Highly active and durable carbon nitride fibers as metal-free bifunctional oxygen electrodes for flexible Zn–air batteries†
Sambhaji S.
Shinde
,
Jin-Young
Yu
,
Jae-Won
Song
,
Yoon-Ho
Nam
,
Dong-Hyung
Kim
and
Jung-Ho
Lee
 *
*
Department of Materials and Chemical Engineering, Hanyang University, Ansan, Kyunggido 426-791, Republic of Korea. E-mail: jungho@hanyang.ac.kr; Fax: +82-31-400-4723; Tel: +82-31-400-5278
First published on 23rd June 2017
Abstract
The design of flexible, highly energetic, and durable bifunctional oxygen electrocatalysts is indispensable for rechargeable metal–air batteries. Herein we present a simple approach for the development of carbon nitride fibers co-doped with phosphorus and sulfur, grown in situ on carbon cloth (PS-CNFs) as a flexible electrode material, and demonstrate its outstanding bifunctional catalytic activities toward ORR and OER compared to those of precious metal-based Pt/C and IrO2 on account of the dual action of P and S, numerous active sites, high surface area, and enhanced charge transfer. Furthermore, we demonstrate the flexibility, suitability, and durability of PS-CNFs as air electrodes for primary and rechargeable Zn–air batteries. Primary Zn–air batteries using this electrode showed high peak power density (231 mW cm−2), specific capacity (698 mA h g−1; analogous energy density of 785 W h kg−1), open circuit potential (1.49 V), and outstanding durability of more than 240 h of operation followed by mechanical recharging. Significantly, three-electrode rechargeable Zn–air batteries revealed a superior charge–discharge voltage polarization of ∼0.82 V at 20 mA cm−2, exceptional reversibility, and continuous charge–discharge cycling stability during 600 cycles. This work provides a pioneering strategy for designing flexible and stretchable metal-free bifunctional catalysts as gas diffusion layers for future portable and wearable renewable energy conversion and storage devices.
Conceptual insightsZn–air batteries suffer from high polarization loss due to sluggish reactions of the oxygen reduction reaction (ORR)/oxygen evolution reaction (OER) at the air-cathodes, severely hindering the rate capability, design rigidity, energy efficiency, and operational life. Since the rechargeable energy storage systems are operated by bifunctional reactions for ORR/OER, designing flexible, cost-efficient, viable bifunctional catalysts is of critical significance. As efficient bifunctional flexible metal-free oxygen electrodes, we present a facile strategy for the in situ fabrication of a porous, three-dimensional heteroatom doped carbon nitride fibrous structure on carbon cloth at a low temperature. This fibrous catalyst revealed a 3D hybrid network of high N content with substantial P and S co-doping, leading to the outstanding ORR/OER performance and durability as well as mechanical strength, superior to those of noble metals, transition metals, and metal-free counterparts. As a result, our fibrous network electrode manifested the exceptional energy/power density, rechargeability, and flexibility that meet or exceed the state-of-the-art Li-ion batteries. |
Introduction
The development of flexible renewable electrochemical energy storage and conversion systems such as metal–air batteries, fuel cells, and supercapacitors is highly desirable in addressing the present challenges regarding global energy and the environment.1–3 The unique features of such systems, such as lightweight, a small unit size, physical flexibility, and stretchability, enable their use in portable, wearable, and flexible energy devices.4–6 Although lithium-ion batteries are considered the most promising candidates for plug-in electric vehicles because of their superior cycle stability, high Coulombic efficiency, and discharge voltage, their inadequate gravimetric storage density and cycle life limits the industrial demand.7–9 Flexible and electrically rechargeable Zn–air batteries (ZABs) are considered as a potential alternative to lithium-ion batteries as a result of their reversibility, high energy density, low cost, a flat discharge profile, and environmentally benign operation.10,11 However, the development of flexible rechargeable ZABs for large-scale applications has been impeded by low rate capability, limited life, and lack of efficient, robust bifunctional catalysts toward electrochemical reactions (ORR/OER). Catalysts containing precious noble metals (e.g., Pt, Ir, and Ru) and/or transition metals (e.g., Ni, Co, Fe, and Mn) are often used for ORR and OER.12–15 Unfortunately, their high cost, electrochemical instability, scarcity, and intrinsically poor electrical conductivity of transition metal catalysts have restricted the commercialization of clean energy technologies.16 Therefore, exploring the strategies to develop flexible, efficient, and stable bifunctional catalysts from metal-free elements remains a technological challenge in the development of metal–air batteries.In recent studies, carbon nanomaterials (graphene, CNTs, activated carbon) doped with heteroatoms (N, P, S, B, F) have been recognized as a promising category of metal-free bifunctional catalysts toward ORR and OER for rechargeable and flexible ZABs.17,18 Specifically, experimental studies and quantum mechanical calculations have confirmed that doping of C with N significantly enhances the activity and stability toward electrochemical reactions because of strong π bonding and the promotion of the electron donor–acceptor properties.19,20 Among N-doped C materials, graphitic carbon nitride (g-C3N4) is highly desirable for the insertion of N into a carbon framework because of its ultrahigh content of N, cost-effectiveness, and a tailorable electronic structure suitable for engineering of potential oxygen reaction catalysts. However, limited efforts have been made to utilize g-C3N4 as the active material for electrochemical reactions because of its poor electrical conductivity. Generally, physical mixing and immobilization of g-C3N4 with carbon supports improve its conductivity; however, the inhomogeneity, poor contact, and high-temperature polymerization of monomers (∼500–600 °C) undesirably leads to substantial loss in nitrogen content.13,21,22 Furthermore, the doping of heteroatoms is a highly desirable strategy to enhance the electrochemical activity of g-C3N4 by tailoring its charge polarization and spin density because of the differences in the electronegativity of carbon, nitrogen, and the heteroatoms.23 Thus, the development of a facile strategy for assuring the in situ growth with rational nanostructures, high surface area, and excellent conductivity at relatively lower temperatures is highly desirable. Recently, a series of molecular-level C3N4-coordinated transition metals (M–C3N4) as one class of M–N/C materials;24 non-metals (X–C3N4)3 and immobilization of the conducting support materials such as CN/graphene, CN/C, CN/CNTs, etc.15,17,22,25–27 for oxygen electrode reactions have been developed. However, lots of unresolved issues still remained: (i) unknown fine structures after metal coordination ligands; thus, the nature of active sites and catalytic centres is unclear,24 (ii) a coordination ability with a variety of metals, non-metals as well as carbon based supports and their chemical interactions is unexplored,25 (iii) the mass transport and access of the proton exchange ionomers are limited due to the inhomogeneous distribution of pores on conducting supports,27 (iv) a low yield and long synthesis time suffer from the interfacial couplings,15 and (v) the potential applications in other energy reactions beyond the scope of oxygen reduction/evolution are restricted. Nevertheless, the optimal use of heteroatom-doped g-C3N4 materials as metal-free bifunctional catalysts for oxygen electrochemistry has rarely been investigated. Preserving the flexibility and outstanding bifunctional catalytic activity for application to air electrodes remains challenging for engineering of flexible ZABs.
