Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An environmentally benign and selective electrochemical oxidation of sulfides and thiols in a continuous-flow microreactor†
Gabriele
Laudadio
 ,
Natan J. W.
Straathof
,
Menno D.
Lanting
,
Benny
Knoops
,
Volker
Hessel
and
Timothy
Noël
,
Natan J. W.
Straathof
,
Menno D.
Lanting
,
Benny
Knoops
,
Volker
Hessel
and
Timothy
Noël
 *
*
Department of Chemical Engineering and Chemistry, Micro Flow Chemistry & Process Technology, Eindhoven University of Technology, Den Dolech 2, 5612 AZ Eindhoven, The Netherlands. E-mail: t.noel@tue.nl
First published on 7th August 2017
Abstract
A practical and environmentally benign electrochemical oxidation of thioethers and thiols in a commercially-available continuous-flow microreactor is presented. Water is used as the source of oxygen to enable the oxidation process. The oxidation reaction utilizes the same reagents in all scenarios and the selectivity is solely governed by the applied potential. The procedure exhibits a broad scope and good functional group compatibility providing access to various sulfoxides (15 examples), sulfones (15 examples) and disulfides (6 examples). The use of continuous flow allows the optimal reaction parameters (e.g. residence time, applied voltage) to be rapidly assessed, to avoid mass- and heat-transfer limitations and to scale the electrochemistry.
Introduction
Sulfoxide and sulfone moieties are widespread in a broad variety of functional organic molecules.1 These moieties have been incorporated in numerous pharmaceutical compounds (e.g. Esomeprazole, Dapsone, Sulmazole, Methionine sulfone and Ponazuril)2 and even in polymeric materials3 (Fig. 1). Moreover, chiral sulfoxides have been employed as chiral auxiliaries (e.g. Ellman's sulfinamide, Oppoltzer camphorsultam)4 and as chiral ligands in asymmetric transition-metal catalyzed transformations (e.g. Skarzewzki's and Hiroi's ligands, Fig. 1).5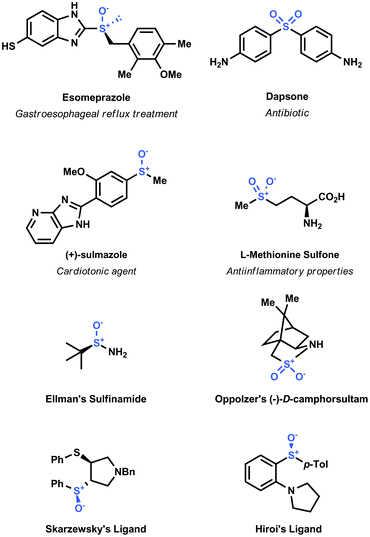 | ||
| Fig. 1 Examples of interesting sulfoxides and sulfones, ranging from pharmaceuticals to chiral ligands. | ||
Typically, sulfoxides and sulfones can be accessed through oxidation of the corresponding thioether.6 Hereto, a wide variety of oxidizing agents have been used, such as H2O2 in combination with metal catalysts,7m-CPBA,8 NaIO4,9 CrO3,9 KMnO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and dioxiranes.11 Unfortunately, these strategies typically suffer from selectivity issues, e.g. overoxidation of the sulfoxide to the sulfone or oxidation of other functional groups within the molecule. And, while hydrogen peroxide is considered to be a green oxidant, its industrial synthesis via the so-called anthraquinone autooxidation process is not sustainable.12
10 and dioxiranes.11 Unfortunately, these strategies typically suffer from selectivity issues, e.g. overoxidation of the sulfoxide to the sulfone or oxidation of other functional groups within the molecule. And, while hydrogen peroxide is considered to be a green oxidant, its industrial synthesis via the so-called anthraquinone autooxidation process is not sustainable.12
Oxidation chemistry can also be achieved using alternative and sustainable technologies, such as photochemistry or electrochemistry, which allow to carry out the desired transformation in the absence of strong oxidants.13 Here, the desired transformation is induced by so-called traceless reagents such as photons or electrons, providing sustainable alternatives for the often hazardous and toxic oxidants.14,15
In addition, these methods are relatively mild and provide good functional group tolerance and high chemoselectivity. Furthermore, sustainable electricity, derived from solar and wind energy, is becoming more abundantly available. Due to the transient nature of these energy sources, small scale electrochemical plants are ideally suited to directly harness this sustainable energy source. Despite the advantages provided by electrochemistry, many organic synthetic practitioners have been discouraged to adopt this technology into their laboratories.16 This is in part due to the apparent complexity of electrochemical transformations which originates from numerous problems, such as the use of specialized equipment and large amounts of tailor-made electrolytes, mass- and heat-transfer limitations, electrodeposition of organic material on the surface of the electrode and limited scalability. However, many of these challenges can be overcome by combining electrochemistry and continuous-flow microreactor technology.17 Due to the small dimensions (100 μm–1 mm), micro-flow technology allows to intensify the contact between the reaction mixture and the electrodes, to eliminate mass-transfer limitations and to avoid hot-spot formation.15b,d,17,18 Furthermore, organic deposition on the electrodes can be minimized due to the continuous-flow operation of the reactor.17
Using a combination of electrochemistry and continuous-flow microreactors, we questioned whether we could selectively access either the sulfoxide or the sulfone starting from their corresponding thioethers.16,19,20 Specifically, we hoped to develop a set of generally applicable conditions which would allow us to access both compounds simply by tuning the applied potential in the electrochemical flow cell, whilst keeping the reaction mixture the same. This would be a great advance compared to traditional synthetic approaches where each product class requires its own set of conditions with often toxic oxidants, metal catalysts and/or elevated temperatures.
