‘Triangulation:’ an expression for stimulating metacognitive reflection regarding the use of ‘triplet’ representations for chemistry learning
Gregory P.
Thomas

The University of Alberta, Canada. E-mail: gthomas1@ualberta.ca
First published on 23rd March 2017
Abstract
Concerns persist regarding high school students' chemistry learning. Learning chemistry is challenging because of chemistry's innate complexity and the need for students to construct associations between different, yet related representations of matter and its changes. Students should be taught to reason about and consider chemical phenomena using ‘triplet’ representations. A meta-language to discuss chemistry learning with students regarding these representations and their use is therefore necessary. This paper reports on a classroom intervention in which the teacher used the term ‘triangulation’ as an expression to stimulate metacognitive reflection in students to consider the importance and use of these representations for their learning of chemistry. Students understood and could elaborate the meaning of triangulation. However, their views of the importance and reported use of cognitive processes associated with it varied across individuals. Despite the variation, this study highlights the potential of developing students' metacognition by explicitly engaging them in considering means of representing the chemistry subject material they are being asked to learn, and how they might learn it using strategies and activities that are aligned with the nature of that material.
Theoretical framework and objectives
A perspective on macroscopic, molecular/submicroscopic and symbolic representations and their use in chemistry education
Concerns persist regarding students' learning of chemistry. Learning chemistry is challenging because of chemistry's innate complexity and the need for students to construct associations between different representations of matter and its chemical and physical changes (Johnstone, 1993; Gabel, 1998; Mahaffy, 2004; Taber, 2013). Chemistry reasoning and the development of understanding should be characterized by conscious consideration of chemical phenomenon at macroscopic, molecular/submicroscopic and symbolic levels (Gabel, 1998; Bucat and Mocerino, 2009; Gilbert and Treagust, 2009; Taber, 2013), even though this may present limitations and problems for students and teachers as suggested by Talanquer (2010). This means, for example, students observing a chemical reaction, being able to, (a) describe changes in macroscopic properties as evidenced by, for example, color and/or enthalpy change/s, (b) envisage and explain corresponding changes happening during the reaction at the molecular, atomic and/or sub-atomic levels, and (c) use the symbols of chemistry communication to describe such changes. There is an inter-relating and connecting of each of the levels to assist achieving conceptual understanding of chemical phenomena.A substantial volume of literature has developed over the past twenty years that considers the importance of the three levels of representation, the chemistry ‘triplet,’ for teaching and learning chemistry. Much of it has explored the influence of teachers attending more to their use of the representations when teaching chemistry; the goal being the development of students' conceptual understanding and/or facilitating students' conceptual change. Given the emphasis on conceptual learning and conceptual change in the chemistry and science education literature (Sinatra and Pintrich, 2003; Duit and Treagust, 2012; Vosniadou, 2012) such goal orientation is not surprising. A consistent finding from this research is that when teachers use the three levels of representation students' conceptual understanding of chemistry is enhanced and their learning improves. Within this literature, of particular note for this paper, are the studies of Treagust et al. (2003) and Madden et al. (2003). Treagust et al. (2003) examined teachers' use of representations in explanations and how such use provided meaning for students. They suggested that, (a) “despite the efforts of the teacher, the role of submicroscopic and symbolic representations may not be understood by the learner…students do not always understand the role of the representation that is assumed by the teacher,” and (b) students can become “familiar with the mode of explanation, learning to use the various representations appropriately and interpreting their meaning accurately” (p. 1367). Madden et al. (2003) explored the representational competence of students engaged in problem solving. They suggested that, “students may benefit from instructional strategies that emphasize the heuristic use of multiple representations in chemistry problem solving” (p. 283). A suggestion that might be drawn from these two studies, is that it may be beneficial to attend explicitly to developing students' knowledge of representations and their use and value for chemistry learning, while at the same time being aware that students may have difficulty understanding the nature of the representations and how they might be used.
If this suggestion were to be explored in chemistry classrooms, it would mean more than teachers being aware of the need to develop students' learning based on the triplet, and teachers planning instruction accordingly. It would mean that teachers also communicate explicitly with students about the levels of representations and explain how classroom activities and experiences relate to the possibility of their learning chemistry and coming to know about what it means to understand chemistry with reference to those representations. The goal of such communication would be to develop students' metacognition, beginning with their construction of metacognitive knowledge, in relation to the triplet and its value and use. This possibility seems not to have been explored in the chemistry education literature with, as previously noted, the predominant focus of research related to the levels of representation being on raising teachers' awareness of them to alter their pedagogy and enhance students' conceptual learning. One recent example of such research is that of Lewthwaite and Weibe (2011) who explored teacher progress towards use of Mahaffy's (2004) tetrahedral model, an extension on that of Johnstone (1991). Lewthwaite and Weibe found that teachers showed limited progress towards the desired orientation, and that any advancement was influenced by contextual and personal factors. Notably, in the Chemistry Teacher Inventory that they developed to map change in teachers' pedagogies over the four years of their project, there is no mention of teachers talking explicitly with students about the representational model.
From the perspective of developing students as chemistry learners, it can be argued that it is students as well as teachers who need to be taught about the ‘triplet’ representations and their potential use. Students should become consciously aware of how their chemistry knowledge base might be organized, how to organize it according to their learning goals, and how to monitor the development of their understanding. This paper investigates a teacher's use of the expression, ‘triangulation,’ for eliciting metacognitive experiences in students' to stimulate their metacognitive reflection regarding the nature and use of macroscopic, molecular/submicroscopic and symbolic representations for chemistry learning. An overview of metacognition, its importance in learning science and chemistry, and the use of language to stimulate and enhance metacognitive reflection is provided in the next sections.
Metacognition: an overview
As explained above, one way for students to improve their chemistry learning is for them to consciously employ strategies that engage their consideration of chemical phenomena using macroscopic, molecular/submicroscopic and symbolic representations. Such conscious application of possibly previously unconsidered strategies requires attending to students' metacognition, i.e., their knowledge, control and awareness of their thinking and learning processes (Flavell, 1976; White, 1998; Thomas, 2012). To do this, teachers need to elicit metacognitive experiences in students. Metacognitive experiences are students' feelings of familiarity, difficulty, learning confidence and satisfaction that are present in and triggered by learning situations (Efklides, 2006). They are the raw material for stimulating metacognitive reflection which involves students reflecting on their thinking and learning processes and the efficacy of those processes.Metacognitive knowledge can be categorized as declarative, procedural or conditional. Declarative metacognitive knowledge takes the form of propositions such as, ‘learning chemistry is about memorizing information and recalling it for exams.’ Procedural metacognitive knowledge takes the form of, for example, ‘when I memorize I write down the first twenty elements of periodic table in order ten times, then repeat that order until I can repeat it without reference to the list.’ It is metacognitive knowledge concerning descriptions of processes individuals consider they use to learn. Conditional metacognitive knowledge takes the form of, for example, ‘I use memorization strategies at the start of a unit when I encounter a lot of new information, but I use concept maps later in a unit when I need to see the connections between ideas.’ Such knowledge concerns when and why a particular learning or cognitive strategy might be used at a particular time or in a particular context.
Scholars have recently begun to conceptualize metacognitive knowledge in new ways. Increased interest in epistemic cognition has resulted in attention being focused on epistemic metacognition and epistemic metacognitive knowledge, which are relevant to this study (Hofer, 2005; Hofer and Sinatra, 2010). As Barzilai and Zohar (2016) state,
People's knowledge, beliefs, ideas and theories about the nature of knowledge and knowing are meta-level epistemic knowledge or EMK…EMK can entail knowledge about persons (EMPK) that includes knowledge about the individual as knower, about other people as knowers, and about human knowledge and knowing in general, and knowledge about strategies and tasks (EMSK) that includes knowledge about strategies of construction and justification of knowledge…EMSK is knowledge about how to carry out an activity that will result in knowing and consists of knowledge about when, why and how to use epistemic strategies and about the reliability of those strategies. (p. 414)
The aforementioned view that understanding chemistry involves consideration of chemical phenomenon at macroscopic, molecular/submicroscopic and symbolic levels clearly relates to EMK about the nature of knowing chemistry, which might be considered a form of declarative metacognitive knowledge. Students knowledge of strategies that they know result in knowing and understanding chemistry clearly relates to EMSK, which might be considered, according to Barzilai and Zohar (2016), to be a blend of declarative metacognitive knowledge and conditional metacognitive knowledge. Therefore, when a teacher elicits metacognitive experiences in students to stimulate their metacognitive reflection regarding the nature and use of macroscopic, molecular/submicroscopic and symbolic representations for learning chemistry then the teacher might be targeting both students' EMK and EMSK.