Herein we report a scalable strategy for the in situ growth of a three-dimensional (3D) architecture of phosphorus and sulfur co-doped carbon nitride interconnected nanofibers (PS-CNFs) on carbon cloth simply by a polymerization reaction, as a flexible oxygen electrode. This new strategy yields a stable polymeric C–N network under polymerization at a relatively low temperature. The prepared 3D hybrid nanofiber architecture displays excellent bifunctional electrochemical performance for ORR and OER, comparable to that of noble metal-based catalysts. Furthermore, advanced flexible and rechargeable ZABs constructed using the PS-CNF air-electrodes demonstrate a high energy efficiency as well as long-term mechanical and cycling durability.
Results and discussion
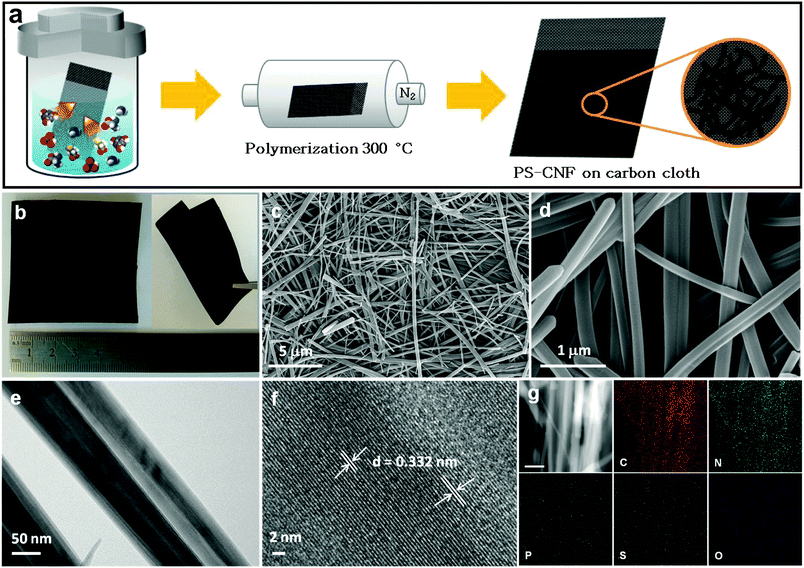
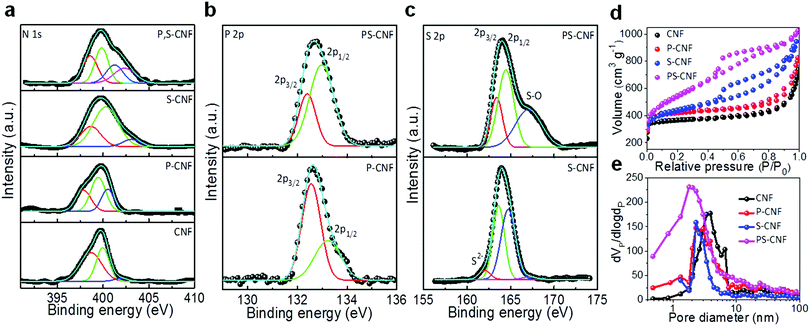
Carbon cloth was first mildly oxidized with piranha solution to generate abundant functional groups to enhance acid–base interactions and hydrophilicity (Fig. S1, ESI†). To obtain flexible, highly active metal-free electrocatalysts with a high surface area, a facile surfactant-assisted precipitation method was carried out followed by a simple polymerization, resulting in the self-assembly of PS-CNFs on the pre-treated carbon cloth (Fig. 1a; see the Experimental methods section in the ESI†). Such a nanostructural design enhances the overall electrochemical performance because of the large number of active site spotlights and the rapid displacement of active ions during the ORR and OER reactions. The cross-stacking of aligned PS-CNFs yields remarkable flexibility, large scale (Fig. 1b), and desirable mechanical properties (such as tensile strength ∼2.04 MPa and tensile modulus ∼0.28 GPa), presumably because of polar interactions of associated carbon, nitrogen, and oxygen (Fig. S2, ESI†), confirming this material as a potential candidate for flexible ZABs. Scanning electron microscopy (SEM) images of the prepared PS-CNFs (Fig. 1c and d) show their typical randomly entangled cross-linked 3D fibrous network structures as densely grown on the carbon cloth. High-resolution SEM and transmission electron microscopy (TEM) (Fig. 1e) images of PS-CNF clearly show a porous framework of interconnected fibers with diameters in the approximate range of 130–173 nm. The surface texture of the carbon cloth and acid–base interactions facilitates the adsorption of precursors and the strongly bonded growth of carbon nitride fibers on the carbon cloth. We attribute the experimentally observed mechanical flexibility of the prepared PS-CNF to its open-pore network structure, in which the free space allows effective attenuation of bending stress without destruction of the fibers. A HRTEM image of a single PS-CNF fibre (Fig. 1f) shows a well crystalline carbon nitride structure with strong orientation of (002) graphitic domains showing a lattice fringe space of ∼0.332 nm, consistent with XRD results (Fig. S3, ESI†). A bright field TEM image and its corresponding energy-dispersive X-ray spectroscopy (EDS) elemental map (Fig. 1g) illustrate the uniform distribution of C, N, P, S, and O throughout the mesoporous fibers, confirming the homogeneous growth of PS-CNF on the carbon cloth. The surfactant-assisted polymerization of melamine and amino acids at a relatively low temperature led to the self-assembly of thermally robust and defect-rich graphitic carbon nitride domains co-doped with P and S on the carbon cloth, as determined by means of microscopy, XRD, TGA, Fourier transform infrared and Raman spectroscopy (Fig. S3–S6, ESI†). The positive charge, electrostatic interaction of the surface oxidized groups, and π–π interaction of conjugated tri-s-triazine motifs can be recognized as the driving forces for the in situ growth of PS-CNF on carbon cloth. Such a flexible nanostructural design can be readily scaled up for commercial applications. The strong coupling and chemical nature of PS-CNF on carbon cloth were investigated by means of X-ray photoelectron spectroscopy (XPS). The survey spectra showed the expected peaks of the C, N, P, S, and O elements together with the corresponding numerical results (Fig. S7a and Table S1, ESI†). The deconvoluted C 1s spectra (Fig. S7b, ESI†) showed a strong C–N coordination at 288.2 eV and a weak graphitic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C coordination at 284.6 eV.22 High-resolution N 1s spectra showed distinct profiles of nitrogen species including pyridinic N1, pyrrolic N2, graphitic N3, quaternary N4, and oxidized pyridinic N5 (Fig. 2a).28 Specifically, the graphitic N3 peak can be assigned to the bridging N atoms in N(–C)3 or to N bonded with H atoms, whereas the pyridinic N1 peak corresponded to the sp2-bonded N species in triazine rings (C