Results and discussion
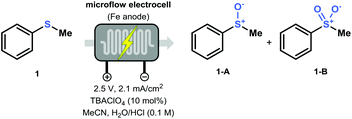
In order to find the optimal conditions for the direct electrochemical oxidation of thioethers, the potentiostatic oxidation of thioanisole (1) was taken as a benchmark (Table 1). The Asia Flux reactor was used as a commercially available electrochemical flow module and was equipped with cheap stainless steel electrodes. At the outset of our investigations, it was found that the use of an electrolyte was crucial to maintain a stable current. Optimal results were obtained using tetrabutylammonium perchlorate (TBAClO4). We also observed that the use of an aqueous acidic solvent was mandatory to lower the pH which allowed to increase the redox potential of the Fe electrodes, as explained by Pourbaix's diagrams (Table 1, entries 3 and 4).21 Consequently, the combination of tetrabutylammonium perchlorate in MeCN with aqueous HCl (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 0.1 M HCl in H2O) with an applied potential of 2.5 V (corresponding to a current of approximately 2.1 mA cm−2), resulted in the oxidation of 1 toward sulfoxide 1-A (63% yield; Table 1, entry 1). Increasing the residence time while keeping the flow rate constant resulted in an increase in yield (entry 2, Table 1). Interestingly, an increase in the applied potential clearly shifted the selectivity towards the double oxidized product 1-B (Table 1, entries 7–9). Finally, a control experiment was carried out using an oxygen-enriched acetonitrile solution without additional water (Table 1, entry 6). Oxidation of the thioether was still observed, indicating that O2 likely serves as the [O]-source. Furthermore, in the presence of water, gas formation can be noticed due to water splitting (see ESI†).
1 v/v, 0.1 M HCl in H2O) with an applied potential of 2.5 V (corresponding to a current of approximately 2.1 mA cm−2), resulted in the oxidation of 1 toward sulfoxide 1-A (63% yield; Table 1, entry 1). Increasing the residence time while keeping the flow rate constant resulted in an increase in yield (entry 2, Table 1). Interestingly, an increase in the applied potential clearly shifted the selectivity towards the double oxidized product 1-B (Table 1, entries 7–9). Finally, a control experiment was carried out using an oxygen-enriched acetonitrile solution without additional water (Table 1, entry 6). Oxidation of the thioether was still observed, indicating that O2 likely serves as the [O]-source. Furthermore, in the presence of water, gas formation can be noticed due to water splitting (see ESI†).
| Entry | Deviation from above | Conv.a (%) | % 1-Aa | % 1-Ba |
|---|---|---|---|---|
Reaction conditions and variations on the electrochemical oxidation of thioanisole. Reagents and conditions: Thioanisole (1 mmol, 0.1 M), TBAClO4 (0.1 mmol), MeCN/HCl (10 mL, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, with 0.1 M HCl in H2O), applied potential: 2.5 V (2.1 mA cm−2, Fe anode/cathode, Vint: 300 μL), residence time: 5 min (at a flowrate of 0.06 mL min−1).a Conversion and selectivity determined by GC-MS with an internal standard. 1 v/v, with 0.1 M HCl in H2O), applied potential: 2.5 V (2.1 mA cm−2, Fe anode/cathode, Vint: 300 μL), residence time: 5 min (at a flowrate of 0.06 mL min−1).a Conversion and selectivity determined by GC-MS with an internal standard. |
||||
| 1 | None | 63 | 62 | 0 |
| 2 | Residence time of 10 min | 75 | 72 | 2 |
| 3 | No H2O/HCl (0.1 M) | n.r. | — | — |
| 4 | No electrolyte | n.r. | — | — |
| 5 | 0.2 M H2O/HCl | 37 | 34 | — |
| 6 | No H2O/HCl, ACN saturated with O2 | 39 | 39 | 0 |
| 7 | Potential at 3.0 V | 92 | 65 | 27 |
| 8 | Potential at 3.5 V | 98 | 59 | 39 |
| 9 | Potential at 4.0 V | 99 | 7 | 92 |
With these preliminary results in hand, the effect of the applied potential on the observed selectivity was investigated in greater detail. Hereto, a single sweep voltammetry experiment was performed at a constant residence time of 5 min (Fig. 2A). The polarogram shows a clear plateau at 2.0–2.5 V,22 which corresponds to the first oxidation step of thioanisole toward 1-A (Fig. 2B). When the applied potential reaches approximately 3.5–4.0 V, another plateau is observed, which corresponds to the second oxidation step (via1-A → 1-B or 1 → 1-B). Further increase in the potential results into a critical oxidation of the stainless steel electrodes and should be avoided. It is clear that such polarograms represent a very useful tool to establish the optimal reaction conditions to obtain either the sulfoxide or the sulfone from a specific thioether (see Fig. 2B and ESI†).