While all students are metacognitive to some extent (Gunstone, 1994), successful chemistry learners typically have a battery of learning strategies that they can employ to achieve learning goals depending on the task and context (Chan and Bauer, 2016; Ye et al., 2016). They engage in inner reflective dialogues with themselves where the dialogues might involve reviewing learning strategies or evaluating the effectiveness of cognitive performance as they engage in a task. Adey and Shayer (1994, p. 71) restrict metacognition to “stressing conscious, reflective awareness about strategies.” This view has also been advocated by Conner and Gunstone (2004), Thomas (2012), and Zohar and David (2009). Therefore, it follows that to develop and enhance metacognition there should be opportunities for students to engage in such conscious metacognitive reflection and become more aware of their learning processes and the strengths and weaknesses of them.
This paper is premised on the view, supported in the existing science education literature, that there is a metacognitive knowledge base that students can be taught explicitly so they develop metacognitive knowledge that is adaptive for their science learning contexts (Baird, 1986; Blank, 2000; Thomas and McRobbie, 2001; Georghiades, 2006; Connor, 2007). This view also has support from the general metacognition community (Flavell, 1987; Schraw, 1998). Studies in science education have consistently found, (a) that students' metacognition is often not adaptive for learning science, including chemistry, and (b) that when their metacognition is enhanced and becomes aligned with understanding and consciously employing strategies associated with quality science learning that their understanding of science is also often enhanced (White, 1998; Georghiades, 2004; Haider and Al Naqabi, 2008; Kaberman and Dori, 2009). However, most classrooms are not sufficiently metacognitively oriented and teachers most often tend to focus predominantly on the material to be learned rather than attending adequately to explicitly teaching students how material might be learned and understood. In particular, the level of metacognitive demand in science classrooms, the extent to which teachers challenge students to consider their existing and potentially new learning strategies, has consistently been found to be insufficient to stimulate adequately the metacognitive reflection and awareness necessary for students to re/consider their thinking and learning processes (Thomas, 2003, 2013; Thomas and Anderson, 2014).
Based on the perspectives above, changes in students' metacognition can be understood in relation to changes in their metacognitive knowledge and an awareness and reflection regarding the value or otherwise of existing or newly proposed learning strategies. As shown in previous studies (Thomas, 1999; Thomas and McRobbie, 2013; Thomas and Anderson, 2014) the extent to which high school students enact new metacognitive knowledge is highly variable and dependent on a range of contextual and personal matters; many of which are beyond the control of the classroom teacher. Even so, it is important for teachers to develop learning environments that elicit metacognitive experiences in students and stimulate their metacognitive reflection regarding the viability of subject appropriate thinking and learning strategies irrespective of whether students opt to employ them. Students need to understand what it means to learn chemistry and this needs to be explicitly communicated to them to reflect upon. The students' thinking and learning processes and proposals to consider or alter those processes become significant ‘objects’ of attention and metacognitive reflection in the classroom, as well as the material to be learned. In addition, the teacher makes metacognitive demands on students “to be aware of how they learn and how they can improve their science learning” (Thomas, 2003, p. 184).
The use of language to elicit metacognitive experiences and stimulate metacognitive reflection
Altering the metacognitive demands of a learning environment requires that teachers use a language of learning to communicate with students about what it means to learn in a specific subject area, in this case chemistry, and about the thinking and learning strategies they might use. The notion of a language of learning centres around developing a shared vocabulary of expressions that the teacher and students both understand as describing ways of thinking or cognitive processes (Tishman and Perkins, 1997; Costa, 2007; Coffield, 2012). This enables classroom participants to employ those terms so that they can discuss how they are thinking as well as what they are thinking. In previous research in chemistry education, Thomas and McRobbie (2001) used the metaphor ‘learning is constructing’ as the basis of a language of learning to discuss constructivist learning processes and to develop students' metacognition in relation to those processes. The students and teacher developed a shared understanding of what it meant to learn and understand chemistry and the cognitive processes that might be employed, and the students' metacognition was developed and enhanced. This study builds on such previous research.Implications for pedagogy and research: study objectives and research question
Based on the previous discussion on ‘triplet’ representations and metacognition and the importance of both in chemistry education; the position in this paper is that both teachers and researchers should be made aware of and conversant with the importance of these representations. In addition, students should be explicitly taught about them using a language of learning to elicit metacognitive experiences that stimulate their metacognitive reflection on the representations and their value for learning chemistry. Currently, there is very little, if any, evidence to support a view that chemistry teachers explicitly use a language of learning as defined above to direct students' attention to consider learning perspectives based on the chemistry ‘triplet.’ Teachers need to make explicit metacognitive demands on their students within chemistry classroom learning environments for this to occur.The objective of this study was to use the expression, ‘triangulation,’ and activities centred on that expression with students to elicit metacognitive experiences to stimulate their metacognitive reflection regarding the use of representations for chemistry learning. This strategy was adopted to encourage their metacognitive development, beginning with their metacognitive knowledge, relevant to learning the chemistry under consideration within their normal curriculum. The following research question was derived from this objective: “Does a change in teacher pedagogy centered on the use of an expression, ‘triangulation’ to explicitly teach the ‘triplet’ model of representations and its use in the classroom stimulate students' metacognitive reflection regarding those representations for learning chemistry?”
Methodology
Research Context
Following the pre-pedagogical change data collection phase outlined below, Craig and the researcher discussed suggestions for pedagogical change in an open and collegial manner. Rather than see teacher autonomy and professional judgment as a concern and something that might jeopardize the fidelity of an intervention or the research, this researcher viewed it as necessary to afford this study, conducted in the naturalistic setting of a chemistry classroom, a reasonable level of contextual validity. It was agreed that initiating changes in students' reasoning would necessitate changes in the classroom environment and development of terminology and/or language that could be used to communicate with students about the ‘triplet’ representations, and what these might mean for their learning of chemistry. Craig was supported to modify his pedagogy by, (a) providing him with literature relevant to understanding the centrality of understanding chemistry at macroscopic, molecular/submicroscopic and symbolic levels, (b) developing classroom scripts and activities for him to consider, adopt and adapt as he saw appropriate, (c) involving him in the development of the mode of presentation of the ‘triplet’ model and terminology for his use with students that reflected the thinking consistent with each of the three levels, and (d) daily debriefings to discuss the intervention's effects and for further planning.
The ethics boards of the University of the researcher and the relevant school district approved the study. Informed consent was obtained from all students and their parents or guardians. Craig consented to being audio and video recorded, however there was no permission sought or given for interviews with him. This was because the research was focused on the teacher as part of the classroom environment, and on the impact of changes in the classroom environment on students' metacognitive reflection and metacognition in relation to learning chemistry. Discussions between the teacher and the researcher were focused on professional issues related to pedagogy and classroom discourse and from the outset it was decided by Craig and the researcher that Craig's intimations to the researcher would not be part of the data corpus. This was to promote the building of a strong collegial and confidential relationship between the teacher and the researcher who were both interested predominantly in the students' progress.
Methods and data sources
There is ongoing debate regarding how best to conduct research into students' metacognition. Some argue that real-time ‘on-line’ methods are most appropriate (Veenman et al., 2006; Meijer et al., 2012). Studies employing on-line methods often involve a single data collection event, often involving the recording of students' think-aloud dialogue or eye-tracking during task performance. They are undertaken typically in quasi-clinical settings in which students are asked to engage with tasks that might not be directly related to what is to be learned as part of their everyday schooling. In many cases, whether or not the research context or the material to be learned is relevant to students' day-to-day schooling and therefore motivating for them is given scant, if any, attention. However, for the purposes of unearthing important findings that inform emerging theoretical perspectives on psychological aspects of metacognition they are useful techniques.The alternative to on-line methods are those categorized as ‘off-line’ which are most commonly used in science education research. These methods usually involve interviews and self-report questionnaires. Across education they have been extensively employed in the study of metacognition (Pintrich et al., 1991; Schraw and Dennison, 1994; Mokhtari and Reichard, 2002). In science education they have been used to evaluate students' metacognition and learning strategies for three decades (Baird, 1986; Case and Gunstone, 2006; Georghiades, 2006; Connor, 2007; Anderson et al., 2009). Off-line measures have been repeatedly used in studies to explore change in metacognition and metacognitive reflection in naturalistic settings. Rather than just learning more about metacognition drawn from only the analysis of one-off measures from experimental studies, researchers use off-line methods to seek to understand the potential influence of interventions within everyday classrooms over time.