C coordination at 284.6 eV.22 High-resolution N 1s spectra showed distinct profiles of nitrogen species including pyridinic N1, pyrrolic N2, graphitic N3, quaternary N4, and oxidized pyridinic N5 (Fig. 2a).28 Specifically, the graphitic N3 peak can be assigned to the bridging N atoms in N(–C)3 or to N bonded with H atoms, whereas the pyridinic N1 peak corresponded to the sp2-bonded N species in triazine rings (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C).29,30 The weaker shoulder peaks observed in the range of 396.7–397.8 eV confirmed the strong coupling of the prepared carbon nitride fibers with the carbon cloth.31 Also, the presence of oxidized pyridinic N5 arose from the protonation of carbon nitride, which produced positively charged CN heterocycles and cyano groups.32 Remarkably, each nitrogen species has different chemical and electronic effects on the adjacent carbon atoms, resulting in different electrochemical activities. The N 1s spectra and their normalized data (Fig. S8 and Table S2, ESI†) demonstrated that the electron-donating quaternary N4 and electron-withdrawing pyridinic N1 in PS-CNF were strongly active sites for ORR and OER, respectively, along with graphitic N3 and oxidized pyridinic N5. The core-level fitting of the P 2p spectra (Fig. 2b) showed the presence of two peaks at 132.4 and 133.1 eV which are corresponding to the P–N and P–O bonding, respectively, indicating that P atoms are selectively substituted on the C site.33,34 The slight shift in the binding energy observed for P-CNF and PS-CNF arose from the condensation and dehydration of phosphoric clusters into polyphosphates. The interaction of phosphate species strongly acts as active species for the oxygen evolution reaction.34 In the S 2p core-level XPS spectra of S-CNF and PS-CNF, two prominent components of S 2p3/2 and S 2p1/2 were observed at ∼163.6 and 164.5 eV, indicating a strong C–S–C interaction (Fig. 2c). This implies that the S core states possess highly active thiol functional groups, because of the stronger electronegativity of S compared to C and P.35 This result confirms that the P atoms can donate partial electrons to S, facilitating the possible interaction of P and S as one of the active species. The observation of a weak S2− peak for S-CNF can be ascribed to residual sulfur during its chemical synthesis, whereas a shoulder observed at 167 eV for S-CNF and PS-CNF was attributed to the formation of sulfate species.36
N–C).29,30 The weaker shoulder peaks observed in the range of 396.7–397.8 eV confirmed the strong coupling of the prepared carbon nitride fibers with the carbon cloth.31 Also, the presence of oxidized pyridinic N5 arose from the protonation of carbon nitride, which produced positively charged CN heterocycles and cyano groups.32 Remarkably, each nitrogen species has different chemical and electronic effects on the adjacent carbon atoms, resulting in different electrochemical activities. The N 1s spectra and their normalized data (Fig. S8 and Table S2, ESI†) demonstrated that the electron-donating quaternary N4 and electron-withdrawing pyridinic N1 in PS-CNF were strongly active sites for ORR and OER, respectively, along with graphitic N3 and oxidized pyridinic N5. The core-level fitting of the P 2p spectra (Fig. 2b) showed the presence of two peaks at 132.4 and 133.1 eV which are corresponding to the P–N and P–O bonding, respectively, indicating that P atoms are selectively substituted on the C site.33,34 The slight shift in the binding energy observed for P-CNF and PS-CNF arose from the condensation and dehydration of phosphoric clusters into polyphosphates. The interaction of phosphate species strongly acts as active species for the oxygen evolution reaction.34 In the S 2p core-level XPS spectra of S-CNF and PS-CNF, two prominent components of S 2p3/2 and S 2p1/2 were observed at ∼163.6 and 164.5 eV, indicating a strong C–S–C interaction (Fig. 2c). This implies that the S core states possess highly active thiol functional groups, because of the stronger electronegativity of S compared to C and P.35 This result confirms that the P atoms can donate partial electrons to S, facilitating the possible interaction of P and S as one of the active species. The observation of a weak S2− peak for S-CNF can be ascribed to residual sulfur during its chemical synthesis, whereas a shoulder observed at 167 eV for S-CNF and PS-CNF was attributed to the formation of sulfate species.36