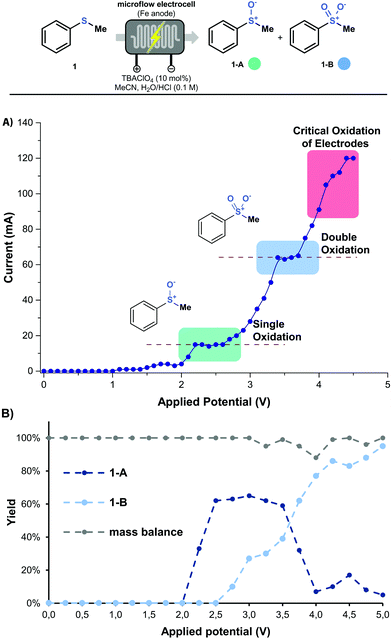 | ||
| Fig. 2 (A) Polarogram of thioanisole at a residence time of 5 min. (B) product distribution of 1-A and 1-B with respect to applied potential. Data recorded by GC-MS with an internal standard. | ||
Next, the effect of flow rate and residence time was investigated (Fig. 3). These investigations were carried out to define the optimal flowrate/residence time ratio in order to avoid mass-transfer limitations. At lower flow rates, mixing is not intense enough to facilitate diffusion of the reactants from the bulk to the electrodes. At higher flow rates, the reaction time is too short to allow for complete conversion. From our data, it is clear that a residence time of 7.5 minutes effects the highest conversion. A further increase in residence time results in a drop in yield (Fig. 3, red zone). Hence, an optimum flowrate regime was found to be situated between 0.04 to 0.06 mL min−1, which corresponds to residence times of 7.5 to 5 minutes, respectively. A residence time of 7.5 minutes gave a slightly higher yield in 1-A, but was accompanied with a low amount of the corresponding sulfone 1-B (<5%). Therefore, a standard residence time of 5 minutes per run was considered optimal and, when full conversion was not achieved within a single run, the reaction mixture was reinjected to increase the overall residence time (see ESI†).
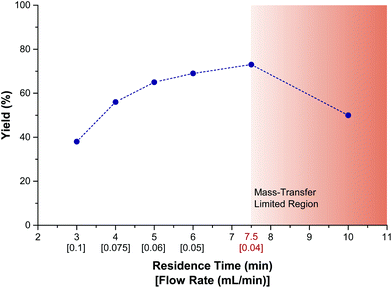 | ||
| Fig. 3 Effect of residence time and flow rate on the yield of 1-A. Data recorded via GC-MS with an internal standard. Internal reactor volume: 300 μm. | ||
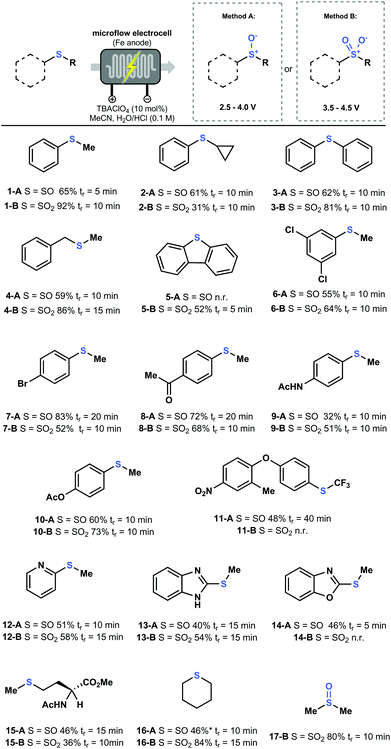
Having established insight in the governing parameters in the electrochemical oxidation of sulfides, we set out to probe the generality of our electrochemical flow protocol. Various thioethers bearing different functional groups were subjected to both Methods A and B, yielding the corresponding sulfoxide A or sulfone B, respectively (Fig. 4). For every compound, a fast potential screening was carried out to obtain the polarogram, which allows us to find the optimal condition for each compound (see ESI†). Notably, the productivity of our electrochemical flow protocol is excellent (i.e. 7.8 mmol h−1 for 1-A and 5.5 mmol h−1 for 1-B) providing means to scale this chemistry to quantities which are sufficient for Medicinal Chemistry applications. Further scale-up can be achieved using larger electrochemical flow reactors or via numbering-up of electrochemical microreators.23
As shown in Fig. 4, a wide variety of aryl, heteroaryl and alkyl thioethers were efficiently converted into their corresponding sulfoxides and sulfones in moderate to excellent yield. Functional groups, such as halides (6, 7, 11), ketones (8), esters (10, 15) and amides (9, 15), were well tolerated under our reaction conditions. Notably, nitrogen-containing heteroaromatic compounds, e.g. pyridine (12) and benzimidazole (13), can be selectively oxidized to produce the corresponding sulfoxides and sulfones without N-oxide formation. However, in the case of benzoxazole (14), nitrogen oxidation was observed at higher potentials prior to the formation