In addition, and of relevance for this paper, making credible assertions regarding the efficacy of interventions targeting students' metacognition requires data in addition to that which is collected on their metacognition. Contextual information, often not visible or considered necessary in clinical or experimental reports, is of vital importance. As has been found in numerous studies in science education (Thomas, 1999; Case and Gunstone, 2006; Anderson et al., 2009) metacognition is socially situated. Context plays an important role in understanding the impact of any intervention. Based on the aforementioned considerations, a mixed-methods methodology concerned with “human social actions and opinion that are locally distinct and situationally contingent” (Erickson, 1998, p. 1155) was adopted for this study.
Both qualitative and quantitative methods were employed over the research period as shown in Table 1. The value of using mixed methods in studies such as this has been suggested by Azevedo (2005), Thomas (2009) and White (1998). Marshall (1996) has argued that collecting data, “with multiple methods over time allows researchers to consider the reciprocal interactions among the psychological, social and cultural aspects, and to shift the focus to foreground any of these, depending on the purpose” (p. 238). Finally, Johnson and Onwuegbuzie (2004, p. 23) support the use of mixed methods as a pragmatic approach allowing researchers to, “select methods and approaches with respect to the underlying research questions, rather than with regard to some preconceived biases about which research paradigm should have hegemony in social science research.” Therefore, the use of mixed methods was appropriate for this study.
| Research target of the method | Method | ||||
|---|---|---|---|---|---|
| Metacognitive demands scale of MOLES-Sa | Interviews with studentsa | Constructivist connectivity scale of SEMLI-Sa | Classroom video and audiob | Classroom observationb | |
| a Completed pre- and post-intervention. b Continuous over the twenty week period of the research. | |||||
| Nature of the classroom environment, esp. metacognitive demands | ✓ | ✓ | ✓ | ✓ | |
| Evidence of metacognitive reflection and metacognitive change | ✓ | ✓ | |||
As previously explained, this study is concerned with understanding the impact of an intervention within a chemistry classroom on stimulating students' metacognitive reflection on their cognition associated with their chemistry learning. Therefore, it was important to collect pre- and post-intervention data on, (a) the nature of the chemistry classroom environment, as well as, (b) the students' metacognition, especially their metacognitive knowledge and their reflections on any changes to that knowledge. Table 1 shows that multiple forms of both qualitative and quantitative data were sought to provide information regarding (a) and (b).
To develop a thick description of the classroom environment the data sources employed were classroom observations and field notes, interviews with students, recording of classroom transactions by a video camera (rear) and a radio microphone on the teacher, and students' responses to the 5-item metacognitive demands scale of the Metacognitive Orientation Learning Environment Scale – Science (MOLES-S) (Thomas, 2003). In the MOLES-S survey these five items are ordered together in one section. These items are shown in Table 2.
| Scale name | Metacognitive demands (MD) | Constructivist connectivity (CC) |
|---|---|---|
| Item 1 | Students are asked by the teacher to think about how they learn science. | I seek to connect what I learn from what happens in the science classroom with out-of-class science activities (e.g. field trips or science visits). |
| Item 2 | Students are asked by the teacher to explain how they solve science problems. | I seek to connect what I learn from out-of-school science activities with what happens in the science classroom. |
| Item 3 | Students are asked by the teacher to think about their difficulties in learning science. | I seek to connect what I learn in my life outside of class with science class. |
| Item 4 | Students are asked by the teacher to think about how they could become better learners of science. | I seek to connect the information in science class with what I already know. |
| Item 5 | Students are asked by the teacher to try new ways of learning science. | I seek to connect what I learn from out-of-class science activities (e.g. field trips or science museum visits) with what happens in the science class. |
| Item 6 | I seek to connect what I learn from what happens in the science classroom with out-of-school science activities. | |
| Item 7 | I seek to connect what I learn in other subject areas with science class. |
To understand the students' metacognitive reflection via changes to their metacognition, especially changes to their metacognitive knowledge and their views on the value of any such changes, interviews and the Constructivist Connectivity Scale of the Self-Efficacy, Metacognition, and Learning Inventory – Science (SEMLI-S) (Thomas et al., 2008) were used. The Constructivist Connectivity scale contains seven-items and explores students' metacognitive knowledge regarding the extent to which they construct connections between information and knowledge elements across their science learning experiences, within and beyond their formal science classes. These items are shown in Table 2. In the SEMLI-S, these items are scattered throughout the 30 items of survey. Given that the triplet model relates to learners consciously seeking connections and relevance between different representations of chemical phenomena (Taber, 2013), it was appropriate to use the Constructivist Connectivity scale as an indicative measure for identifying changes in students' cognition and then, through interviews, their metacognition in relation to such cognition. Both quantitative sub-scales are scored using a 5-item Likert scale from 1 (almost never) to 5 (almost always).
Thirteen students were interviewed individually pre- and post-intervention for about 20 minutes on each occasion. A common interview structure and questions were employed across the study. The interviews explored, (a) students' perceptions of the pre- and post-intervention classroom environments, especially the metacognitive demands made on them and the language Craig used and (b) their pre- and post-knowledge, control and awareness of their chemistry learning processes and metacognitive reflection on such matters. Interviews were sequenced using a hermeneutic dialectic circle (Guba and Lincoln, 1989). This meant that responses to questions were sought from individual students, beginning with one student randomly selected at the start, and continuing until no new views were forthcoming from subsequent interviewees; their responses falling into one or more categories that had emerged through the daily analysis of the interviews.
The research involved eight weeks of pre-intervention data collection, then collaborative development of the intervention and discourse scripts with Craig over the following two weeks, and also as the need arose during the intervention. Ten weeks of intervention followed, giving a total of 20 weeks. The researcher attended 80% of the lessons of the Chemistry class over the entire research period. Lessons devoted to in-class assessment, revision or where large numbers of students were absent for non-chemistry-related school activities were not observed at Craig's request. This constitutes prolonged engagement and persistent observation. The topics addressed during the research period included: gases and gas laws, stoichiometry, solutions, chemical equilibrium, and acids and bases.
As the research proceeded interviews and video and audio data from the classroom were transcribed and analyzed. Member checking was undertaken. The range of data sources attending to the classroom's environment and students' the metacognitive reflection facilitated the opportunity for thorough cross-checking and triangulation. Along with prolonged engagement, persistent observation, and member checking, cross-checking and triangulation are means to achieve credibility for the research (Guba and Lincoln, 1989, 1997; Yin, 2016).
In this study transferability, the “equivalent to generalisabilty to the extent that there are similarities between sending and receiving contexts,” (Guba and Lincoln, 1997, p. 89) of the findings is sought through the development of a description that seeks to provide an extensive and careful description of the context within which the assertions were salient. While it is not possible to know in an a priori way about the contexts to which knowledge from interpretive studies might be applied, the range of research methods employed and the description of the classroom and its members provides reference information for those seeking possible transferability to other contexts.
Data analysis
No particular form of data was afforded priority or status in either analysis or reporting in this study. As various forms of data became available they were reviewed and analyzed in a timely manner, usually on the same day they were collected. This enabled assertions to emerge continuously in relation to the foci of the study, and for confirming and/or disconfirming data to be sought in subsequent data collection and analyses. A characteristic of the assertions reported in this paper is they were supported by all forms of data that were collected. When an emerging assertion could not be confirmed through data from each of the data sources relevant to the issue being explored, that assertion was laid aside and not reported in the results. Any such assertions are not reported in the results that follow. The analysis of all data was undertaken by the author, the sole researcher in this study. To assist ascertain the validity of assertions he engaged in peer debriefing as recommended by Guba and Lincoln (1989) and Cresswell and Miller (2000). Prior to debriefing, all identifying data was removed so as to maintain the anonymity of participants. Peer debriefing allowed potential biases and assumptions of the researcher as well as possible misinterpretations of data to be explored. This process occurred with impartial colleagues at the researcher's university and also with his and other colleagues' graduate students.To facilitate the analysis of the data from classroom transactions recorded as video, audio of the teacher, and field notes the researcher considered all three forms of data. In the field notes the researcher recorded events pertaining to the aims of the study. The researcher reviewed the video and audio-tapes with reference to the field notes to direct his attention to particular events, for example, Craig talking about learning chemistry to the whole class, or his interactions with individuals or small groups. However, while the field notes were used as a guide, all video and audio records were reviewed in their entirety so as not to privilege any one event or events so that the tenor of the classroom environment might be understood as well as possible. The examples of Craig's dialogue with the students found later in this paper are drawn from these data sets.