The N2 sorption measurements of the CNF-based catalysts were carried out to investigate their pore distribution and specific surface area. The as-prepared CNF-based catalysts showed typical type-IV isotherms with a H3 hysteresis loop (Fig. 2d), confirming the presence of a mesoporous network. The observed BET surface area of PS-CNF (1649 m2 g−1) was much higher than those of P-CNF (1124 m2 g−1) and S-CNF (1268 m2 g−1), as well as that previously reported for hard templated porous carbons (∼500–1200 m2 g−1).10,13,37 The fast N2 uptake at a relatively higher pressure (P/P0 > 0.9) illustrated the presence of larger secondary pores. Furthermore, a BJH pore size distribution analysis based on N2 desorption isotherms showed sharp peaks of diameters <2 nm for PS-CNF, <3 nm for P-CNF, S-CNF, and <4 nm for pristine CNF (Fig. 2e). Notably, the pore volumes of the prepared catalysts showed an increasing trend with doping: 0.8, 1.1, 1.34, and 1.68 cm3 g−1, respectively, for pristine CNF, P-CNF, S-CNF, and PS-CNF. Because the flexible carbon nitride 3D fibrous networks co-doped by P and S showed an ultrahigh specific surface area, mesopores, enhanced pore volume and large electrical conductivity (viz. ∼165 S m−1; compared with ∼52 S m−1 for CNF), and were formed by means of a facile one-step polymerization, they appear to be very promising for electrocatalytic applications.
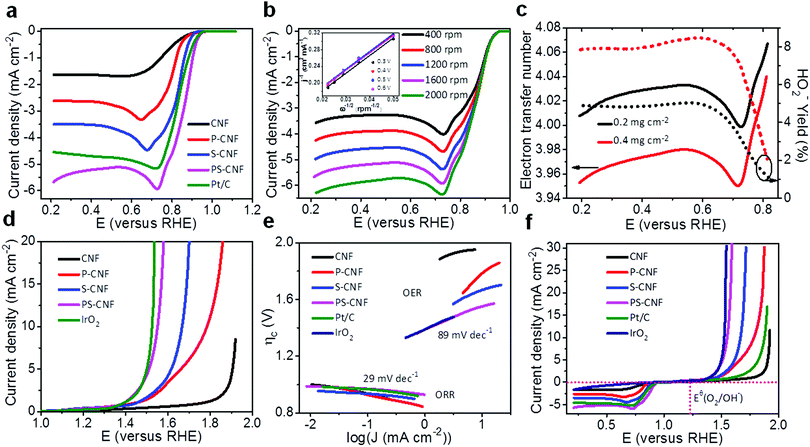
Cyclic voltammetry (CV) (Fig. S9, ESI†) showed a cathodic peak for PS-CNF in O2-saturated KOH solution; however, none was observed in N2-saturated KOH solution, a similar result to that observed for Pt/C. The oxygen reduction peak for PS-CNF was observed at 0.82 V, a slightly less positive value compared to that of Pt/C, 0.84 V. Furthermore, the reduction current density of PS-CNF (1.2 mA cm−2) was noticeably superior to that of Pt/C (0.61 mA cm−2), suggesting that the PS-CNF catalysts had excellent catalytic activity. The observation of high electrical conductivity and the consequent fast charge transfer (Table S1 and Fig. S10, ESI†) confirmed that the PS-CNF catalyst was highly active compared to CNF, P-CNF, and S-CNF catalysts. As shown in linear sweep voltammograms (LSVs), the pristine CNF demonstrated negligible ORR performance, whereas PS-CNF showed a positive onset potential of 0.94 V and a half-wave potential of 0.86 V (Fig. 3a). These potentials outperform those of Pt/C and the previously reported metal-free catalysts.3,10,17,38,39 Remarkably, the limiting current density of PS-CNF (5.63 mA cm−2) outperformed those of Pt/C (4.55 mA cm−2), P-CNF, and S-CNF catalysts. Fast oxygen consumption causes a slight decrement in the current density for higher overpotentials. The higher electrocatalytic activity of PS-CNF compared to the other aforementioned metal-free catalysts, concerning half-wave potential and reaction current density, confirms the significance of co-doping with P, S and the nanostructural network for facile ORR. The ORR kinetics was determined according to the Koutechy–Levich (K–L) equation from the RDE curves collected at different rotation speeds (Fig. 3b and Fig. S11, ESI†). Furthermore, a linear increment in the current density was observed with increasing rotation speed, evidencing a first-order reaction toward oxygen reduction in PS-CNF. The linear behaviour of K–L plots (inset, Fig. 3b) with similar slopes for PS-CNF and Pt/C catalysts yields the electron transfer number (n) of ∼4.02, indicating a four-electron reaction pathway for ORR.40 As P-CNF and S-CNF have the highest P and S contents, they could possess relatively poor catalytic activity compared to PS-CNF, leading to a greater charge transfer resistance (Table S1 and Fig. S10, ESI†). Furthermore, the ORR pathways of PS-CNF catalysts were evaluated by performing the rotating ring-disk electrode (RRDE) measurements. The PS-CNF electrodes (Fig. 3c and Fig. S12a, ESI†) showed higher disk current densities (5.63 and 6.77 mA cm−2) for ORR reactions as well as lower ring current densities (∼0.012 and 0.029 mA cm−2) in the cases using two different