different research skills.
The next suggested insertion point is when teaching the periodic table. In this topic, teachers teach about metals, non-metals and semi-conductors. Therefore, they suggested that they teach applications related to semi-conductors, such as photovoltaic solar cells (discussing their contributions, pros and cons), and about LEDs. It is suggested that these applications be integrated later into one of the optional curricular modules. Another insertion point for the concept was in the structure and bonding topic. Teachers suggested relating to the self-cleaning, mimicking nature (the lotus effect), and applications of carbon nanotubes (CNTs) in different daily products, when teaching hydrophilic and hydrophobic, intermolecular forces, and the carbon allotropy concepts. In the oxidation–reduction topic the teachers suggested integrating the concept while teaching about anti-oxidants as natural materials. Teachers think they can use the working mechanism underlying the materials to teach the students how nanotechnology researchers can learn from nature and make new materials that work using the same mechanism. Note that in this topic the teachers integrated three of the four sub-concepts (current and future applications, nature's mimics, and benefits and risks of nanotechnology). The fourth sub-concept, tailoring nano-materials to the application, was suggested to be integrated into the topic acids and bases when teaching about indicators. Teachers explained that they use the indicator's property of changing its color, to get information regarding the pH level of the base or acid. Therefore, they can teach about tailoring nanoparticles’ properties to obtain new properties according to the needed application. The chemistry of food topic was the next suggested insertion point. Teachers suggested integrating the concept when teaching about saccharides, and other food ingredients and connecting the subject with nano-sensors that were developed to measure the concentration of these materials. In the Kinetics topic, teachers suggested integrating the concept while teaching about catalysis and catalyzers, so they will be able to teach about the photo-catalysis of the nanotechnology self-cleaning application.
The first insertion point suggested in the optional curricular modules was in the polymers module. In this module teachers mentioned the everyday uses of polymers (e.g., food packaging, anti-bacterial products, and strong glues, among others). Therefore, they think it is an excellent context to teach about nanocomposite polymers, their contribution to humanity, and relate them to the RRI (Responsible research and innovation) dimensions and discuss it with the students. Teachers believe that they will cultivate students’ awareness regarding ethical and social aspects along with scientific/technological applications. The next insertion point is in the physical chemistry optional module. Also in this module, teachers suggested demonstrating different semi-conductor-based nanotechnology applications (e.g., LEDs and photovoltaic cells). They felt that solar cells are very practical and relevant and that many ethical and social issues are associated with environmental issues. The last suggested insertion point was in the biochemistry module, when the hydrophilic and hydrophobic concepts in proteins were taught. Again, teachers related to the self-cleaning surfaces application, and linked it to technological applications that are based on mimicking nature (the lotus effect) or processes that occur in the cell, which are used as the basis for producing nano devices designed for the same purpose as nature provides.
3. Size and scale
The essential concept size and scale has two components that are defined as follows: Size is the extent or amount of an object. Scale is a comparison of the size of an object to a reference object. The NST essential concept size and scale was suggested to be inserted into different places in the high-school chemistry curriculum, as presented in Table 2. Their explanations regarding each insertion point are detailed in Appendix 1.Teachers suggested several insertion points for the essential NST concept size and scale in different topics of the high-school chemistry curriculum. They suggested integrating the concept into five of the ten chemistry curricular topics (basic concepts, atomic structure, structure and bonding, stoichiometry, and chemistry of food) and in all four optional curricular modules (polymers, physical chemistry, biochemistry, and environmental chemistry) of the Israeli high-school chemistry curriculum.
The first suggested insertion point of the concept was within the “basic concepts”. Teachers suggested integrating the concept size and scale when teaching about “the state of matter” and the “macroscopic and microscopic levels” topics. By teaching the concept, students will be able to distinguish between the macro and micro sizes and scales. One teacher wrote that she mentions the concept in the first introduction to the chemistry lesson to give the students an idea about the scales that the field of chemistry deals with. A second suggested insertion point was in the atomic structure topic. According to teachers, when students learn about the atom's structure (e.g., protons, electrons, neutrons, atoms, and molecules) they find it hard to estimate their sizes. So it is important to make students realize the differences in size and scale of each particle in order for students to make sense of the atomic structure. Another explanation, given by teachers, claims that teaching this concept in the atomic structure context will provide teachers with more tools to make students more deeply understand the different scales of different particles in chemistry and will also make students demonstrate and describe, in their head, the scales of different visible and invisible objects. The topic “structure and bonding” was the next suggested insertion point of the concept. Teachers suggested integrating the concept when teaching about the length of chemical bonds, because it will help students overcome their misconceptions regarding the chemical bond length and its characteristics, and make them realize the connection between bond length and its strength. Another suggested insertion point of the concept was when teaching stoichiometry. Teachers suggested integrating the concept when teaching about concentrations and dilution, as well as dealing with negative exponents, hence, small scale numbers. But they also suggested teaching the concept when dealing with large positive exponents, such as the number of particles in one mole. The next suggested insertion point was in the “chemistry of food” topic. In teaching about food macromolecules (e.g., saccharides, proteins) as ‘huge’ molecules, teachers explained that the size and scale concept will provide the students with tools that will help them perceive the meaning of “huge” in the context of macromolecules.
The concept size and scale was also suggested to be integrated into several optional curricular modules. In the polymers module it was suggested to be taught when teaching about macromolecules and their long, twisted chains, similar to what was described in connecting this concept to biological macromolecules. This concept was also suggested in the physical chemistry optional module, when the physical chemistry topics are introduced before teaching about wavelengths and colors. In the biochemistry optional module, teachers suggested integrating the concept while teaching about the packing process of DNA molecules, macromolecules that are packed in a very small area in the cell, which is also very small. Students need to understand the meaning of small and the relative size of each part in the system. The last suggested insertion point is in the environmental chemistry module, when teaching about water quality and the analytical methods used for determining the concentration of ions in water. Students learn that when a colorful solution is diluted until its color is invisible, it does not mean that the water has no dissolved substance in it. In fact, it still contains the colorful solution but in a very low concentration, hence the concentration scale of the dissolved substance is changed. Therefore, special analytical tools are needed to determine its existence.
4. Characterization methods
The essential concept characterization methods was defined as: “Tools for observing, imaging, studying, and manipulating the nanomaterial's size, along with techniques for characterizing nanomaterials”. This concept has three sub-concepts: SPM (scanning probe microscopy) and mostly STM (scanning tunneling microscopy) and AFM (atomic force microscopy), EM (electron microscopy), which includes TEM (transmission electron microscopy) and SEM (scanning electron microscopy) and resolution. For the curriculum insertion points, we focused only on AFM and the resolution sub-concepts. The NST essential concept AFM and resolution (characterization methods) was suggested to be inserted into different places in the high-school chemistry curriculum as summarized in Table 2. Detailed explanations regarding the insertion point are presented in Appendix 3.Teachers suggested several insertion points of the essential NST concept characterization methods and especially the sub concepts AFM and resolution in different topics of the high-school chemistry curriculum. They suggested integrating the concept into five of the ten chemistry curricular topics (basic concepts, atomic structure, structure and bonding, kinetics, and inquiry laboratories) and in three of the four optional curricular modules (polymers, physical chemistry, and biochemistry) of the Israeli high-school chemistry curriculum.‡
The first place was in the “basic concepts” topic of the curriculum. Teachers suggested integrating the concept when they teach about elements, compounds, mixtures, and pure material. In the teachers’ opinion, students should learn about the characterization methods that are used to see at the atomic level in order to deepen the four levels of understanding needed in chemistry lessons (the macro, micro, symbol, and process levels). The second place was in the atomic structure topic. Teachers know from experience that it is difficult to teach about atoms and explain that they are the particles that compose the material. By using AFM images, students can actually see what they have learned and they can validate models and theories that were constructed by scientists explaining the structure of matter. These images will support students in constructing a mental model of the structure of matter. The next suggested insertion point referred to the topic “structure and bonding”. Teachers suggested connecting the intermolecular bonds and their strengths to the working principle of the AFM microscope based on vdW forces between the atoms, at the end of the tip, and the atoms (and molecules) on the scanned surface. Teachers suggested integrating the concept also when teaching about the geometry of molecules by showing the students’ results from the AFM that scientists used to see the geometry of molecules, which helped them support their theoretical knowledge and examine their hypothesis. The next suggested insertion point was in the kinetics topic because the surface area of matter is one of the factors that affects a reaction rate. Therefore, teachers suggested relating to the AFM concept as a research method used for characterizing the surface area of different kinds of matter including catalysts and estimating their roughness. The last suggested insertion point was in the inquiry laboratories topic. Teachers suggested relating to the concept when students conduct experiments and get different products. It is a great opportunity to teach the students that these differences can be characterized by using advanced instrumentations such as AFM, which is used as a research tool for characterization and for learning about the products’ structures.