The interview data were attended to so that each interview was reviewed prior to the interview that followed it. This timing of such review is an important element of the hermeneutic dialectic circle, affording the researcher evidence to support or challenge emerging assertions and to propose new assertions for investigation via future data collection. Students' responses were interpreted and sorted into categories pertaining to their, (a) classroom environment, particularly the extent of the metacognitive demands within that environment, (b) metacognitive knowledge and any changes to that knowledge over the period of the study, and (c) metacognitive reflections on either or both of (a) and (b). Once this categorization had been completed the responses were further sorted into sub-sets that reflected variations within those larger categories. For example, two students may have reported that they noted that Craig had begun to use a particular terminology over the course of the study, but they may have differed in whether they considered it of any value for them personally. The quotes in the results section are drawn from the interview data and are selected as representative exemplars of students' reports regarding (a), (b) and (c) above. They are chosen to elucidate the range of students' views.
T-tests and effect size calculations were conducted for the pre- and post-test scores for each of the two quantitative sub-scales. The use of effect size was chosen because of its sensitivity to sample size, in this case a small sample of 27 students, and its resistance to sample size influence (Coe, 2002; Ferguson, 2009). Coe states that effect sizes are “particularly valuable for quantifying the effectiveness of a particular intervention” (p. 1). In regard to interpretation of effect sizes, Ferguson notes that, “as with all statistical tools, effect size estimates are just that, estimates” and that “there is no agreement on what magnitude of effect size is necessary to establish practical significance” (p. 532). General guidelines rather than rigid numerical criteria exist to aid the interpretation of effect sizes. These guidelines centre around recommendations that effect sizes of 0.20, 0.50, and 0.80 could be interpreted respectively as small, medium and large (Cohen, 1988). Ferguson (2009) however, cautioned against strict adherence to the use of these numerical values for interpreting the extent of the effect of an intervention. Further, Durlak (2009) argues the importance of considering the practical significance of the findings in addition to the magnitude of the effect size, stating that “an intervention with a weak effect size but no risks may be valuable,” and that the “same intervention may be less desirable if the risks are considerable” (p. 536). In this study, the cut-offs for effect sizes suggested by Cohen were acknowledged as rules of thumb, and the risks associated with the intervention were carefully considered when suggesting any practical significance of the intervention based on the calculated effect sizes.
Results
The results section is composed of three parts. The first attends to the pre- and post-intervention chemistry classroom learning environment and a brief overview of Craig's pedagogy. The pre- and post-data and interpretations are separated by the second part which reports the development and enactment of pedagogical changes that Craig engaged in. This is positioned in the sequence so that readers can understand the evolution and nature of the changes in Craig's pedagogy and the considerations that led to those changes. The third part comprises 4 student cases that exemplify variations across students regarding their metacognitive knowledge and their metacognitive reflections on the metacognitive experiences elicited over the period of the research.Nature of the classroom environment
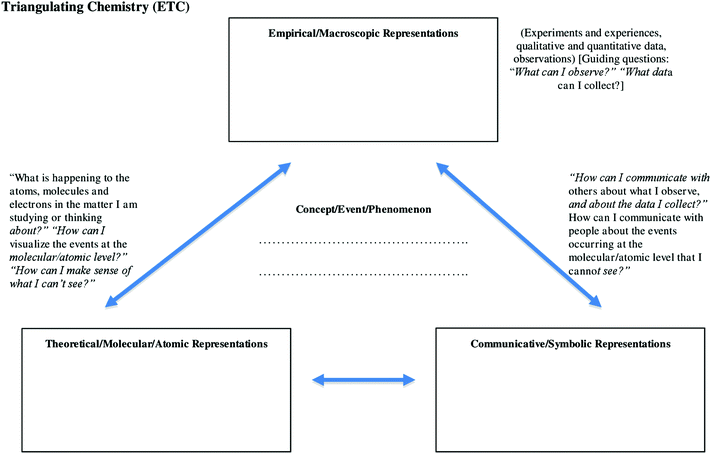
Triangulating is the conscious mental process of trying to relate and connect all three types of representation regarding chemical species and phenomena so that we develop an understanding of chemistry. We can begin to think about how we relate and connect what we know about any chemical phenomenon, change, reaction, substance, system or event using these three types of representation. When we do this we can start to think about how we develop our understanding of chemistry.
Fig. 1 shows a worksheet that was developed as an heuristic for Craig's use with students. It was developed by the researcher based on Craig's suggestion to develop a resource that would support students' understanding and use of the cognition associated with ‘triangulating’ the representations of chemical entities and phenomena. The intention was to direct students' cognition, in an explicit way, to attend to the separate nature of each of the representations and the relationships between them. This was seen as important because it was considered that the students needed opportunities to enact any newly acquired metacognitive knowledge so that they could assess the viability of that knowledge and its enactment in their learning contexts. Students' agency needs to be considered in all classroom interventions and it was not assumed that they would passively and unquestioningly accept changes in their classroom environment.
It is evident that some of the terminology on Fig. 1 varies somewhat from that typically used in the chemistry education literature. ‘Macroscopic’ is also termed ‘empirical (E).’ Molecular is also termed ‘atomic’ and ‘theoretical (T).’ Symbolic is also referred to as ‘communicative (C).’ Missing from Fig. 1 is the term ‘submicroscopic’ which is a term recommended for use by Gilbert and Treagust (2009). These variations, including the omission of the term ‘submicroscopic,’ arose out of discussions between Craig and the researcher and reflected Craig's preference for the language he would use in class. Craig considered that the language eventually selected should be consistent with what he was familiar with personally from his previous experience teaching the Theory of Knowledge aspect of the International Baccalaureate syllabus. Therefore, as had been agreed upon as a condition of the research and consent, and given that the chemistry triplet “has been reconceptualised in diverse ways” (Taber, 2013, p. 156), this modification was seen as reasonable and in keeping with the research intentions. Craig influenced and maintained control over the precise nature of his pedagogy and his autonomy and professional judgment was respected. Future research aimed at stimulating metacognitive reflection in chemistry education might further consider the inclusion of terms like ‘submicroscopic’ into classroom discourse, as might be consented to by the teacher.
Craig's changes to his pedagogy and his revised way of talking with students about chemistry and chemistry learning where noticeably absent from his pre-intervention discourse. In introducing the notion of ‘triangulation’ to students using a topic from a previous class he drew their attention to the schematic, and the dialogue was as follows:
Craig: What do you see?
Ss: A triangle
Craig: A triangle. It looks like a triangle. So, I want you to recognize, “OK, I've got some arrows and they seem to make a triangle.” Here's our goal. Our goal is to take concepts that we are learning in chemistry and triangulate them. I'm going to say, “Everyone triangulate” from time to time, and you need to know what I mean by triangulate. We're going to do an example today and we're going to start to learn the process. Greg and I have been working out what words to use for the three points of the triangle. At the top of the triangle you'll see the word ‘macroscopic’ and that means, to me, visible. I can see that. What do I see happening? Now, we've used a word ‘empirical’…anything we do down [at] the lab, anything I can measure, anything I can write down both qualitatively and quantitatively…we get empirical. And so, at the top of that [triangle] we've got empirical. I've separated this [on the diagram]…any time we are talking about stuff that we can see we are talking about empirical. For example, a molecular compound in solution does not conduct electricity. Now why is that? Now, we know the theoretical. [Some] molecular compounds, when they go into solution they dissolve; neutral molecules surrounded by H2O molecules. Does that explain why it doesn't conduct? Now we want to make those connections between empirical and theoretical regarding the conductivity of this molecular substance. It doesn't conduct in solution because, theoretical, the other corner of the triangle, says that when it dissolves there are no ions in solution and ions in solution are the reason for conductivity. Now, can we draw that…get a picture of that? There's a picture of that in your textbook. You've got a little sphere for a sugar molecule and you've got H2O molecules all the way around it. We can draw a dissolving equation. We can communicate what we know empirically and theoretically by drawing some things down on paper. We've got empirical, theoretical and communication. What we want to do to a concept like we've just talked about is bring them together, to make connections. Concepts have an empirical side to them, a theoretical side to them, and a communication side to them. So we've got macroscopic on one corner, molecular, theoretical, atomic on the other corner, and we've got “how are we representing this?, How are we communicating this?” And so, we've got the three corners here…we're going to triangulate stuff. We're making connections. This is something we're presenting to you so that we can all grasp it.