mass loadings, i.e., 0.2 and 0.4 mg cm−2, respectively. Notably, the RRDE ring current densities for PS-CNF electrodes confirmed the minimal amount of peroxide oxidation (i.e., <6 and 8% for 0.2 and 0.4 mg cm−2 mass loadings, respectively). Larger mass loading resulted in high current densities with an increase in peroxide oxidation, implying more degradation in the electrodes. The achieved electron transfer number per oxygen molecule according to RRDE analysis was about 3.96–4.07. Thus, both RDE and RRDE analyses confirmed the reduction of oxygen molecules to water by means of a highly desirable four-electron pathway. As we know, the incorporation of heteroatoms stimulates the charge redistribution that is strongly responsible for ORR activity in carbon-based metal catalysts,12 and co-doping by two heteroatoms of different electronegativities to that of carbon demonstrates a synergistic effect for further enhancement of ORR activity.41
The chronoamperometric response demonstrated a slight attenuation in the current density (∼0.4% in the initial current density) for PS-CNF, whereas the response of Pt/C degraded rapidly by 24.45% in just 9000 s, thus demonstrating the superior stability in an alkaline environment of the PS-CNF catalyst grown in situ, compared to that of Pt/C (Fig. S12b, ESI†). Hence, PS-CNF appears to be favourable for future development of alkaline fuel cells. The high catalytic selectivity should be considered against fuel oxidation (especially, for organic fuels such as methanol) in the anode, because it can be permeated through the polymer electrolyte membrane to the cathode, showing a serious effect on the overall cell performance.42 Accordingly, the electro-oxidation of methanol over PS-CNF and Pt/C was evaluated. Upon addition of 3 M methanol in O2-saturated 0.1 M KOH solution, the PS-CNF catalyst retained a stable current response whereas the Pt/C catalyst immediately jumped to a negative current, showing the oxidation of the Pt/C surface (Fig. S12c, ESI†). Indeed, the PS-CNF catalyst showed an outstanding catalytic selectivity for ORR and a remarkable tolerance of the crossover effects, demonstrating its superiority as a catalyst compared to Pt/C. Similarly, in a test of catalytic poisoning, Pt/C showed a strong negative current response upon addition of CO, whereas PS-CNF showed no noticeable response under similar test conditions (Fig. S12d, ESI†), demonstrating the vulnerability of Pt/C and suggesting better suitability of PS-CNF as a catalyst. The observed excellent stability, methanol crossover, and the resistance to CO poisoning arise from the unique 3D interconnected fibrous structure of PS-CNF.
Having demonstrated the excellent ORR performance of CNF-based catalysts, we also investigated their OER activity (Fig. 3d). As expected, the required overpotential for driving the current density of 10 mA cm−2 (a metric related to solar fuel synthesis)42 for PS-CNF was 320 mV, significantly lower than those of Pt/C (644 mV), P-CNF (550 mV), and S-CNF (430 mV), and comparable to that of IrO2 (300 mV). PS-CNF also exhibited a lower onset potential (1.32 V) compared to IrO2 (1.34 V) and Pt/C. PS-CNF demonstrated the smallest Tafel value of 29 mV dec−1 in the region of ORR compared to those of Pt/C (38 mV dec−1), P-CNF, and S-CNF (Fig. 3e). Furthermore, it showed the lowest Tafel slope of 89 mV dec−1 in the region of OER compared to all other prepared electrodes: P-CNF, S-CNF, Pt/C, and IrO2. These results confirm that PS-CNF has better reaction kinetics for ORR and OER. Fig. 3f illustrates the rapid increment in the anodic current related to OER, of over ∼1.3 V. The lower onset potentials and higher current densities of PS-CNF compared to those of Pt/C further reflected the former's better OER performance. The reversibility and overall bifunctionality toward oxygen reactions of PS-CNF was assessed by calculating the variance of the ORR and OER metrics (ΔE = Ej=10 − E1/2). Ideally, lower values of ΔE correspond to the excellent bifunctional performance of an oxygen electrode.43 As listed in Table S3 (ESI†), PS-CNF shows a lower ΔE value (0.69 V) than the noble metal-containing catalysts (Pt/C: 0.94 V;44 Ir/C: 0.92 V43); Table S3 (ESI†) also compares other performance parameters of PS-CNF with those reported for other state-of-the-art bifunctional catalysts toward ORR and OER, including catalysts based on metal oxides as well as metal-free catalysts.