In the optional curricular modules, the microscopy concept was suggested for the polymers module, used by teachers to teach about fibers. They believe that by integrating the concept and introducing another kind of microscope such as TEM, students will learn the importance of these microscopes in providing information regarding how the fibers are organized in a polymer, along with the polymer's structure, its characteristics, and how they affect its applications. The next suggested insertion point was in the physical chemistry module when teaching the duality of an electron, in spectroscopy, which is the basis of the working mechanism of the electronic microscope. Therefore, teachers suggested presenting electronic microscopies as breakthrough applications based on the duality principle. Another insertion point was suggested in the biochemistry module when teaching about the processes inside a living cell. Teachers suggested introducing different microscopic techniques and discussing the resolution that can be achieved by each technique while visualizing living cells. They also suggested discussing the limitations of the different techniques when working with these living cells (e.g., using the electron microscopy techniques we cannot work with living cells. They should be treated first, hence destroying their biological activity). In the biochemistry module, they also suggested using AFM for identifying the secondary structure of a protein that is absorbed on a surface and identifying mutations in it.
5. Functionality
The essential concept functionality can be defined as a property that is provided for a material or for a specific area in it. This property endows the material with a specific activity or endows it with bonding ability. Functionality transforms nanoscience into nanotechnology.As presented in Table 2 teachers suggested several insertion points for the essential NST concept functionality in different topics of the high-school chemistry curriculum. They suggested integrating the concept into seven of the ten chemistry curricular topics (atomic structure, the periodic table, structure and bonding, oxidation–reduction reactions, acids and bases, the chemistry of food, and inquiry laboratories) and into all four of the optional curricular modules (polymers, physical chemistry, environmental chemistry, and biochemistry) of the Israeli high-school chemistry curriculum. Teachers’ explanations are presented in Appendix 4.
The first suggested insertion point of the concept was in the “Atomic structure” topic. Teachers suggested integrating the concept functionality when teaching about the ability of a nucleus of an atom, as a positively charged particle, to attract electrons. This ability stabilizes the electronic structure of the atom. The second place was in the periodic table topic. Teachers mentioned that determining the location of each element in a specific column of the periodic table provides it with specific chemical characteristics according to the column and its functionality. The next insertion point was in the structure and bonding topic. Teachers suggested teaching the concept because the properties of matter depend on the characteristics of the functional groups that are included in the molecules. For example, a molecule with a hydroxyl group (–OH), probably dissolves in water. Oxidation–reduction reactions was another suggested insertion point. According to the teachers, the oxidizing and reducing materials’ characteristics provide the materials with their ability to react in oxidation or reduction reactions, namely, they provide the materials with some functionality. The acids and bases topic was the next insertion point. Teachers emphasized that the characteristics of an acid as a proton donor and of a base as a proton acceptor govern the activity of acid–base reactions and provide the materials with some functionality. The next suggested insertion point was in the chemistry of food topic. Teachers explained that teaching about antioxidants; their ability to lose electrons and function as a reducing material terminates the free radicals that are obtained in different processes that occur in the human body. The inquiry laboratories topic was the last suggested insertion point for the functionality concept. Teachers suggested demonstrating the “copper plates experiment” whereby they change the wetting properties of the metal, consequently affecting its functionality.
This concept was also suggested to be integrated into several optional curricular modules. In the polymers module, they suggested mentioning the field of composite materials and the importance of providing a nanoparticle with special functionality, thus enabling it to bind much better to a polymeric matrix. In the physical chemistry module, they related to functionality when teaching about semi-conductors in photovoltaic cells. Teaching about the structure of the energy levels leads to specific conductivity characteristics of semi-conductors that enable them to function as a photovoltaic cell. Teachers considered integrating the concept when teaching the biochemistry module. In this module, teachers teach the proteins topic and discuss the enzyme's catalytic site. They stress the uniqueness of the enzyme's structure, which provides the protein with its catalytic nature. The last suggested insertion point was in the environmental chemistry module. For example, when teaching about water quality, there are many developments that enable purification of water and they are based on some functionality of the nanomaterials, such as the ability of nanomaterials to bind metal ions and to remove them from the water.
6. Classification of nanomaterials
The essential concept classification of nanomaterials was defined as the classification of nanomaterials according to the four following sub-concept characteristics: (1) classifying nanomaterials according to their chemical composition (for example, carbon nanocompounds, inorganic NPs, and organic nano-compounds), (2) classifying nanomaterials according to their electrical conductivity (semiconductors, conductors, and insulators), (3) classifying nanomaterials according to their source (natural nanomaterials, organic molecules, and synthetic nanomaterials), and (4) dimensionality (the number of dimensions in which a nano-structure expands beyond 100 nm (0D, 1D, 2D, and 3D)).As presented in Table 2, teachers suggested several insertion points for the essential NST concept classifications of nanomaterials in different topics of the high-school chemistry curriculum. They suggested integrating the concept into five of the ten chemistry curricular topics (basic concepts, the periodic table, structure and bonding, stoichiometry, and inquiry laboratories) and in two of the four optional curricular modules (physical chemistry and biochemistry) of the Israeli high-school chemistry curriculum. Teachers’ explanations regarding the insertion points are presented in Appendix 5.
The first suggested insertion point of the concept was within the basic concepts topic. Teachers suggested integrating the concept classifications of nanomaterials when relating to carbon and its various forms. There, they can teach about nanoparticles that are composed of carbon atoms. The second place was in the periodic table topic. Teachers suggested integrating the concept when teaching about the periodic table. The classification of nanomaterials is analogous to classifying the elements in the periodic table, where the elements are presented in columns and rows. Therefore, one can compare and see what can be learned from each of the nanomaterials’ classifications and can determine their differences and similarities. They also suggested integrating the concept according to the materials’ conductivity (conductors, semi-conductors, and insulators). The next insertion point was in the structure and bonding topic.
Since this topic deals with allotropic structures of carbon, it is an appropriate place to represent different carbon nanoparticles (fullerene, carbon nanotubes, and graphene). Dealing with this family of materials enables one to consider other nanomaterials that are not carbon-based, such as inorganic nanomaterials, but have the same structure. Stoichiometry was the next topic that teachers suggested integrating the concept into. They suggested mentioning, for example, reactions for creating nanoparticles (categorizing nanomaterials according to their chemical composition). The next insertion point was in the inquiry laboratories, where gold nanoparticles can be prepared and then the different types of nanoparticles, representing different types of classification, can be discussed.
This concept was also suggested to be integrated into two optional curricular modules. In the physical chemistry module, teachers can teach about the difference between conductive, insulating, and semi-conductive materials. Teachers also considered integrating the concept when teaching the biochemistry module. In this module, teachers can mention nanoparticles that are based on DNA, and then can relate to the classification of materials according to the organic and inorganic classification.
7. Fabrication approaches for nanotechnology
The essential concept fabrication approaches for nanomaterials was defined as the wide variety of options that can be used for fabricating nanomaterials. This concept has two sub-concepts: (1) top-down vs. bottom-up approaches for fabricating nanomaterials and (2) a self-assembly approach for fabricating nanomaterials. Teachers were asked to suggest insertion points only for the self-assembly sub-concept.Teachers suggested several insertion points of the NST concept fabrication approaches for nanomaterials (and focused on the sub-concept of self-assembly) in different topics of the high-school chemistry curriculum, which are presented in Table 2 and explained in Appendix 6. They suggested integrating the concept into seven of the ten chemistry curricular topics (basic concepts, atomic structure, structure, and bonding, oxidation–reduction, the chemistry of food, thermodynamics, and inquiry laboratories) and in three of the four optional curricular modules (polymers, physical chemistry, and biochemistry) of the Israeli high-school chemistry curriculum.