Craig used the expression with students in numerous ways. One exemplar of this discourse, taken from classroom discussion aimed to prepare students to learn about strong and weak acids, is given below. Students were asked to complete a ‘triangulation’ sheet (Fig. 1) that directed them to think about strong acids using representations of the chemistry triplet. Such language was used frequently with students to direct their thinking and activity in the classroom. In this case he indicates that students might use their prior knowledge or information from their own notes to inform their learning of the current topic.
“We've got our ‘triangles’ (triangulation sheets) here. We are going to try triangulating a few concepts that we are going to be using over the next few weeks. The challenge is that the concepts are not what we are currently talking about. You will need to go back to your notes, I might give you an idea where, pull something out that we have already looked at and triangulate it.”
Another exemplar of his use of the language and heuristic is taken from his teaching of solubility and phase equilibria. After a brief explanation of major ideas on the white board, he stated, “Now what I want you to do is to take a look in your textbook at page 487.” Then after standing at the board and thinking about what he might write, he said, “Hmmm. You know what? This calls for this. This will make this easier,” after which he moved to the side of the classroom and took a handful of sheets of paper with the worksheet (Fig. 1) printed on both sides. After distributing them to the class he gave the following instructions, “On one side write phase equilibrium as the central concept, and on the other side write solubility equilibrium. Take a look at page 487, fill in the three boxes, empirical, theoretical, and communicative descriptions for a phase equilibrium and a solubility equilibrium. And where do you find out that information? On page 487.” This example suggests that Craig felt comfortable with using the worksheet heuristic for tasks that might normally be done by students using note taking and summarizing.
In attending to the students' work and the chemistry concepts under consideration Craig made reference to all forms of representation, not focusing on any particular form. On regular occasions he would ask students to move to the whiteboard and draw their completed triangulation sheets and then direct them to explain their drawings to the class. He also used the contents of their individual sheets as examplars that he would direct the whole class to consider. In addition, he used the expression occasionally with individuals and small groups of students as he moved around the classroom. Where appropriate, Craig would draw submicroscopic diagrams on the board or draw students' attention to past examples of molecular structures to add to the triangulation diagrams the students had provided. This happened, for example, in discussions regarding the solubility of ethanol in water and phase solubility. Through these interactions and responses to students' understandings he initiated discussion between students and between himself and students on how the ‘triplet’ representations were related and connected. In doing so, he used their work on and discussion related to the triangulation sheets as a form of formative assessment of their emerging chemistry understanding. Such interactions were not present in the pre-intervention classroom. Craig's engagement with students regarding the representations seemed to prompt him to consider and attend to their understandings that became apparent through these classroom activities. The researcher did not have consent to access the summative assessment tasks for the class as these were departmentally sanctioned. Therefore it is not possible to establish the extent to which the summative assessment reflected the intent of Craig's pedagogical change.
As shown in Table 3, there were changes in students' reports on the constructivist connectivity scale of the SEMLI-S. While not statistically significant, the effect size calculated of 0.18 suggests this change might be interpreted as a small change. This small change is worth noting for, as will be discussed later, it suggests that some students reported seeking to connect ideas and concepts more following the intervention than prior to it, an indication of a change in their enacted metacognitive procedural knowledge. If this was the case it would be an important finding, even if not the case for all students. This is because integrating and connecting the triplet representations or parts of them would be considered a cognitive process that, as previously explained, would be useful for students to learn about and engage in.
Nine of the thirteen students interviewed suggested Craig's pedagogical change was beneficial for them. Katy, Dillon, and Cecilia are representatives of that nine. The other four did not and Amber is a representative voice for them.
Katy. Katy represents students who expressed the view that they considered that learning and understanding chemistry involved seeking and acknowledging relationships between ideas and concepts. Their self-reports of the extent of their constructivist connectivity where unaltered or only marginally changed; in Katy's case her pre-score was 24/35 and post was 25/35. The intervention stimulated them to metacognitively reflect on their views and in the post-intervention interviews they reported qualitatively changed notions of what it meant to learn and understand chemistry.
Pre-intervention. For Katy, to learn chemistry was, “to understand how things (substances) are combined, how things are made up, to learn the way things are formed. She stated that as she was learning chemistry she was “thinking how ‘this’ relates to other things that can help me remember ‘this’ better, like life situations where I've seen this type of thing.” To understand chemistry was “understanding what's going on…whatever concept you get, you relate it to something else, even if you don't know what's going on. You get that ‘click’ in your head and you're like, “Oh. I get it!” When you get a formula and you (know) “that's this, and this is why it affects something.”
Post-intervention. Katy described her chemistry learning processes as being essentially the same as pre-intervention: “I try to relate (new material) to other things I know I'm confident about because that will help me remember what I'm learning. I think the most important thing is relating what I'm learning to something I have done previously because I obviously have more knowledge on it, so it helps. This (her view) hasn't changed much because even back then (pre-intervention) I tried to do that for myself because I know how I learned and I know that relating things to other things helps me.”
However, at the same time, Katy could articulate, at least partly, the suggestions made by Craig and see some value in them for herself: “Triangulation means a combination of those three different aspects…I never thought of it this way…we've been thinking empirically and theoretically…he's providing us with more ways of learning. I didn't like it at the beginning and I thought, “This is difficult.” I wasn't that into it. Now it's getting better. It helps a lot with visuals like diagrams; drawing it out for each (concept). I'm liking it better now. I think learning empirically and theoretically is a great way of knowing what you're talking about.”
She also claimed that Craig's pedagogy change had stimulated her to reflect on other aspects of her learning processes: “I started to think more of other things when Mr Craig presented us with one of these (worksheets, Fig. 1). I thought, “Oh, this is a neat way of combining ideas and thinking. If you know that you're learning something and you know the empirical, you can see it, and if you don't know the theoretical aspect this thing (thinking with representations worksheet) is good because you can actually write it down. And you think, “Oh, I actually never thought of it like that” and it makes you bring out all of your ideas.”
Dillon. Dillon represents students who did not express, pre-intervention, the view that they considered that learning and understanding chemistry involved seeking and acknowledging relationships between ideas and concepts. Their self-reports of the extent of their constructivist connectivity where unaltered or only marginally changed; in Dillon's case his pre-score was 24/35 and post was 26/35. The intervention stimulated them to metacognitively reflect on their views and in the post-intervention interviews they reported qualitatively changed notions of what it meant to learn and understand chemistry.
Pre-intervention. Dillon stated:
“To learn chemistry I just go over my notes and make sure I understand them. When you can actually read the notes Mr Craig gives you and understand what they mean, then I guess that's how I learn chemistry. If it doesn't make sense then I'll try to figure it out. I don't know what happens inside my head when I learn chemistry…I haven't thought about that much before. I don't think there's much to change. A lot of it comes from just individual study. I know I understand it when I can do the homework and get the answers correct without having to stop and think, “Was this right or not?” If I can do it and check my answer and it's all correct then I usually know I understand it. Also, if I can talk about the concepts, that's when I can probably understand it…explaining the concepts in my own terms.”
Post-intervention. Post-intervention, Dillon reported, using the language that Craig had used in class, how he understood the three levels of thinking, and how his knowledge and awareness of his learning processes related to learning chemistry had changed.
“The triangulation process is probably THE (student's emphasis) most useful thing I have learned all year. Basically. It's the idea that everything you learn in chemistry has three aspects to it: empirical, theoretical and communicative. When Mr (Craig) talks about the theoretical, the empirical, and you can follow it, and when you can communicate it as well; when you have all these three things, I think you can legitimately say you understand it (the concept). You might have learned a concept solely empirically or solely theoretically, but until you can understand it both ways and be able to communicate it you haven't really understood it. I didn't actually think that way previously. The whole triangulating thing (sic) that Mr (Craig) used, that's what's changed my view. For learning chemistry that's perfect.”