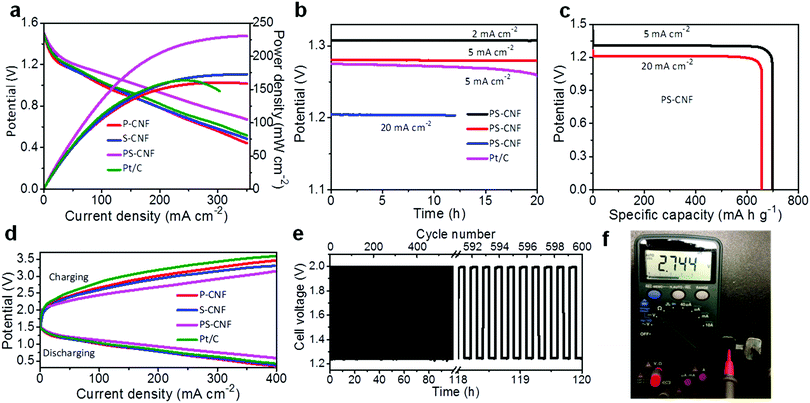
To demonstrate the potential applications of the PS-CNF bifunctional catalyst in real energy devices, a primary Zn–air battery was assembled using PS-CNF grown in situ on carbon cloth as the air cathode and the zinc plate as the anode, with atmospheric oxygen as a fuel source (Fig. S13, ESI†). Fig. 4a shows polarization and power density curves obtained for liquid ZABs with the PS-CNF catalysts. The PS-CNF cathode showed a higher potential and power density (∼231 mW cm−2) than its Pt/C-based counterpart over a wide range of current densities (i.e., up to 350 mA cm−2), signifying a superior rate performance. The open-circuit voltage of the primary ZABs with PS-CNF air cathodes was quite high, at ∼1.49 V. The power density observed for the PS-CNF electrode was nearly 1.5 times that of the Pt/C electrode because of its good ORR activity and a unique 3D mesoporous structure that enabled fast mass and charge transfer. Furthermore, no potential decrease was observed for the ZABs using PS-CNF electrodes under long-term galvanostatic discharging for 20 h at 2 and 5 mA cm−2, or for 12 h at 20 mA cm−2 (Fig. 4b), unlike the case of Pt/C electrodes. The cell performance and ZAB stability observed with the use of the PS-CNF electrode were consistent with its superior bifunctional catalytic activity and stability as described above. Upon continuous discharge, the Zn plate is gradually consumed, and the electrolyte can collect the increasingly soluble zinc, causing the battery to eventually cease the functioning when all Zn is consumed. Therefore, the battery was mechanically recovered by refilling of the Zn foil and the KOH electrolyte. The ZABs with PS-CNF electrodes showed a continuous performance of over 240 h without a potential decrease during two and many cycles (>5) compared to that of the recently reported primary ZABs, demonstrating the mechanical recharging capability (Fig. S14 and Table S4, ESI†). The full-cell ZABs using PS-CNF air electrodes demonstrated the voltage plateaus of ∼1.3 and 1.21 V with the specific capacities of 698 and 657 mA h g−1 for the discharge current densities of 5 and 20 mA cm−2, respectively. The resultant gravimetric energy densities were also 785 and 753 W h kg−1 for each case. Notably, the present values are comparable to or higher than those of the reported primary ZABs (Table S4, ESI†),3,10,17,45–47 confirming that the presently reported PS-CNF networks grown in situ hold great promise as bifunctional catalysts. Furthermore, we also developed rechargeable ZABs having a similar configuration except that 0.2 M of zinc oxide was added to the KOH electrolyte. Fig. 4d illustrates the discharge and charge LSV curves of self-assembled bifunctional electrodes using a three-electrode cell configuration. Specifically, PS-CNF showed a lower sum of charge and discharge overpotentials compared to those of Pt/C, P-CNF, and S-CNF, confirming its excellent rechargeability. In particular, the rechargeable ZABs in the three-electrode configuration with PS-CNF air electrodes illustrated the highest round-trip efficiency, with no obvious potential drop observed during 600 discharge/charge cycles conducted over a 120 h period with the constant current density of 2 mA cm−2 (Fig. 4e). Contrastingly, Pt/C + IrO2 electrodes showed the inferior cycle stability of less than 50 h under continuous operation (Fig. S15, ESI†), including a significant loss in charge and discharge potentials. This degradation arises from carbon corrosion and the removal of Pt particles from the carbon cloth. These results are comparable to or even better than those of the recently studied metal oxide catalysts for rechargeable ZABs (Table S5, ESI†).1,3,10,17,48,49 Thus, the presently reported PS-CNF material is a more realistically usable catalyst for ZABs, having the potential to reduce the charge–discharge overpotential gap and offering substantial long-term cycling stability. To test its feasibility for practical energy devices, we prepared multiple ZABs using PS-CNF electrodes. Moreover, connecting in-series two solid-state micro ZABs including PS-CNF air electrodes showed the maximum open cell potential of 2.744 V without a current collector in atmospheric air (Fig. 4f), even after operating overnight, exemplifying superior working stability.
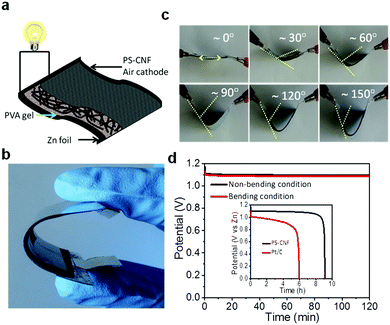
Apart from liquid ZABs, we constructed flexible solid-state ZABs consisting of in situ grown PS-CNF as a free-standing air electrode, an alkaline PVA gel polymer electrolyte, and a Zn foil to illustrate the potential applications in flexible, portable, and wearable electronic technologies (Fig. 5a and b). The prepared solid-state ZABs with PS-CNF showed a relatively inferior discharge voltage because of the high contact resistance and poor ionic conductivity of the PVA polymer gel, but illustrated its excellent flexibility and cycling durability. This assembled battery was bent to angles of 30°, 60°, 90°, 120°, and 150° without causing damage to the original structure (Fig. 5c). The discharge performance of the prepared ZABs with PS-CNF was tested under discharge at 2 mA cm−2 before and after bending (bending angle ∼120° for 100 cycles); the resulting discharge voltage profiles illustrated a negligible potential difference before and after bending, confirming that the prepared flexible battery can remain operational under external strain/stress (Fig. 5d). Remarkably, the PS-CNF air electrode (inset, Fig. 5d) exhibited outstanding electrochemical performance, with a higher voltage plateau (1.1 V) and a longer discharge time (9 h) compared to that of Pt/C (1.0 V plateau, 6 h discharge time). The electrode design is a crucial factor apart from its good flexibility to simplify the fabrication method and to remove the polymer binder than that of reported flexible ZABs.50 Compared to the conventional drop-cast air electrodes, the self-assembled PS-CNF electrodes reported herein have a unique 3D mesoporous structure and an ultrahigh surface area, thereby offering abundant channels and fast electron/ion paths for gas diffusion. Moreover, the highly conductive and flexible PS-CNF can act as a self-supporting electrode or current collector to reduce the cost and cell size. This noteworthy performance arose from the exceptional electronic properties of the cross-stacked network structure of the PS-CNF catalyst grown in situ, the flexible and stretchable components, and the use of a solid-state hydrogel polymer as a separator to avoid the electrolyte leakage. Clearly, these results indicate that the PS-CNF air electrode reported herein is a promising candidate for future portable and wearable electronic devices.