The first suggested insertion point of the concept fabrication approaches of nanomaterials (focusing on the sub-concept of self-assembly) was in the “basic concepts” of the chemistry curriculum. Teachers suggested integrating the concept when they discuss mixtures and solutions and when they explain the hydrophilic and hydrophobic concepts and relate to detergents’ ability to remove fatty spots as an example of mixtures. Teachers can go deeper and connect the micelles’ structures that are self-assembled in terms of nanotechnology. They also suggested connecting the topic when teaching the four basic levels of chemistry, especially when describing the micro level. In the structure and bonding topic, teachers suggested integrating the concept while teaching intermolecular bonds. They referred to intermolecular bonds as crucial for building a bottom-up structure, hence, self-assembly. They also suggested integrating the concept while discussing the materials’ properties in relation to intermolecular bonds by explaining how the properties are determined by the surface interactions and how the molecules are spontaneously organized. The next insertion point was in the oxidation–reduction topic. Teachers used the lab experiments they generally conduct with their student for stimulating the self-assembly process. For example, they prepare gold nanoparticles with their students and perform the bottom-up process by reducing gold ions with citrate and gradually producing gold nanoparticles. Chemistry of food is another suggested insertion point. Teachers suggested relating to the self-assembly concept when teaching fatty acids, saccharides, and proteins. Teachers use the membrane as a structure that is created by a self-assembly process based on the chemical properties of the fatty acids. They use the saccharides’ structures (mono, di, and poly saccharides) for comparing their structures to a bottom-up process. Lastly, the thermodynamics topic was another suggested insertion point, when teaching the entropy and free energy subjects. Teachers wrote that these subjects provide explanations for the self-assembly concept. Molecules are usually arranged in structures that spontaneously lead to decreased free energy, so the structure is self-assembled into a more stable state of the system. The inquiry laboratories topic was the last insertion point of the concept. Interestingly, teachers related to the concept as an analogy of how the inquiry laboratory process is built in general. It is a bottom-up structure: students start from small pieces of data followed by an experiment, results, and conclusions that complete the picture (the full assembly). They also suggested using several experiments (e.g., CuCl2 electrolysis) in which students can visualize the bottom-up process of creating copper metal at the macro-scale.
This concept was also suggested to be integrated into several optional curricular modules. In the polymers module it was suggested to be taught when teaching how an amorphous polymer is transformed into a well-arranged and crystallized structure in a slow cooling process involving polymer chains self-assembled, as opposed to a fast cooling process. Teachers suggested integrating the concept top-down and discussing self-assembly when teaching about microelectronics in the physical chemistry topic. They relate to photolithographic principles as an example of a top-down process. In the biochemistry module, teachers suggested using the bottom-up and self-assembly concept when teaching the lipids topic and when discussing the phospholipids organizing into shapes (e.g., liposome, micelles, and cell membrane). These topics demonstrate how the membrane's structure (which is in nanometric sizes) is organized. They also considered integrating the concept when teaching the quarternary structure of a protein, as a self-assembly structure. This protein is folded in a specific way because of thermodynamics and the final structure leads to some functionality.
8. The making of nanotechnology
The essential concept of the making of nanotechnology was defined as revealing the mystery of nanotechnology, or in other words, how nanoscience research is performed and how innovations are transformed into applications. This concept has three sub-concepts: multidisciplinary science and technology, team work, and the development of nanotechnology. The NST essential concept, the making of nanotechnology, was suggested to be inserted into different places in the high-school chemistry curriculum, as presented in Appendix 5. This concept is described in detail in Blonder and Sakhnini (2015).Teachers suggested integrating the concept into seven of the ten chemistry curricular topics (basic concepts, atomic structure, structure and bonding, stoichiometry, oxidation–reduction, chemistry of food, and inquiry laboratories) and in three of the four optional curricular modules (polymers, physical chemistry, and biochemistry) of the Israeli high-school chemistry curriculum, as presented in Table 2 and explained in Appendix 7.
The first suggested insertion point of the concept was in the basic chemistry concepts. Teachers suggested integrating the concept when they relate to chemistry as an interdisciplinary field that deals with astronomy, physics, material engineering, biology, geology, and others and it is considered a central science. Teachers considered using this concept as an example to demonstrate the scientific research process. The topic of atomic structure was the second suggested insertion point. In this topic, teachers referred to several sub-concepts (multidisciplinary science and technology, team work, and the development of nanotechnology) to explain the scientific cooperation between scientists from different disciplines (chemists, physicists) and its contribution in scientific developments. The third insertion point was in the structure and bonding topic. When teaching about the allotropic forms of carbon and about graphite, teachers suggested discussing the historical development of carbon nanoparticles such as CNTs and graphene. Stoichiometry was another suggested insertion point for the concept. Students feel that stoichiometry is a mixture of chemistry and mathematics. Therefore, it provides a great opportunity to present the interdisciplinary nature of contemporary science and more specifically, nanotechnology. The next suggested insertion point was oxidation–reduction when teaching corrosion protection methods. Here, teachers can present the new nanotechnology method used for corrosion protection and the collaboration needed among scientists from different scientific disciplines (chemistry and materials engineering) to make this nanotechnology application work. In the topic chemistry of food, teachers suggested integrating the concept while teaching about saccharides, proteins, and fatty acids, which serve as good examples of collaboration between chemists, biologists, physicians, and nutritionists, and which support research for finding medications for different diseases. They also suggested integrating the concept when teaching fatty acids, hydrophilicity, and hydrophobicity characteristics. In addition, they suggested introducing the self-cleaning nanotechnology application, which, again, exemplifies the multidisciplinary nature of nanoscience and nanotechnology. They stressed the importance of exposing students to the work of modern chemists, the interdisciplinary knowledge they need (e.g., biology and food engineering) and to the fact that modern research labs employ scientists from different scientific fields that work together and provide answers to related questions. The last suggested insertion point of the concept was in the inquiry laboratories topic. Teachers suggested relating to the concept when explaining the team work nature of lab work, stressing the importance of team work in the lab for the success of the team, and emphasizing the importance of cooperation within different scientific disciplines in the research process as a basis for its success.
The concept was also suggested to be integrated into several optional curricular modules. In the polymers module it was suggested to be taught when discussing integrating nanoparticles into polymers to obtain polymers with different properties. This requires team work among scientists from different scientific disciplines such as medicine, ecology, and food packaging. They also suggested integrating it when teaching tensile strength where it is needed to emphasize that this concept comes from the world of engineering and nanotechnology and links the world of science to the world of technology. The concept was also suggested in the physical chemistry topic, when teaching about semi-conductors and describing the semi-conductor industry. Teachers suggested describing the historical development of the electronics industry and how nanomaterials contributed to it. In the biochemistry module, teachers used the combination of chemistry and biology to explain that in science, it is difficult to separate scientific disciplines, because each discipline complements other disciplines. For example, in biochemistry, how the chemical structure affects the biological function.
Note that the nature of the concept the making of nanotechnology is different from other concepts that were identified in this research, as was emphasized in Blonder and Sakhnini (2015); and Blonder and Sakhnini (2016). The teachers spontaneously dealt with this difference by suggesting how to integrate the concept into the pedagogical level of chemistry teaching and not necessarily into specific parts of the chemistry curriculum. They wrote that the concept has great potential to attract students to learn chemistry, motivate them, and expose them to contemporary science, with no need to indicate a specific insertion point. Several ideas demonstrating this notion followed. Teachers suggested using the concept for chemistry orientation by using materials and conducting experiments that are considered as the cutting-edge of science. Teachers believe that by doing so, they will increase students’ interest and motivation in sciences in the future and will also expose them not only to the academic level of sciences but also to make them realize what constitutes research work. Exposing students to scientific language will encourage them to read scientific papers and develop their scientific literacy. In addition, based on the making of nanotechnology, one can connect between philosophy, history, and science to more deeply understand how science has developed. This is very important for deepening the students’ understanding of nano-size objects and for effectively promoting science teaching.
Discussion
According to the results, all of the NST essential concepts were integrated into the different chemistry curriculum topics in 74 different insertion points. In three of the compulsory chemistry topics (basic concepts, atomic structure, structure and bonding) teachers integrated all the concepts. In the other topics teachers found at least two NST essential concepts to be integrated into each of the curricular topics. In addition to Table 2, a graphical summary of the results is presented in Fig. 1.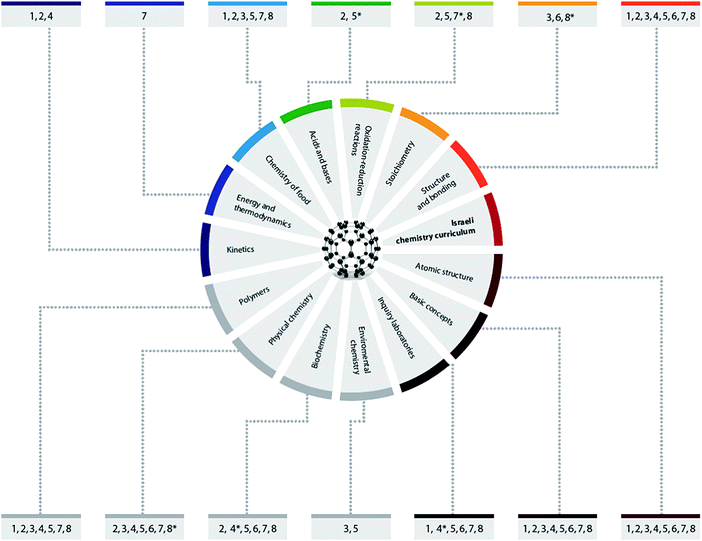 | ||
| Fig. 1 Insertion points of NST essential concepts in the Israeli high-school chemistry curriculum. The numbers of the 8 NST essential concepts are according to Table 2. *Insertion points that were not confirmed at the validation stage. | ||
The two-stage methodology served as a platform to examine the reliability of the insertion points of the NST essential concepts in the high-school chemistry curriculum in Israel. We found that 95% of the insertion points were validated in the second stage. The other 5% (3 insertion points) are ones that were suggested only by 1–2 teachers in the identification stage. These insertion points are denoted in Table 2 (as well as in Table 1 and Appendices 1–7). According to this finding, we suggest that these three non-validated insertion points be re-examined before they are recommended to other teachers. In addition, the low number of new insertion points that were raised in the second stage of the validation reveals that the process of identifying the insertion points of the NST essential concepts in the Israeli high-school chemistry curriculum is about to a reach saturation point. In order to fully meet the aim of the study to identify all the insertion points using the NST essential concepts in the high-school chemistry curriculum in Israel, an additional study should be conducted until saturation is reached.