Cecilia. Cecilia represents students who had difficulty, pre-intervention, explaining at all how they learned or understood chemistry. They were unsure and tentative about what they thought it mean to learn and understand chemistry and often hinted only generally at the importance of seeking and acknowledging relationships between ideas and concepts. Their self-reports of the extent of their constructivist connectivity increased, and this was supported by new intimations about the thinking they employed; in Cecilia's case her pre-score was 26/35 and post was 29/35. The intervention stimulated them to metacognitively reflect on their views and in the post-intervention interviews they reported qualitatively changed notions of what it meant to learn and understand chemistry and how they might go about it.
Pre-intervention. When asked, “What happens inside your head when you learn chemistry, Cecilia replied, “That's a good question. I'm not really sure.” Cecilia stated in relation to her tentative metacognitive knowledge of learning and understanding chemistry:
“I'm not really sure how I would explain learning chemistry. I answer questions in the textbook or something. Also, reading notes, I find that really useful. I think I'm more of a visual learner…usually I learn stuff better when I'm reading it…I try to remember it and connect it to the other stuff I've learned in previous classes. I usually just read it but I don't actually wonder why it's that way. To understand it means to understand how it works and how to find the answer that you’re looking for…if it makes sense, sort of…if you're getting good marks. It's like a puzzle, it all fits together sort of…if you're missing something you won't get it.”
Post-intervention. Cecilia reported how she understood the forms of representation, how they helped her reconceptualise elements of her chemistry learning and how her knowledge, control and awareness of their learning processes related to what it meant to understand chemistry had changed. She stated:
I think that the triangulation stuff is good. [Previously] I didn't pay attention to the empirical stuff, the stuff I was learning in the lab…I didn't really relate it. Now I see the similarities between that [the empirical], and what we learned in class. Before I would just do a lab and think about it separately. I'd do a lab write-up and hand it in. Now I can see how it relates to the stuff we are learning [in class] and it helps me see the relevance of doing it. Before if I didn't understand something I wouldn't know until I got it wrong. Now I think I can realize that I don't understand it [an idea]…when I don't see the relevance of the thing [idea] to what I've been learning it means I'm not connecting it properly which means I don't understand it. I picked up this idea about connecting things from the triangulating. In class he'll (Craig) give us these papers [worksheets, Fig. 1] and it helps us show what we’ve been learning in different ways. I found that really helpful.
Some students found the idea of revising their learning processes to be uncomfortable, as they considered the new proposals as contrary to their existing processes and what they valued in chemistry learning.
Amber. Amber represents students who, on the basis of the evidence collected, were unchanged in their views of what it mean to learn and understand chemistry and how they did so. Their self-reports on the constructivist connectivity sub-scale either remained unchanged or changed only slightly. In Amber's case her score was 31, pre-intervention, to 32 post-intervention. Further, they were often ambivalent to or dismissive of the ideas presented.
Pre-intervention. Amber described how she learned chemistry as follows:
“I just read over my notes and I memorise it, and then if I don't understand I go back in the textbook and see what they say, and then I’ll ask the teacher if I still don't understand it. I don't really think about how I process the information, because when I get it I just understand what it is, and I just put it somewhere in my long-term memory. When I see notes, I read over them and I understand because they [the authors] have put them into their own words…and when I read other people's thoughts I process them into my own thoughts. It's like a habit, I just do it, I don't think about it…I worked it out for myself.”
She added that it was about, “going further into the basic subject of chemistry and then you're looking at how this relates to certain elements [aspects] of the world and then you can apply it to real life.” In relation to how she considered what it meant to understand chemistry she stated, “When I say I understand chemistry I mean understanding the contents of the textbook of chemistry and the equations and what the elements do when they combine…the light bulb goes on, then I can answer a question more easily and I don’t have to spend much time on it.”
Post-intervention. Amber stated,
“My view of what it means to learn and understand chemistry hasn't changed. I don't think about this much. When I do the equations and I get the right answer, that's when I know I understand it. For theories, when I can explain them in my own words, that's when I understand them. I get this “light bulb feeling,” like “that's how you do it” feeling…like it all makes sense now. Mr Craig made us write that triangular charts ‘thingy’ with empirical observations, and then there's theoretical, and then it's like, I can't remember the other one. Triangulation is putting [information] into that triangle thing. I think it's a little annoying, but it kind of helps, because you have to think, “Well, what can I associate ‘this’ with for communication?”, and then for empirical it would be, “What makes it an empirical observation?” And for the theory, you just read the textbook. It's annoying that you have to do it. It doesn't help with memorization. I've always connected ideas. I do it automatically. I just learned more about it.”
These changes are evidence of metacognitive reflection and revision, and suggest that even if there is reflection, there may not be revision of some students' views.
Discussion and study significance
Taber (2013) argued for the importance of modeling how chemists operate with and between macroscopic descriptions and categories and theoretical submicroscopic models “using the symbolic language of the subject as the means to readily represent and communicate these concepts” (p. 166). This study, in part, takes up Taber's suggestion. The teacher's changing of the chemistry classroom learning environment directed students to consider, what were for some, alternative views of chemistry learning and chemistry learning processes. The ideas suggested by Craig were commensurate with the chemistry education literature that identifies the need for students to build relationships between macroscopic, molecular/submicroscopic, and symbolic levels. Craig's revised pedagogy employed a meta-language reflecting a strong theoretical perspective on chemistry learning and his use of an heuristic to ‘activate’ that language elicited metacognitive experiences for students, openly challenging them to consider the nature of chemistry and how it might best be learned. These metacognitive experiences stimulated them to metacognitively reflect on their knowledge, control and awareness of their learning process. The consequences of these reflections were students' conscious personal considerations of, (a) their existing chemistry learning processes and whether these might be modified, and (b) their conceptions of chemistry as a form of knowledge and what it might mean to learn and understand chemistry. All students learnt terminology regarding chemistry and chemistry learning and these became elements of their metacognitive knowledge that they were able to explain to the researcher.Katy explained that, after an initial feeling of unease and uncertainty, she had developed a new perspective on how procedural metacognitive elements of triangulation could be valuable for her chemistry learning. Her suggestion that she had never thought of chemistry and chemistry learning “in such a way” suggests shifts in her metacognitive knowledge, including her meta-level epistemic knowledge (EMK). Notably, her knowledge about how to perform an activity that results in knowing a subject (EMSK) has changed. In Dillon's case this shift seems much more marked. Dillon clearly identified the teacher as a key element in inducing his changes. He refers to the legitimacy of his new view of what it means to learn and understand chemistry using the cognition and strategies taught by Craig. As with Katy, we see evidence of shifts in Dillon's EMK and EMSK. In Cecilia's case we see changes in her metacognitive knowledge, and EMK and EMSK and, again, a clear statement about the usefulness of triangulation as a process. Her metacognitive reflection clearly identifies specific aspects of her chemistry learning that have been attended to as a consequence of Craig's changed pedagogy. In the case of all these students, a change in the teacher's pedagogy centered on the use of an expression, ‘triangulation’ to explicitly teach them regarding the ‘triplet’ model of representations and its use stimulated their metacognitive reflection regarding those representations for learning chemistry.
While all students identified changes to the classroom discourse and activities and some responded favorably to those changes, others such as Amber were resistant to considering or adopting the ideas presented to them over the research period. That there were variations across students should come as no surprise. Students are not passive recipients of teacher suggestions, especially at the higher levels of schooling. Rather, they compare the viability of new ideas for learning against their existing ideas and against what they consider are the demands of the learning context. Craig's explicit teaching of how to think and learn about chemistry learning induced metacognitive conflict (Thomas, 2012) in students such as Amber and Katy. Metacognitive conflict is a form of metacognitive experience in which information presented to students regarding thinking and learning triggers negative emotions and/or responses. Such explicit teaching of the nature of chemistry and how it might be learned and the potential inducement of metacognitive conflict can trigger metacognitive reflection, but not always lead to changes in metacognition or changes in learning strategies. Even so, direct and authoritative instruction from the teacher for students to engage in such considerations increases the level of metacognitive demand in classrooms and the possibility for students' revisions of their adaptive metacognition. The alternative to such direct instruction is to expect students to learn about such matters in a process metaphorically akin to diffusion and not have their existing, possibly tacit views ever openly explored and perhaps challenged.