Conclusions
In summary, we proposed a facile and scalable approach for the in situ growth of a carbon nitride nanofibrous network co-doped with phosphorus and sulfur on carbon cloth as a 3D flexible oxygen electrode. Such flexible PS-CNFs have an ultrahigh surface area (1649 m2 g−1), superior electrical conductivity (165 S m−1), remarkable tensile modulus (∼0.28 GPa), and high tensile strength (∼2.04 MPa). These PS-CNF catalysts illustrated superb bifunctional catalytic activity, stability, and reversibility toward ORR and OER, outperforming most of the noble metal-based, transition metal-based, and metal-free catalysts. This electrode shows great promise as a flexible bifunctional air electrode for both primary and rechargeable ZABs, offering low overpotentials and a long lifetime. Liquid primary ZABs using the P,S-CNF air electrode demonstrated high discharge potential and power density (231 mW cm−2), high energy densities (785 and 753 W h kg−1 for 5 and 20 mA cm−2), and an exceptional lifetime without a decrease in voltage over 240 h by means of mechanical refilling. Rechargeable ZABs with a three-electrode configuration including PS-CNF electrodes exemplified remarkable reversibility and stability for 600 cycles over 120 h of operation. Significantly, a flexible solid-state rechargeable ZAB was constructed with PS-CNF and demonstrated a high discharge voltage of 1.1 V at 2 mA cm−2 with excellent mechanical and cycling stability under external strain. The present work represents a key advancement in the design of flexible bifunctional air electrodes for renewable energy technologies such as portable and wearable optoelectronic devices.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2017R1A2B3006941). This work was also supported by the Human Resources Development program (No. 20154030200680) of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government Ministry of Trade, Industry and Energy.References
- H. Yang, J. Miao, S. Hung, J. Chen, H. Tao, X. Wang, L. Zhang, R. Chen, J. Gao, H. Chen, L. Dai and B. Liu, Sci. Adv., 2016, 2, e1501122 Search PubMed.
- D. Lee, J. Fu, M. Park, H. Liu, A. Kashkooli and Z. Chen, Nano Lett., 2016, 16, 1794–1802 CrossRef CAS PubMed.
- S. Shinde, C. Lee, A. Sami, D. Kim, S. Lee and J. Lee, ACS Nano, 2017, 11, 347–357 CrossRef CAS PubMed.
- L. Li, Z. Wu, S. Yuan and X. B. Zhang, Energy Environ. Sci., 2014, 7, 2101–2122 CAS.
- J. Liu, C. Guan, C. Zhou, Z. Fan, Q. Ke, G. Zhang, C. Liu and J. Wang, Adv. Mater., 2016, 28, 8732–8739 CrossRef CAS PubMed.
- W. Zuo, R. Li, C. Zhou, Y. Li, J. Xia and J. Liu, Adv. Sci., 2017, 1600539, DOI:10.1002/advs.201600539.
- J. Jung, M. Risch, S. Park, M. Kim, G. Nam, H. Jeong, Y. Horn and J. Cho, Energy Environ. Sci., 2016, 9, 176–183 CAS.
- P. Bruce, S. Freunberger, L. Hardwick and J. Tarascon, Nat. Mater., 2012, 11, 19–29 CrossRef CAS PubMed.
- N. Liu, Z. Lu, J. Zhao, M. McDowell, H. Lee, W. Zhao and Y. Cui, Nat. Nanotechnol., 2014, 9, 187–192 CrossRef CAS PubMed.
- J. Zhang, Z. Zhao, Z. Xia and L. Dai, Nat. Nanotechnol., 2015, 10, 444–452 CrossRef CAS PubMed.
- Y. Li, M. Gong, Y. Liang, J. Feng, J. Kim, H. Wang, G. Hong, B. Zhang and H. Dai, Nat. Commun., 2013, 4, 1805 CrossRef PubMed.
- J. Zhang, L. Qu, G. Shi, J. Liu, J. Chen and L. Dai, Angew. Chem., Int. Ed., 2016, 55, 2230–2234 CrossRef CAS PubMed.
- T. Ma, S. Dai, M. Jaroniec and S. Qiao, Angew. Chem., 2014, 126, 7409–7413 CrossRef.
- Y. Jiao, Y. Zheng, M. Jaroniec and S. Qiao, Chem. Soc. Rev., 2015, 44, 2060–2086 RSC.
- T. Ma, J. Ran, S. Dai, M. Jaroniec and S. Qiao, Angew. Chem., Int. Ed., 2015, 54, 4646–4650 CrossRef CAS PubMed.
- G. Li, X. Wang, J. Fu, J. Li, M. Park, Y. Zhang, G. Lui and Z. Chen, Angew. Chem., Int. Ed., 2016, 55, 4977–4982 CrossRef CAS PubMed.
- Q. Liu, Y. Wang, L. Dai and J. Yao, Adv. Mater., 2016, 28, 3000–3006 CrossRef CAS PubMed.