As stated above, in the first three basic topics in the high-school chemistry curriculum: basic concepts, atomic structure, and structure and bonding, teachers suggested that all 8 NST essential concepts be inserted. Trying to analyze the meaning of this finding led us to two possible explanations. These are the basic chemistry topics and teachers spend a long time dealing with them since they support students’ understanding of the other chemistry topics. As basic chemistry topics, they are also related to all the NST essential concepts. In addition, when teachers devote many hours to teach a topic, they know many of its aspects and therefore, it is easier for them to find insertion points with the modern NST essential concepts they just learned in the course.
In the other chemistry curricular topics (e.g., stoichiometry, acids and bases, and oxidation–reduction) the teachers are more focused on teaching the students to solve exercises. Here it is not trivial to suggest that any external additional NST concepts be added to the curriculum. The results of the study might reflect on the way teachers teach different topics in the chemistry curriculum, and not only the insertion of a body of knowledge (namely, chemistry and NST). This might also be the case with the optional chemistry curricular topics. All the topics involve understanding the topic, as well as performing relevant calculations. However, the topic of environmental chemistry in the Israeli curriculum includes analytical chemistry methods (Mandler et al., 2012, 2014). Namely, the focus can be easily shifted from environmental issues to calculating the metal ion concentration in water. When a teacher emphasizes the calculation aspects, it is difficult for her to suggest many insertion points.
The concept the making of nanotechnology did not reach a consensus during the Delphi study (Sakhnini and Blonder, 2015) but it was decided to be included for educational purposes. It is interesting to note that this concept was most frequently integrated into the chemistry curriculum. Teachers suggested the concept in 7 of the curriculum topics (of which one was not validated). They stressed its uniqueness in terms of the pedagogical level: its great potential for attracting students to learn chemistry, increasing their motivation, and exposing them to contemporary science, as reflected from teachers’ explanations that are presented in Appendix 7. This result is in agreement with a previous study (Blonder and Sakhnini, 2015).
The results of the study highlight several important aspects related to teachers’ knowledge and development. The first aspect is related to the important role the teachers played in the process of identifying and validating the insertion points using the NST essential concepts in the high-school chemistry curriculum. The teachers utilized their pedagogical content knowledge (PCK) (Shulman, 1986, 1987); according to Shulman (1986, 1987) and others (Kind, 2009), one component of PCK is knowledge about the curriculum. In the current study, the teachers’ deep curricular knowledge of the high-school chemistry curriculum enabled them to suggest insertion points using the NST essential concepts they just learned in the course “Introduction to materials and nanotechnology” for subjects in the chemistry curriculum. It is interesting to compare these results to a study that was conducted in Taiwan. In their study Lin et al. (2015) found that teachers reported that their understanding of teaching nanotechnology does not reach a very satisfactory level. They also stated their strong intention to pursue professional development for teaching nanotechnology. We suggest that asking the teachers to suggest insertion points using the NST essential concepts achieved two different goals: (1) it was a research tool used to collect the insertion points for the current study; (2) it also promoted teachers’ knowledge in two dimensions: the content knowledge of NST and their PCK. When the teachers who studied the course needed to look for insertion points using the NST essential concepts, they first needed to understand them (namely, strengthening the CK). The next stage of looking for insertion points within the chemistry curriculum highlights the teachers’ connection of the CK to their own teaching and supports them in any future attempt to integrate NST essential concepts into their chemistry teaching. The finding that teachers suggested the insertion points using several (2–8) NST essential concepts in each subject in the mandatory chemistry curriculum shows that the chemistry curriculum is a suitable medium for teaching about nanotechnology, and that the teachers who participated in the study know how to identify suitable places for this integration.
In addition, integrating the insertion points of the NST concepts into the current high-school chemistry curriculum, by experienced teachers, reflects the relevance of these concepts from the teachers’ perspective. The importance of supplementing the curriculum with scientific materials suggested by the teachers has long been recognized (Connelly and Ben-Perez, 1980; Sabar and Shafriri, 1982). Bennett et al. (2005) referred to several factors that seem to be relevant for teachers in adopting curricular changes, such as the teachers’ perceptions of its effects on students’ learning and their effects on students’ interests and motivation (Blonder et al., 2008b).
The two different forms of courses that the teachers underwent (Blonder, 2011) adequately supported developing teachers’ content knowledge of the NST essential concepts and helped them integrate the NST learned concepts into the curricular topics. According to Bryan et al. (2015), teachers need to be given the time and resources to develop the knowledge bases necessary to effectively integrate and implement NST instruction into existing course curricula. However, content knowledge alone is not sufficient. Blonder's group reported in several studies that adapting scientific knowledge to education is an important stage of the teachers’ professional development (Blonder, 2010; Mamlok-Naaman et al., 2010; Blonder and Mamlok-Naaman, 2016).
Implementation
The results of the current study constitute a research-based mapping of the Israeli high-school chemistry curriculum for areas into which NST essential concepts could be integrated. This mapping may vary for each specific curriculum that is used in other countries. However, most of the curricula presented here represent basic chemistry concepts that are common to chemistry curricula world-wide. Therefore, this study is also applicable to high-school chemistry curricula in other countries. This mapping is therefore useful to chemistry educators who wish to integrate NST concepts into the chemistry curriculum. This integration could be an innovative step towards renovating the chemistry curriculum by adding basic concepts of a contemporary research innovative field, NST.Conflicts of interest
There are no conflicts to declare.Appendix 1
Suggested insertion points of the essential concept applications and innovations in nanotechnology into different topics of the high-school chemistry curriculum and the teachers’ quotations explaining the insertion points.| Curriculum subjecta | Teacher's explanation |
|---|---|
a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Number of teachers who suggested the concept. b Number of teachers who suggested the concept. b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Insertion points that were confirmed at the validation stage. Insertion points that were confirmed at the validation stage. | |
| Basic concepts (2/11)b | “In the first lesson in chemistry, students are exposed to the definition of chemistry. Therefore, we can relate to the notion that chemistry is a continuously developing science that produces new materials with new properties that fit a desired application. We can relate to this idea all the time as we progress through the curriculum contents”. “We can teach research skills by providing the students with an article dealing with nanotechnology applications or by asking them to bring an article that interests them and that deals with the subject and by practicing different research skills”. |
| Atomic structure (4/11)b | “When teaching the periodic table, we discuss metals, non-metals, and semi-conductors. Then we can relate to photovoltaic solar cells and their contributions and relate to LEDs too”. “When teaching the periodic table, we can teach semiconductors and their applications in photovoltaic solar cells. It will motivate and provide them with tools that will help them in the future to acquire knowledge independently”. |
| Structure and bonding (7/11)b | “When teaching molecular materials/organic compounds/functional groups, here we also teach hydrophilic and hydrophobic concepts and also discuss intermolecular forces. Therefore, we can relate to self-cleaning applications and to mimicking nature (the lotus effect) at the same time”. “In atomic materials, we teach carbon allotropy, graphite, and diamonds. Therefore, we can relate to carbon nanotubes (CNTs), their integration into different products, and their contributions to the development of nanotechnology. In addition, we can relate to the benefits and risks of these products to human beings and to humanity”. “While teaching about metallic materials and alloys, we can relate to steel alloy as a material with very special strength properties and can compare its strength to the CNT strength, which is 100 times stronger than a steel alloy, and has different applications in the aircraft industry and other industries. This topic is suitable because it adds social and educational values, bigger doesn't necessarily mean stronger. Sometimes “small” can be very powerful and strong”. |
| Oxidation–reduction (3/11)b | “When teaching about anti-oxidants as natural materials, we can teach that there are many materials that naturally work as anti-oxidants. Based on this knowledge and by studying these materials’ mechanisms, we can use nanotechnology and mimic nature to make new materials that work using the same mechanism for different purposes”. |
| Acids and bases (2/11)b | “Teaching about indicators, we can explain that we use the indicator's property of changing its color to get information regarding the pH level of the base or acid. Therefore, we can teach about Tailoring nano-materials to the requested application. We can teach about tailoring nanoparticles’ properties to generate new properties according to our needed application”. |
| Chemistry of food (2/11)b | “When we teach about saccharides, we can teach about their nano application, which is used in medicine”. “When teaching about a food's ingredients, it is suitable to teach about nano metric sensors that were developed to sense or measure these materials’ concentrations in the body”. |