The change in the scores on the Constructivist Connectivity sub-scale of the SEMLI-S was positive for the class as a whole, although there was variation between individuals pre- and post-intervention. The modest quantitative shift in the class score is potentially important given that students consciously ‘connecting ideas’ is a sought after key component of their chemistry learning strategies, and this intervention was able to be woven into Craig's pedagogy. However, the quantitative shift is not informative regarding the obvious qualitative shifts in the nature of what some students reported regarding the ideas and information they were seeking to more actively connect as a consequence of the intervention. Some students, for example, Katy and Amber reported, pre-intervention, that they sought to connect ideas in order to learn. However, in their post-intervention interviews they reported new insights regarding what ideas, the representations, they might connect and what the benefits for their learning might be. Therefore, while results from such instruments might show only minor quantitative shifts, there is a need to consider qualitative shifts in students' learning processes as well.
Despite the variations between students, this study highlights the potential of collaborating with chemistry teachers to develop students' metacognition and learning strategies that are commensurate with the nature of the material to be learned. This study did not set out to explore whether Craig's pedagogical changes would alter students' conceptual knowledge. Rather, its focus is on students' metacognitive reflection and revisions to their metacognitive knowledge. Based on the findings of this study Fig. 2 is proposed as a means of conceptualising the chemistry triplet, the explanations of each level of representation, and the metacognitive self-talk questioning that students might engage in as they consider chemistry phenomena or concepts that they are asked to learn about. Fig. 2 complements Fig. 1. It has more detail included about what each type of information is relevant for each representation. It is based on the aforementioned chemistry education literature and the language used by Craig that emerged as a consequence of the discussions between Craig and the researcher. It is expected that it might be modified for future use in other studies and by other teachers. Such modification will be determined according to the needs of future users and their interpretation of, for example, the chemistry triplet and the nature of students' chemistry learning processes.
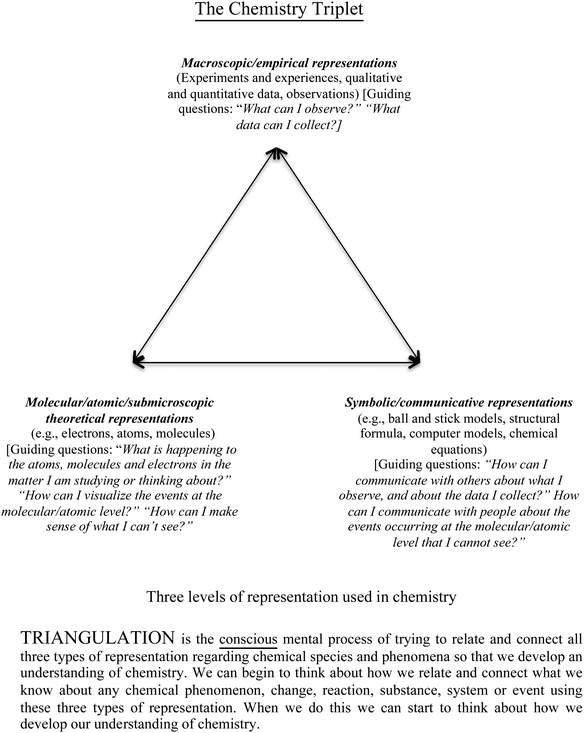 | ||
| Fig. 2 A diagram describing the chemistry triplet including triangulation in terms of (meta)cognitive ‘self-talk’ (after Johnstone, 1993). | ||
Over the past 35 years the field of chemistry education has seen the development of a considerable base of ideas about the nature of chemistry and its learning. It could be suggested that these ideas have been used predominantly to educate teachers regarding how to structure instruction. This study suggests that there may be value in being open and explicit with students too about the nature of chemistry so that they can begin to construct adaptive metacognition, including viable conceptions of chemistry and chemistry learning processes. Students in this study were able to understand the teacher's explanation regarding the nature of chemistry and how it might be learned, and even though they had to comply with Craig's instructions, they were authorised to adopt, or otherwise, elements of his views. It may be time to begin to increasingly consider sharing what we know about the nature of chemistry and chemistry learning explicitly with students so that they can become more knowledgeable and have opportunities to metacognitively reflect regarding such matters.
Acknowledgements
This study was part of the ‘Using metaphor to develop metacognition in relation to scientific inquiry in high school science laboratories project’ supported by the Social Science and Humanities Research Council of Canada. Contract Grant Number: SSHRCC File Number 410-2008-2442.References
- Adey P. and Shayer M., (1994), Really raising standards: cognitive intervention and academic achievement, London: Routledge.
- Anderson D., Thomas G. P. and Nashon S., (2009), Social barriers to meaningful engagement in biology field trip group work, Sci. Educ., 93(3), 511–534.
- Azevedo R., (2005), Using hypermedia as a metacognitive tool for enhancing student learning. The role of self-regulated learning, Educ. Psychol., 40(4), 199–209.
- Baird J. R., (1986), Improving learning through enhanced metacognition: a classroom study, Eur. J. Sci. Educ., 8(3), 263–282.
- Barzilai S. and Zohar A., (2016), Epistemic meta(cognition): ways of thinking about knowledge and knowing, in Greene J. A., Sandoval W. A. and Bråten I. (ed.), Handbook of epistemic cognition, New York, NY: Routledge, pp. 409–424.
- Blank L. M., (2000), A metacognitive learning cycle: a better warranty for student understanding? Sci. Educ., 84(4), 486–506.
- Bucat B. and Mocerino M., (2009), Learning at the sub-micro level: structural representations, in Gilbert J. K. and Treagust D. (ed.), Multiple Representations in Chemistry Education, Dordrecht: Springer, pp. 11–29.
- Case J. and Gunstone R. F., (2006), Metacognitive development: a view beyond cognition, Res. Sci. Educ., 36(1–2), 51–67.
- Chan J. Y. K. and Bauer C. F., (2016), Learning and studying strategies used by general chemistry students with different affective characteristics, Chem. Educ. Res. Pract., 17, 675–684.
- Coe R., (2002), It's the effect size stupid: what effect size is an why it is important, paper presented at the Annual Council of the British Educational Research Association, Exeter, England.
- Coffield F., (2012), Learning styles; unreliable, invalid and impractical and yet still widely used, in Adey P. and Dillon J. (ed.), Bad education: debunking myths in education, Maidenhead: Open University Press, pp. 215–230.
- Cohen J., (1988), Statistical power analysis for the behavioral sciences, 2nd edn, Hillsdale, NJ: Erlbaum.
- Connor L. N., (2007), Cueing metacognition to improve researching and essay writing in a final year biology class, Res. Sci. Educ., 37(1), 1–16.
- Conner L. and Gunstone R. F., (2004), Conscious knowledge of learning: accessing learning strategies in a final year high school biology class, Int. J. Sci. Educ., 26(12), 1427–1443.
- Costa A., (2007), The school as home for the mind: creating mindful curriculum, instruction and dialogue, 2nd edn, Thousand Oaks, CA: Corwin Press.
- Cresswell J. W. and Miller D. L., (2000), Determining validity in qualitative inquiry, Theor. Pract., 39(3), 124–130.
- Duit R. and Treagust D., (2012), How can conceptual change contribute in theory and practice in science education, in Fraser B. J., Tobin K. G. and McRobbie C. J. (ed.), Second international handbook of science education, Dordrecht: Springer, pp. 107–118.
- Durlak J. A., (2009), How to select, calculate, and interpret effect sizes, J. Pediatr. Psychol., 34(9), 917–928.
- Efklides A., (2006), Metacognition and affect: What can metacognitive experiences tell us about the learning process? Educ. Res. Rev., 1(1), 3–14.
- Erickson F., (1998), Qualitative methods for science education, in Fraser B. J. and Tobin K. G. (ed.), International handbook of science education, Dordrecht, The Netherlands: Kluwer Academic Publishers, pp. 1155–1173.
- Ferguson C. J., (2009), An effect size primer: a guide for clinicians and researchers, Prof. Psychol.: Res. Pract., 40(5), 532–538.
- Flavell J. H., (1976), Metacognitive aspects of problem solving, in Resnick L. B. (ed.), The nature of intelligence, Hillsdale, NJ: John Wiley, pp. 231–235.