- Y. Zheng, Y. Jiao, L. Ge, M. Jaroniec and S. Qiao, Angew. Chem., Int. Ed., 2013, 52, 3110–3116 CrossRef CAS PubMed.
- K. Gong, F. Du, Z. Xia, M. Durstock and L. Dai, Science, 2009, 323, 760–764 CrossRef CAS PubMed.
- Y. Jiao, Y. Zheng, M. Jaroniec and S. Qiao, J. Am. Chem. Soc., 2014, 136, 4394–4403 CrossRef CAS PubMed.
- S. Shinde, A. Sami and J.-H. Lee, Carbon, 2016, 96, 929–936 CrossRef CAS.
- S. Shinde, A. Sami and J.-H. Lee, ChemCatChem, 2015, 7, 3873–3880 CrossRef CAS.
- T. Ma, S. Dai, M. Jaroniec and S. Qiao, Angew. Chem., Int. Ed., 2014, 53, 7281–7285 CrossRef CAS PubMed.
- S. Yang, X. Feng, X. Wang and K. Mullen, Angew. Chem., Int. Ed., 2011, 50, 5339–5343 CrossRef CAS PubMed.
- H. Chang, S. H. Joo and C. Pak, J. Mater. Chem., 2007, 17, 3078 RSC.
- S. Yang, X. Feng, X. Wang and K. Mullen, Angew. Chem., 2011, 123, 5451–5455 CrossRef.
- J. Masa, W. Xia, M. Muhler and W. Schuhmann, Angew. Chem., Int. Ed., 2015, 54, 10102 CrossRef CAS PubMed.
- T. Palaniselvam, M. Valappil, R. Illathvalappil and S. Kurungot, Energy Environ. Sci., 2014, 7, 1059–1067 CAS.
- G. Liu, P. Niu, C. Sun, S. Smith, Z. Chen, G. Lu and H. Cheng, J. Am. Chem. Soc., 2010, 132, 11642–11648 CrossRef CAS PubMed.
- S. Shinde, A. Sami and J. Lee, J. Mater. Chem. A, 2015, 3, 12810–12819 CAS.
- J. Tian, Q. Liu, A. Asiri, K. Alamry and X. Sun, ChemSusChem, 2014, 7, 2125–2132 CrossRef CAS PubMed.
- F. Su, C. K. Poh, J. S. Chen, G. Xu, D. Wang, Q. Li, J. Lin and X. W. Lou, Energy Environ. Sci., 2011, 4, 717 CAS.
- Y. Zhang, T. Mori, J. Ye and M. Antonietti, J. Am. Chem. Soc., 2010, 132, 6294–6295 CrossRef CAS PubMed.
- A. Puziy, O. Poddubnaya, R. Socha, J. Gurgul and M. Wisniewski, Carbon, 2008, 46, 2113–2123 CrossRef CAS.
- S. Cho, J. Seo, S. Park, S. Beaupre, M. Leclerc and A. Heeger, Adv. Mater., 2010, 22, 1253–1257 CrossRef CAS PubMed.
- L. Ji, M. Rao, H. Zheng, L. Zhang, Y. Li, W. Duan, J. Guo, E. J. Carirns and Y. Zhang, J. Am. Chem. Soc., 2011, 133, 18522 CrossRef CAS PubMed.
- Z. Li, M. Shao, L. Zhou, Q. Yang, C. Zhang, M. Wei, D. Evans and X. Duan, Nano Energy, 2016, 25, 100–109 CrossRef.
- H. Liang, Z. Wu, L. Chen, C. Li and S. Yu, Nano Energy, 2015, 11, 366–376 CrossRef CAS.
- R. Liu, D. Wu, X. Feng and K. Müllen, Angew. Chem., Int. Ed., 2010, 49, 2565–2569 CrossRef CAS PubMed.
- S. Wang, L. Zhang, Z. Xia, A. Roy, D. W. Chang, J. B. Baek and L. Dai, Angew. Chem., Int. Ed., 2012, 51, 4209–4212 CrossRef CAS PubMed.
- L. Qu, Y. Liu, J. B. Baek and L. Dai, ACS Nano, 2010, 4, 1321 CrossRef CAS PubMed.
- C. McCrory, S. Jung, J. Peters and T. Jaramillo, J. Am. Chem. Soc., 2013, 135, 16977–16987 CrossRef CAS PubMed.
- Y. Gorlin and T. F. Jaramillo, J. Am. Chem. Soc., 2010, 132, 13612–13614 CrossRef CAS PubMed.
- J. Masa, W. Xia, I. Sinev, A. Zhao, Z. Sun, S. Grîtzke, P. Weide, M. Muhler and W. Schuhmann, Angew. Chem., Int. Ed., 2014, 53, 8508–8512 CrossRef CAS PubMed.
- J. Park, M. Park, G. Nam, J. Lee and J. Cho, Adv. Mater., 2015, 27, 1396–1401 CrossRef CAS PubMed.
- R. Cao, R. Thapa, H. Kim, X. Xu, M. Kim, Q. Li, N. Park, M. Liu and J. Cho, Nat. Commun., 2013, 4, 2076 Search PubMed.
- M. Prabu, K. Ketpang and S. Shanmugam, Nanoscale, 2014, 6, 3173–3181 RSC.
- C. Li, X. Han, F. Cheng, Y. Hu, C. Chen and J. Chen, Nat. Commun., 2015, 6, 7345 CrossRef CAS PubMed.
- X. Liu, M. Park, M. Kim, S. Gupta, G. Wu and J. Cho, Angew. Chem., Int. Ed., 2015, 54, 9654–9658 CrossRef CAS PubMed.
- J. Fu, D. Lee, F. Hassan, L. Yang, Z. Bai, M. Park and Z. Chen, Adv. Mater., 2015, 27, 5617 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7nh00058h |
| This journal is © The Royal Society of Chemistry 2017 |