| Kinetics (2/11)b | “When teaching about catalysis and catalyzers, we can teach about photo-catalysis and a nanotechnology self-cleaning application”. |
| Optional curricular modules | |
| Polymers (7/11)b | “The last chapter of the polymers module relates to the everyday uses of polymers. I think it is an excellent context to teach about OLED and nanocomposite polymers and their contribution to humanity”. “In the polymer module we can teach about nano-composite polymers and the multiple applications it suggests (e.g., food packaging, anti-bacterial products, strong glues, and many more). When teaching these applications, we can relate to the RRI (Responsible research and innovation) dimensions (Schomberg and Von Schomberg, 2013; Blonder et al., 2016) and discuss it with the students. By applying this, we cultivate the students’ awareness of the ethical and social aspects that accompany scientific/technological applications”. |
| Physical chemistry (6/11)b | “We can demonstrate to the students different nanotechnology applications that are produced and applicable based on semi-conductors; we already teach about LEDs and we can also teach about photovoltaic cells”. “When teaching the electronic structure of solids in physical chemistry, we teach about conductors, semi-conductors, insulators, doping, and much more. We can demonstrate the nanotechnology application of solar cells. It is very practical and relevant. In addition, we can relate to many ethical and social issues related to the nanotechnology applications”. |
| Biochemistry (3/11)b | “When teaching the hydrophilic and hydrophobic concepts in proteins, we can relate to the nanotechnology application ‘self-cleaning surfaces’, and link it to technological applications that are based on mimicking nature (The lotus effect)”. “We can relate to nanotechnology applications that mimic nature's processes that occur in the cell. These processes are used as the basis for producing nano devices designed for the same purpose that nature provides”. |
Appendix 2
Suggested insertion points of the essential concept size and scale in different topics of the high-school chemistry curriculum and teachers’ quotations explaining the insertion points.| Curriculum subjecta | Teacher's explanation |
|---|---|
a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Number of teachers who suggested the concept. b Number of teachers who suggested the concept. b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Insertion points that were confirmed at the validation stage. c Insertion points that were confirmed at the validation stage. c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Insertion point that was added during the validation process. Insertion point that was added during the validation process. | |
| Basic concepts (3/11)b | “Teaching about the state of matter in the macroscopic (what you can see and measure) and microscopic levels (the particle level). By teaching the size and scale concept, the students will be able to distinguish between the macro and micro sizes and scales”. “I teach the concept “size and scale” in the first chemistry lesson. I mention the concept in the first lesson to give the students an idea about the scales that chemistry deals with”. |
| Atomic structure (11/11)b | “In the atomic structure topic, students are exposed to many particles whose sizes are hard to estimate (e.g., protons, electrons, neutrons, atoms, and molecules). So it is important to make students realize the differences in the size and scale of each particle and for them to make sense of the atomic structure”. “We are trying to explain to students the meaning of size and scale, using and comparing different objects, so we can manage to help them come closer to the real size of the particles or objects that chemistry deals with. I think that teaching this concept will provide us with more tools to make students more deeply understand different scales of different particles in chemistry”. “When teaching about molecules, atomic structure, and finally the atomic radius, I explain that when we’re talking about an isolated molecule, its size is very small, but when we talk about many of these molecules, we can visualize the quantity but we cannot visualize the molecules. Then we teach about the atomic radius, which is measured using an angstrom scale that is smaller than the nano scale. By doing this, I can provide the students with a tool they can use to demonstrate and describe, in their head, the scales of the different visible and invisible objects”. |
| Structure and bonding (7/11)b | “Using the length of chemical bonds, we can relate to the concept. It will help students understand what scale we are talking about, help them in categorizing their knowledge and the different scales and in realizing the connection between the bonds’ length and strength”. “Students have misconception regarding the chemical bonding length and its characteristics. Therefore, it is necessary to relate to the scale of its size”. “We can relate to the concept, when teaching about atomic materials (CNT, fullerene, carbon allotropy…), metallic and ionic lattices. In all these structures, we talk about “huge” structures and we mention the macromolecule concept. So when mentioning the word “huge”, we should define what the scale of that “huge” structure is. The word huge might create a misconception. Therefore, it is important to relate to the size and scale concept”. |
| Stoichiometry (8/11)b | “When teaching about concentration and dilution, we deal with negative exponents. Students face difficulties in understanding the negative exponents. In discussing the nonmetric size and scale, we can help them to better understand this topic as well as the solution and dilution topics”. “In chemistry we use the mole concept a lot. We use the sizes 1, 2, and 0.5 mole… Students can estimate the size of one mole if we relate it to other sizes in their everyday life. When assigning these numbers to the mole concept, the whole scale changes. We start talking about the tremendous number of particles that participate in a reaction. These scales are very hard for the students to perceive, and they constitute the essence of chemistry”. |
| Chemistry of food (3/11)b | “Teaching about saccharides, proteins, and fats, we talk about macromolecules as huge molecules. The size and scale concept will provide the students with tools that will help them perceive the meaning of a “huge” molecule and relate to what is considered huge”. |
| Optional curricular modules | |
| Polymers (5/11)b | “In polymers and macromolecules, we use a lot the concept macromolecules, a long and twisted chain. Therefore, it is important to realize the different sizes of these molecules and their scale”. |
| Physical chemistry (2/11)b | “I suggest talking about the concept size and scale when introducing the physical chemistry topics before teaching about wavelengths and colors”. |
| Biochemistryc | “Regarding the protein separation process, in the proteins’ solutions we can’t differentiate by the naked eye between those proteins that we intend to separate because of their small nanometric sizes. Therefore, we need to understand and utilize their characteristics”. “The DNA molecule is a macromolecule that is packed in a very small area in the cell. Here it is important to realize the different sizes of these molecules and their scale”. |
| Environmental chemistry (2/11)b | “In this chapter we teach about water quality and analytical methods to determine the concentration of dissolved materials. When we dilute a colorful solution until we’re not able to see the color it seems that the water has no substance in it. However, in fact it is still there but in a very small amount in comparison with the number of water molecules and we need special analytical tools to determine their existence”. |
Appendix 3
Suggested insertion points of the essential concept characterization methods and especially the sub-concepts AFM and resolution in different topics of the high-school chemistry curriculum and the teachers’ quotations explaining the insertion points.| Curriculum subjectsa | Teacher's explanation |
|---|---|
a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Number of teachers who suggested the concept. b Number of teachers who suggested the concept. b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Insertion points that were confirmed at the validation stage. Insertion points that were confirmed at the validation stage. | |
| Basic concepts (3/11)b | “We can relate to the concept when we teach about elements, compounds, mixtures, and pure material. Students should learn about the characterization methods used to see at the atomic level”. “In chemistry lessons we stress the four levels of understanding: the macro, micro, symbol, and process levels. We can term resolution as analogous to the level of understanding. The macro level relates to low-resolution observing and the micro level to a high-resolution observing level”. |
| Atomic structure (2/11)b | “When teaching about the atomic structure, we can relate to the AFM and resolution concept. There is difficulty in teaching about atoms and explaining that they are the particles that compose the material. By using AFM images, students can visualize the material's structure and its surface area topography, although they still cannot “see” the atoms”. “We can stress the importance of the different microscopes as tools that provide us with images regarding the particulate structure of matter. These images provide evidence for the theoretical knowledge we teach, and we validate models and theories that were constructed by scientists that explain the structure of matter”. |
| Structure and bonding (8/11)b | “When teaching about intermolecular bonds and their strength, we can connect to the working principle of the AFM microscope that is based on vdW forces between the atoms at the end of the tip and the atoms and molecules on the scanned surface”. “Teaching about the geometry of molecules, we can teach the students how developments in the field of microscopy enabled scientists to improve their theoretical knowledge and to examine their hypothesis in a visualized, definite way. Therefore, by using AFM, scientists could see the geometry of molecules. These geometries can teach us a lot about the characteristics of the material and its chemical reactivity”. “While teaching the topic ‘interactions between radiation and matter’, we can explain that: (1) by using the electronic microscope, we can measure at the nanometric scale and at high resolution the distance between the atoms in matter. (2) We can scan the surface topography of matter using AFM, which teaches us about the intermolecular forces between the tip of the microscope and the atom, which enables us to determine the surface area's topography, and the electrons and atoms’ density on the surfaces according to the intermolecular interaction forces”. |