- Flavell J. H., (1987), Speculations about the nature and development of metacognition, in Weinert F. E. and Kluwe R. H. (ed.), Metacognition, motivation, and understanding, Hillsdale, NJ: Lawrence Erlbaum, pp. 21–29.
- Gabel D., (1998) The complexity of chemistry and its implications for teaching, in Fraser B. J. and Tobin K. G. (ed.), International Handbook of Science Education, Dordrecht, The Netherlands: Kluwer, pp. 233–248.
- Georghiades P., (2004), From the general to the situated: three decades of metacognition, Int. J. Sci. Educ., 26(3), 365–383.
- Georghiades P., (2006), The role of metacognitive activities in the contextual use of primary pupils' conceptions of science, Res. Sci. Educ., 36(1–2), 29–49.
- Gilbert J. and Treagust D. F., (2009), Towards a coherent model for macro, submicro, and symbolic representations in chemical education, in Gilbert J. K. and Treagust D. (ed.), Multiple Representations in Chemistry Education, Dordrecht: Springer, pp. 333–350.
- Guba E. G. and Lincoln Y. S., (1989), Fourth generation evaluation, Beverley Hills, CA: Sage.
- Guba E. G. and Lincoln Y. S., (1997), Naturalistic and rationalistic inquiry, in Keeves J. P. (ed.), Educational research, methodology, and measurement: an international handbook, Oxford: Pergamon, pp. 86–91.
- Gunstone R. F., (1994), The importance of specific science content in the enhancement of metacognition, in Fensham P., Gunstone R. F. and White R. T. (ed.), The content of science: a constructivist approach to its teaching and learning, London, UK: Routledge, pp. 131–146.
- Haider A. H. and Al Naqabi A. K., (2008), Emiratii high school students' understandings of stoichiometry and the influence of metacognition on their understanding, Res. Sci. Technol. Educ., 26(2), 215–237.
- Hofer B. K., (2005), The legacy and the challenges: Paul Pintrich's contributions to personal epistemology research, Educ. Psychol., 40, 95–105.
- Hofer B. K. and Sinatra G. M., (2010), Epistemology, metacognition, and self-regulation: musings on an emerging field, Metacogn. Learn., 5, 113–120.
- Johnson R. B. and Onwuegbuzie A. J., (2004), Mixed Methods Research: A Research Paradigm Whose Time Has Come, Educ. Res., 33(7), 14–26.
- Johnstone A. H., (1991), Why is chemistry difficult to learn? Things are seldom what they seem, J. Comput. Assist. Learn., 7, 701–710.
- Johnstone A. H., (1993), The development of chemistry teaching: a changing response to changing demand, J. Chem. Educ., 70(9), 701–705.
- Kaberman Z. and Dori Y. J., (2009), Metacognition in chemical education: question posing in the case-based computerized learning environment, Instr. Sci., 37, 403–436.
- Lewthwaite B. and Weibe R., (2011), Fostering teacher development to a tetrahedral orientation in the teaching of chemistry, Res. Sci. Educ., 41, 667–689.
- Madden S. P., Jones L. L. and Rahm J., (2003), The role of multiple representations in the understanding of ideal gas problems, Chem. Educ. Res. Pract., 12, 283–293.
- Mahaffy P., (2004), The future shape of chemistry education, Chem. Educ. Res. Pract., 5(3), 229–245.
- Marshall H. H., (1996), Implications of differentiating and understanding constructivist approaches, Educ. Psychol., 31(3/4), 235–240.
- Meijer J., Veenman M. V. J. and Van Hout-Wolters B., (2012), Multi-domain, multi-method measures of metacognitive activity: what is all the fuss about metacognition…indeed, Res. Pap. Educ., 27(5), 597–627.
- Mokhtari K. and Reichard C. A., (2002), Assessing students' metacognitive awareness of reading strategies, J. Educ. Psychol., 94, 680–690.
- Pintrich P. R., Smith D. A. F., Garcia T. and McKeachie W. J., (1991), A manual for the use of the Motivated Strategies for Learning Questionnaire (MSLQ) (Technical Report No. 91-B-004), Ann Arbor, MI: University of Michigan: National Center for Research to Improve Postsecondary Teaching and Learning.
- Schraw G., (1998), Promoting general metacognitive awareness, Instr. Sci., 26(1–22), 113–125.
- Schraw G. and Dennison R., (1994), Assessing metacognitive awareness, Contemp. Educ. Psychol., 19, 460–475.
- Sinatra G. M. and Pintrich P. R., (ed.), (2003), Intentional conceptual change, Mahweh, NJ: Erlbaum.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14, 156–168.
- Talanquer V., (2010), Macro, Submicro, and symbolic: the many faces of the chemistry “triplet”, Int. J. Sci. Educ., 1(1), 1–17.
- Thomas G. P., (1999), Student restraints to reform: conceptual change issues in enhancing students' learning processes, Res. Sci. Educ., 29(1), 89–109.
- Thomas G. P., (2003), Conceptualisation, development and validation of an instrument for evaluating the metacognitive orientation of science classroom learning environments: the Metacognitive Orientation Learning Environment Scale – Science (MOLES-S), Learn. Environ. Res., 6(2), 175–197.
- Thomas G. P., (2009), Interpretive and mixed methods approaches to metacognition research: providing context, presented at the SIG 16 (Metacognition) Invited Symposium at the conference of the European Association for Research on Learning and Instruction, Amsterdam, The Netherlands.
- Thomas G. P., (2012), Metacognition in Science Education: Past, present and future considerations, in Fraser B. J., Tobin K. G. and McRobbie C. J. (ed.), Second International Handbook of Science Education, Dordrecht: Springer, pp. 131–144.
- Thomas G. P., (2013), Changing the metacognitive orientation of a classroom learning environment to stimulate metacognitive reflection regarding the nature of physics learning, Int. J. Sci. Educ., 35(7), 1183–1207.
- Thomas G. P. and Anderson D., (2014), Changing the Metacognitive Orientation of a Classroom Environment to Enhance Students' Metacognition Regarding Chemistry Learning, Learn. Environ. Res., 17(1), 139–155.
- Thomas G. P. and McRobbie C. J., (2001), Using a metaphor for learning to improve students' metacognition in the chemistry classroom, J. Res. Sci. Teach., 38(2), 222–259.
- Thomas G. P. and McRobbie C. J., (2013), Eliciting Metacognitive Experiences and Reflection in a Year 11 Chemistry Classroom: An Activity Theory Perspective, J. Sci. Educ. Technol., 22(3), 300–313.
- Thomas G. P., Anderson D. and Nashon S., (2008), Development of an instrument designed to investigate elements of students' metacognition, self-efficacy and learning processes: the SEMLI-S, Int. J. Sci. Educ., 30(13), 1701–1724.
- Tishman S. and Perkins D. N., (1997), The language of thinking, Phi Delta Kappan, 78(5), 368–374.
- Treagust D. F., Chittleborough G. and Mamiala T. L., (2003), The role of submicroscopic and symbolic representations in chemical explanations, Int. J. Sci. Educ., 25(11), 1353–1368.
- Veenman M. V. J., Van Hout-Wolters B. H. A. M. and Afflerbach P., (2006), Metacognition and learning: conceptual and methodological considerations, Metacogn. Learn., 1(1), 3–14.
- Vosniadou S., (2012), Reframing the classical approach to conceptual change: preconceptions, misconceptions and synthetic models, in Fraser B. J., Tobin K. G. and McRobbie C. J. (ed.), Second international handbook of science education, Dordrecht, The Netherlands: Springer, pp. 119–130.
- White R. T., (1998), Decisions and problems in research on metacognition, in Fraser B. J. and Tobin K. G. (ed.), International handbook of science education, Dordrecht, The Netherlands: Kluwer Academic Publishers, pp. 1207–1212.
- Ye L., Shuniak C., Oueni R., Robert J. and Lewis S., (2016), Can they succeed? Exploring at-risk students' study habits in college general chemistry, Chem. Educ. Res. Pract., 17, 878–892.
- Yin R. K., (2016), Qualitative research from start to finish, 2nd edn, New York, NY: The Guilford Press.
- Zohar A. and David A. B., (2009), Paving a clear path in a thick forest: a conceptual analysis of a metacognitive component, Metacognition & Learning, 4(3), 177–195 Search PubMed.
| This journal is © The Royal Society of Chemistry 2017 |