| Kinetics (3/11)b | “One of the factors that affects a reaction rate is the surface area. We can relate to the concept AFM as a research method used for characterizing the surface area of different kinds of matter including catalysts, in the same way that we did with aluminum foil in the lab”. |
| Inquiry laboratories (2/11) | “We can relate to the AFM, electron microscope, and resolution concept in the inquiry laboratory module. Students conduct experiments and get different products. These products can be characterized by advanced instrumentation such as AFM. AFM can be used as a research tool for characterization and for learning about the products’ structures”. |
| Optional curricular modules | |
| Polymers (2/11)b | “In polymers we teach about fibers. Using TEM we observe how the fibers are organized in a polymer. This will inform us about the structure of the polymer, its characteristics, and how they will affect its applications”. |
| Physical chemistry (3/11)b | “Teaching the duality of an electron, in spectroscopy. The working mechanism of the electronic microscope is based on this duality. We can present electronic microscopy as a breakthrough application based on the duality principle”. |
| Biochemistry (2/11) | “When teaching about processes inside a living cell, we can introduce different microscopic techniques and discuss the resolution that can be achieved by each and the possibility of visualizing living cells. Using the electron microscopic techniques, we cannot work with living cells before treating them and actually destroying their biological activity”. “In biochemistry, we can use AFM for identifying the secondary structure of a protein that is absorbed on a surface: the alpha helix, the beta structure, and the spherical structure, the DNA and RNA structure and identifying mutations”. |
Appendix 4
Suggested insertion points of the essential concept functionality into different topics of the high-school chemistry curriculum and teachers’ quotations explaining the insertion points.| Curriculum subjectsa | Teacher's explanation |
|---|---|
a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Number of teachers who suggested the concept. b Number of teachers who suggested the concept. b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Insertion points that were confirmed at the validation stage. c Insertion points that were confirmed at the validation stage. c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) The teacher refers to an experiment that was conducted in the course that is based on: (Qinmin et al., 2007). The teacher refers to an experiment that was conducted in the course that is based on: (Qinmin et al., 2007). | |
| Atomic structure (2/11)b | “The idea is to emphasize the unique characteristics that provide matter with properties. The ability of the nucleus of an atom, as a positively charged particle, to attract electrons stabilizes the electronic structure of the atom”. |
| Atomic structure (4/11)b | “The location of each element in a certain column of the periodic table provides it with specific chemical characteristics according to the column”. |
| Structure and bonding (10/11)b | “The properties of matter depend on the characteristics of the functional groups that are included in the molecules. For example, a molecule with a hydroxyl group (–OH) most probably dissolves in water”. “When we teach the structure and bonding topic, we can connect the concept “functionality” because the ability to create intermolecular bonds between molecules explains part of the functionality concept. Hydrogen bonds, for instance, interact specifically and in certain orientations to each other via specific sites”. “The specific ability of intermolecular bonding can provide functionality to a nanoparticle and can enable specific and targeted binding”. |
| Oxidation–reduction reactions (3/11)b | “An oxidizing material has the ability to gain electrons. A reducing material has the ability to lose electrons. This characteristic provides materials with their ability to react in oxidation or reduction reactions, namely, it provides the materials with some functionality”. |
| Acids and bases (2/11) | “An acid is a proton donor. A base is a proton acceptor. This characteristic causes the activity of acid–base reactions and provides the materials with some functionality”. |
| Chemistry of food (7/11)b | “Antioxidants and their ability to lose electrons and react as reductants terminating the free radicals that are obtained in different processes that occur in the human body”. |
| Inquiry laboratories (4/11)b | “We can demonstrate the functionality concept as a part of the inquiry lab, for example, in the “copper plates experiment” where we change the wetting properties of the metal”. |
| Optional curricular modules | |
| Polymers (6/11)b | “We should mention the field of composite materials. When we provide a nanoparticle with special functionality, it will bind much better to a polymeric matrix”. |
| Physical chemistry (7/11)b | “We can relate to functionality when teaching about semi-conductors in photovoltaic cells. Teaching about the structure of the energy levels leads to the specific conductivity characteristics of semi-conductors, which enable them to function as a photovoltaic cell”. |
| Biochemistry (6/11)b | “In this chapter we teach about DNA. Binding different DNA bases and different sequences provide the nanoparticle with very specific binding ability with the complementary DNA strand”. “In the proteins topic, we teach about the enzyme's catalytic site. Its unique structure provides the protein with its catalytic nature and this function converts a protein into an enzyme”. |
| Environmental chemistry (3/11)b | “In environmental chemistry when we teach about water quality, there are many processes that enable purification of water that are based on the functionality of nanomaterials. This includes, for example, the ability of nanomaterials to bind metal ions and to remove them from the water”. |
Appendix 5
Suggested insertion points of the essential concept classification of nanomaterials into different topics of the high-school chemistry curriculum and teachers’ quotations explaining the insertion points.| Curriculum subjecta | Teacher's explanation |
|---|---|
a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Number of teachers who suggested the concept. b Number of teachers who suggested the concept. b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Insertion points that were confirmed at the validation stage. Insertion points that were confirmed at the validation stage. | |
| Basic concepts (3/11)b | “As a part of the basic concepts, we discuss carbon and its various forms. Therefore, we can integrate nanoparticles that are composed of carbon atoms”. |
| Atomic structure (11/11)b | “It is appropriate to integrate the concept when teaching about the periodic table. The classification of nanomaterials is analogous to classifying the elements in the periodic table, where the elements are presented in columns and rows. We can compare and see what we learn from each of the nanomaterials’ classifications and what are the differences and the similarities in them”. “When we teach about the elements’ groups in the periodic table, we can integrate the concept “classification of nanomaterials” according to their conductivity (conductors, semi-conductor, and insulators)”. |
| Structure and bonding (10/11)b | “When dealing with the structure and bonding topic, we discuss metallic bonds and metals. It is appropriate to relate to nanomaterials and their electrical conductivity. Then we can expand and examine what causes conductivity in general”. “In this chapter, we deal with allotropic structures of carbon. It is an appropriate place to represent different carbon nanoparticles (fullerene, carbon nanotubes, and graphene). Dealing with this family of materials enables us to consider other nanomaterials that are not carbon-based, such as inorganic nanomaterials, but ones having the same structure”. |
| Stoichiometry (3/11)b | “We can mention, for example, reactions for creating nanoparticles (categorizing nanomaterials according to their chemical composition)”. |
| Inquiry laboratory (3/11)b | “In the Inquiry laboratories, we can prepare gold nanoparticles and then discuss the different types of nanoparticles representing different types of classification”. |
| Optional curricular modules | |
| Physical chemistry (7/11)b | “We can learn about the electrical conductivity of nanomaterials when teaching this topic. We can learn about the difference between conductive, insulating, and semi-conductive materials”. |
| Biochemistry (3/11)b | “We can mention nanoparticles that are based on DNA, and then relate to the classification of materials according to the organic and inorganic classification”. |
Appendix 6
Suggested insertion points for the essential concept fabrication approaches for nanomaterials in different topics of the high-school chemistry curriculum and teachers’ quotations explaining the insertion points.| Curriculum subjectsa | Teacher's explanation |
|---|---|
a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Number of teachers who suggested the concept. b Number of teachers who suggested the concept. b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Insertion points that were confirmed at the validation stage. Insertion points that were confirmed at the validation stage. | |
| Basic concepts (3/11)b | “When I teach about mixtures and solutions, I explain the hydrophilic and hydrophobic concepts and relate to the detergent property of removing fatty spots as an example for mixtures. Here we can go deeper and connect the micelles’ structure as a self-assembled structure”. “When teaching the description at the micro level, we can talk about the self-assembly that is observed only at the micro level, but we can influence the phenomena at the macro level”. |
| Atomic structure (4/11)b | “We can discuss an analogy to the historical development of the scientific understanding of the atom's structure as a top-down developmental process. Scientists deepened their understanding until they reached the current atomic model structure”. |
| Structure and bonding (6/11)b | “When teaching the intermolecular bonding topic, we can teach the self-assembly concept. For building a bottom-up structure, intermolecular bonds need to be built”. “We can integrate this concept when teaching about materials’ properties and intermolecular bonds. We give examples of detergents, emulsions, and how the properties of a material are determined by the surface interactions and how the molecules are organized; this is actually self-assembly”. |
| Oxidation–reduction (1/11) | “In the laboratory reaction for creating gold nanoparticles, gold ions are reduced to gold atoms and the gold nanoparticles are gradually created in a self-assembly process. We can teach this reaction in class”. “In this topic we apply an experiment called the tree of silver. In the experiment a copper wire is placed inside a solution of silver ions. The silver ions are reduced to produce solid silver that is crystallized around the copper wire in a tree shape: Cu0(s) + Ag(aq)1+ → Cu2+ + Ag0(s) The growth of the silver tree simulates the self-assembly process”. |
| Chemistry of food (3/11)b | “By teaching fatty acid and protein topics, we can relate to the self-assembly concept. The membrane is created by a self-assembly process based on the chemical properties of the fatty acids”. “In teaching about saccharides we start teaching the mono-saccharides structure, then the di-saccharides and the poly-saccharides. This can be compared to a bottom-up process”. |
| Energy (4/11)b | “While teaching the entropy and free energy topics, the self-assembly concept can be integrated. Molecules are usually arranged in structures that spontaneously lead to decreased free energy, so the structure is self-assembled into a more stable state of the system. This explains self-assembly”. |
| Inquiry laboratories (3/11)b | “I suggest preparing gold nanoparticles in the chemistry lab, and actually performing the bottom-up process by reducing gold ions with citrate and gradually producing the gold nanoparticles”. “The process of the inquiry laboratory is built in a bottom-up structure: We start from certain small pieces of data followed by an experiment, the results and conclusions, which are the big picture. We can talk with our students about this analogy”. “In the experiment of the CuCl2 electrolysis we can see the bottom-up process. In this experiment, a blue CuCl2 solution undergoes the process of electrolysis. Students can see the creation of copper metal, and the solution's color disappears as a result of the decreased copper ion concentration in the solution. This experiment is an example of a macroscopic bottom-up process”. |
| Optional curricular modules | |
| Polymers (5/11)b | “When teaching how an amorphous polymer is transformed into a well-arranged and crystallized structure proceeding as a slow cooling process as opposed to a fast cooling process, we are actually talking about the self-assembly of a polymer chain”. “Polymerization methods are a bottom-up process, whereas the hydrolysis of a polymer back to monomers is a top-down process”. |
| Physical chemistry (2/11)b | “We can integrate the concepts top-down and self-assembly when teaching about microelectronics. The photolithography principle (in which the industry creates chips) is a top-down process”. |
| Biochemistry (3/11)b | “In my opinion, we can use the bottom-up and self-assembly concept when teaching the lipids topic and when discussing the phospholipids organizing into shapes (e.g., liposome, micelles, and cell membrane). These topics demonstrate how the membrane's structure (which is in nanometric sizes) is organized in contrast to the other structures”. “We can consider the quarternary structure of a protein as a self-assembled structure. The protein is folded this way because of thermodynamics and that leads to some functionality”. |
Appendix 7
Suggested insertion points of the essential concept the making of nanotechnology into different topics of the high-school chemistry curriculum and teachers’ quotations explaining the insertion points.| Curriculum subjectsa | Teacher's explanation |
|---|---|
a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Number of teachers who suggested the concept. b Number of teachers who suggested the concept. b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Insertion points that were confirmed at the validation stage. Insertion points that were confirmed at the validation stage. | |
| Basic concepts (3/11)b | “In the first lesson in chemistry, we can relate to the fact that chemistry is an interdisciplinary field that deals with astronomy, physics, material engineering, biology, geology, and others. Chemistry is considered an essential core science. Nanotechnology is a field in which chemists play an important role”. “We can use this concept as an example to explain the process of scientific research”. |
| Atomic structure (4/11)b | “In the atomic structure and the periodic table, the data collection process requires cooperation among scientists. Also in the historical development of atomic structure, we encounter scientific cooperation between scientists from different disciplines (chemists, physicists). This can be compared to the interdisciplinary nature of nanotechnology”. |
| Structure and bonding (3/11)b | “When we teach about allotropic forms of carbon and about graphite, we can add the development of carbon nanoparticles such as CNTs and graphene”. |
| Stoichiometry (1/11) | “When we teach stoichiometry, students feel that this subject is based on chemistry and mathematics. The teacher can use this opportunity to present the interdisciplinary nature of contemporary science and more specifically, nanotechnology”. |
| Oxidation–reduction (1/11)b | “When teaching corrosion protection methods, we can present the new nanotechnology method used for corrosion protection and discuss the collaboration needed between scientists from different scientific disciplines (chemistry and materials engineering) to make this nanotechnology application work”. |
| Chemistry of food (3/11)b | “Learning about saccharides, proteins, and fatty acids is an example of collaboration between chemists, biologists, physicians, and nutritionists. This collaboration also supports research for finding medications for different diseases”. “When we teach about fatty acids and discuss hydrophilicity and hydrophobicity, we can introduce the nanotechnology application of self-cleaning. This will exemplify the multidisciplinary nature of nanoscience and nanotechnology”. “Students learn about the story of how margarine was invented. In this context, it is important to expose them to the work of a modern chemist, the interdisciplinary knowledge needed (e.g., biology and food engineering) and to the fact that modern research labs employ scientists from different scientific fields that work together to give answers to questions in common”. “We teach about saccharides, fatty acids, and proteins. In these macromolecules, the connection between structure and function has an important role in biological systems. Here we need team work between scientists from different scientific disciplines (chemists, biologists, mathematicians, and nutritionists) to create scientific understanding”. |
| Inquiry laboratories (4/11)b | “This concept can be integrated when explaining the team work nature of lab work. Each person in the team does his best and contributes to the success of the work”. “We can use this concept to explain where a research idea comes from… Observations, research questions…”. “As an introduction for the inquiry lab, we can stress the importance of team work in the lab. We can demonstrate an example of applicative/basic research and stress the importance of cooperation within different scientific disciplines in the research process as a basis for its success”. |
| Optional curricular modules | |
| Polymers (4/11)b | “We can teach about integrating nanoparticles into polymers to get different products with different properties. This requires team work between scientists from different scientific disciplines such as doctors, ecologists, and food packaging”. “When teaching tensile strength we should emphasize that this concept comes from the engineering world and that nanotechnology connects the world of science and the world of technology”. |
| Physical chemistry (2/11) | “When we teach about semi-conductors and describe the semi-conductor industry, we can describe the historical development of the electronics industry and the contribution of nano materials to this industry. This will refer to the historical development of nanotechnology and also to the interdisciplinary nature of the field”. |
| Biochemistry (3/11)b | “In biochemistry, one can see the link between chemistry and biology. We can explain that in science, it is difficult to separate scientific disciplines, because each discipline complements other disciplines. For example, in biochemistry, the chemical structure influences the biological function”. |
Acknowledgements
We acknowledge Mr Ziv Ariely for the design of Fig. 1. This work was partially funded by Helen and Martin Kimmel Center for Nanoscale Science.References
- Barak M. and Hussein-Farraj R., (2013), Integrating model-based learning and animations for enhancing students' understanding of proteins' structure and function, Research in Science Education, 43(2), 619–636.
- Bennett J., Gräsel C., Parchmann I. and Waddington D., (2005), Context-based and conventional approaches to teaching chemistry: comparing teachers' views, Int. J. Sci. Educ., 27(13), 1521–1547.
- Blonder R., (2010), The influence of a teaching model in nanotechnology on chemistry teachers' knowledge and their teaching attitudes, J. Nano Educ., 2, 67–75. DOI: http://10.1166/jne.2010.1004.
- Blonder R., (2011), The story of nanomaterials in modern technology: an advanced course for chemistry teachers, J. Chem. Educ., 88, 49–52. DOI: http://10.1021/ed100614f.
- Blonder R. and Dinur M., (2011), Teaching nanotechnology using student-centered pedagogy for increasing students' continuing motivation, J. Nano Educ., 3, 51–61. DOI: http://10.1166/jne.2011.1016.
- Blonder R. and Mamlok-Naaman R., (2016), Learning about teaching the extracurricular topic of nanotechnology as a vehicle for achieving a sustainable change in science education, Int. J. Sci. Math. Educ., 14, 345–372. DOI: http://10.1007/s10763-014-9579-0.
- Blonder R. and Sakhnini S., (2012), Teaching two basic nanotechnology concepts in secondary school by using a variety of teaching methods, Chem. Educ. Res. Pract., 13, 500–516. DOI: 10.1039/C2RP20026K.
- Blonder R. and Sakhnini S., (2015), The making of nanotechnology: exposing high-school students to behind-the-scenes of nanotechnology by inviting them to a nanotechnology conference, Nanotechnol. Rev., 4(1), 103–116. DOI: http://10.1515/ntrev-2014-0016.
- Blonder R. and Sakhnini S., (2016), What are the basic concepts of nanoscale science and technology (NST) that should be included in NST educational programs, in Winkelmann K. and Bhushan B. (ed.), Global Perspectives of Nanoscience and Engineering Education, AG Switzerland: Springer International Publishing, pp. 117–127.
- Blonder R., Mamlok-Naaman R. and Hofstein A., (2008a), Analyzing inquiry questions of high-school students in a gas chromatography open-ended laboratory experiment, Chem. Educ. Res. Pract., 9, 250–258. DOI: 10.1039/B812414K.
- Blonder R., Mamlok-Naaman R., Kipnis M. and Hofstein A., (2008b), Increasing science teachers' ownership through the adaptation of the PARSEL modules: a “bottom-up” approach, Sci. Educ. Int., 19, 285–301.
- Blonder R., Joselevich E. and Cohen S. R., (2010), Atomic force microscopy: opening the teaching laboratory to the nanoworld, J. Chem. Educ., 87(12), 1290–1293. DOI: http://10.1021/ed100963z.
- Blonder R., Zemler E. and Rosenfeld S., (2016), The story of lead: A context for learning about responsible research and innovation (RRI) in the chemistry classroom, Chem. Educ. Res. Pract., 17, 1145–1155, DOI: 10.1039/C6RP00177G.
- Bryan L. A. and Giordano N. J., (2015), Special issue on Pre-college nanoscale science, engineering, and technology learning, Nanotechnol. Rev., 4(1), 1–6. DOI: http://10.1515/ntrev-2014-0051.
- Bryan L. A., Magana A. J. and Sederberg D., (2015), Published research on pre-college students' and teachers' nanoscale science, engineering, and technology learning, Nanotechnol. Rev., 4(1), 7–32. DOI: http://10.1515/ntrev-2014-0029.
- Cohen S., Blonder R., Rap S. and Barokas J., (2016), Online nanoeducation resources, in Winkelmann K. and Bhushan B. (ed.), Global perspectives of nanoscience and engineering education, AG Switzerland: Springer International Publishing, pp. 171–194.
- Connelly F. M. and Ben-Perez M., (1980), Teacher's role in the using and doing of research and curriculum development, J. Curric. Stud., 12, 95–107.
- Dangur V., Avargil S., Peskin U. and Dori Y. J., (2014), Learning quantum chemistry via a visual-conceptual approach: students' bidirectional textual and visual understanding, Chem. Educ. Res. Pract., 15, 297–310. DOI: 10.1039/c4rp00025k.
- Dori Y. J., Dangur V., Avargil S. and Peskin U., (2014), Assessing advanced high school and undergraduate students' thinking skills: the chemistry—from the nanoscale to microelectronics module, J. Chem. Educ., 91(9), 1306–1317. DOI: http://10.1021/ed500007s.
- Flynn L., Johnson P. and Penn R. L., (2007), Building a successful middle school outreach effort: microscopy camp, J. Chem. Educ., 84(6), 955. DOI: http://10.1021/ed084p955.
- Gilbert J. K., (2006), On the nature of “context” in chemical education, Int. J. Sci. Educ., 28, 957–976.
- Gilbert J. K. and Lin H. S., (2012), How might adults learn about new science and technology? The case of nanoscience and nanotechnology, Int. J. Sci. Educ., Part B, 1–26. DOI: http://10.1080/21548455.2012.736035.
- Herscoviz O., Kaberman Z. and Dori Y. J., (2007), The “Taste of Chemistry” Unit, Bulletin of Chemistry Teachers Alchemia (Hebrew), 11, 39–47.
- Hofstein A., Mamlok R. and Rosenberg O., (2006), Varying instructional methods and students assessment methods in high school chemistry, in McMahon M., Simmons P., Sommers R., DeBaets D. and Crawley F. (ed.), Assessment in Science, Arlington, Virginia: NSTA Press, pp. 139–148.
- Hutchinson K., Bodner G. M. and Bryan L. A., (2011), Middle-and high-school students' interest in nanoscale science and engineering topics and phenomena, J Precoll Eng Educ Res, 1(1), 30–39. DOI: http://10.7771/2157-9288.1028.
- Jones M. G., Blonder R., Gardner G. E., Albe V., Falvo M. and Chevrier J., (2013), Nanotechnology and nanoscale science: educational challenges, Int. J. Sci. Educ., 35, 1490–1512. DOI: http://10.1080/09500693.2013.771828.
- Katchevich D., Hofstein A. and Mamlok-Naaman R., (2013), Argumentation in the chemistry laboratory: inquiry and confirmatory experiments, Res. Sci. Educ., 43, 317–345. DOI: http://10.1007/s11165-011-9267-9.
- Kind V., (2009), Pedagogical content knowledge in science education: perspectives and potential for progress, Stud. Sci. Educ., 45(2), 169–204.
- Laherto A., (2012), Informing the development of science exhibitions through educational research, Int. J. Sci. Educ., Part B, 1–23. DOI: http://10.1080/21548455.2012.694490.
- Lin S.-F., Chen J.-Y., Shih K.-Y., Wang K.-H. and Chang H.-P., (2015), Science teachers' perceptions of nanotechnology teaching and professional development: a survey study in Taiwan, Nanotechnol. Rev., 4(1), 71–80. DOI: http://10.1515/ntrev-2014-0019.
- Mamlok-Naaman R., Blonder R. and Hofstein A., (2010), Providing chemistry teachers with opportunities to enhance their knowledge in contemporary scientific areas: a three-stage model, Chem. Educ. Res. Pract., 11, 241–252. DOI: 10.1039/C0RP90005B.
- Mandler D., Mamlok-Naaman R., Blonder R., Yayon M. and Hofstein A., (2012), High-school chemistry teaching through environmentally oriented curricula, Chem. Educ. Res. Pract., 13, 80–92. DOI: 10.1039/c1rp90071d.
- Mandler D., Blonder R., Yayon M., Mamlok-Naaman R. and Hofstein A., (2014), Developing and implementing inquiry-based, water quality laboratory experiments for high school students to explore real environmental issues using analytical chemistry, J. Chem. Educ., 91, 492–496. DOI: http://10.1021/ed200586r.
- Moosavifazel V., Kumar A., Cho H. J. and Seal S., (2014), Laboratory research motivated chemistry classroom activity to promote interests among students towards science, J. Nano Educ., 6, 25–29. DOI: http://10.1166/jne.2014.1050.
- Murriello S., Contier D. and Knobel M., (2009), NanoAventura: an interactive exhibition on nanoscience and nanotechnology as an educational tool, J. Nano Educ., 1, 96–105.
- NAP, (2016), Triennial Review of the National Nanotechnology Initiative, Retrieved from Washington, DC.
- Qinmin P., Haizu J. and Hongbo W., (2007), Fabrication of superhydrophobic surfaces on interconnected Cu(OH)2 nanowires via solution-immersion, Nanotechnology, 18(35), 355605, Retrieved from http://stacks.iop.org/0957-4484/18/i=35/a=355605.
- Sabar N. and Shafriri N., (1982), On the need for teachers training in curriculum development, Stud. Educ. Eval., 7, 307–315.
- Sakhnini S. and Blonder R., (2015), Essential concepts of nanoscale science and technology for high school students based on a Delphi study by the expert community, Int. J. Sci. Educ., 37(11), 1699–1738. DOI: http://10.1080/09500693.2015.1035687.
- Sakhnini S. and Blonder R., (2015), Essential concepts of nanoscale science and technology for high school students based on a Delphi study by the expert community, Int. J. Sci. Educ., 37(11), 1699–1738. DOI: http://10.1080/09500693.2015.1035687.
- Schomberg V. and Von Schomberg R., (2013), A vision of responsible research and innovation, in Owen R., Heintz M. and Bessant J. (ed.), Responsible Innovation, London: John Wiley & Sons, Ltd, pp. 51–74.
- Shulman L. S., (1986), Those who understand: Knowledge growth in teaching, Educ. Res., 15, 4–14.
- Shulman L. S., (1987), Knowledge and teaching – foundations of the new reform, Harvard Educ. Rev., 57, 1–22.
- Taylor A. and Jones G., (2009), Proportional reasoning ability and concepts of scale: surface area to volume relationships in science, Int. J. Sci. Educ., 31(9), 1231–1247. DOI: http://10.1080/09500690802017545.
- Tenne R., (2006), Inorganic nanotubes and fullerene-like nanoparticles, J. Mater. Res.J. Mater. Res., 11, 2726–2743. DOI: http://10.1557/jmr.2006.0354.
Footnotes |
| † The definitions of the NST essential concepts are taken from Sakhnini and Blonder (2015). |
| ‡ The chemistry teachers who participated in the study studied the course “Introduction to materials and nanotechnology” at the Weizmann Institute of Science. In this course (Blonder, 2011) they work with an AFM (Blonder, 2010; Blonder et al., 2010) that is used for teaching purposes only. After they complete the course, they can come with their students to work with this AFM instrument. |
| This journal is © The Royal Society of Chemistry 2017 |