of the sulfone. Interestingly, biologically relevant compounds such as the amino acid Methionine (15) and a precursor of the antiprotozoal agent Toltrazuril24 (11) were efficiently oxidized in good yield. However, it is important to note that for some substrates it was not possible to access both the sulfoxide and sulfone. As an example, dibenzothiophene (5) is directly oxidized to the sulfone. It is however plausible that a double oxidation is immediately occurring, since it is known that aromatic sulfoxides are relatively reactive.25 The Toltrazuril precursor (11) is oxidized only to the sulfoxide form, which can be explained by the strong electron-withdrawing character of the CF3 group, making it less prone to oxidation. Despite the broad substrate scope, some limitations do exist to our methodology (see ESI†). Free amines, alcohols and carboxylic acids do not yield any product. In addition, thioethers bearing nitriles, aldehydes and hydroxyls at the γ-position underwent retro-Michael reactions.
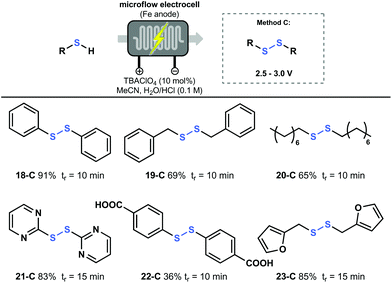
Finally, the oxidation of thiols to symmetric disulfides was performed applying an analogous approach as for the oxidation of thioethers. Also here, a potential screening was carried out after which the proper voltage value was set to perform the reaction (Fig. 5). As a result, simple thiols such as thiophenol (18), benzylthiol (19) and octanethiol (20), were all converted to the corresponding disulfide in good to excellent yield. Notably, 2-mercaptopyrimidine (21) and 2-furanylmethanethiol (23) could also be converted to their disulfide derivative and were obtained in good yield. It should be noted that disulfide 23-C is prone to overoxidation under photochemical oxidation reactions, however, this was not observed using our electrochemical method.26 This highlights the efficiency of electrochemistry and its complementarity compared to photoredox catalysis.27 Furthermore, poorly soluble starting materials like 4-mercaptobenzoic acid (22) could also be converted using this protocol.
Conclusion
A straightforward, green and broadly applicable electrochemical continuous-flow procedure to oxidize thioethers and thiols has been developed. Using a commercially available electrochemical flow setup (Asia Flux), a wide variety of functionalized sulfoxides (15 examples) and sulfones (15 examples) could be accessed selectively, simply by changing the applied voltage. Similarly, aryl and alkyl thiols could be efficiently oxidized to their corresponding disulfides (6 examples). Because of the sustainable nature of our developed protocol, we believe that our method is highly attractive for technical applications.Acknowledgements
Financial support is provided by the Dutch Science Foundation (NWO) via a VIDI grant for T. N. (Grant No. 14150).Notes and references
- D. S. Johnson and J. J. Li, Modern Drug Synthesis, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2010 Search PubMed.
- (a) L. Dirikolu, R. Yohn, E. F. Garrett, T. Chakkath and D. C. Ferguson, J. Vet. Pharmacol. Ther., 2009, 32, 280–288 CrossRef CAS PubMed; (b) Y. I. Zhu and M. J. Stiller, J. Am. Acad. Dermatol., 2001, 45, 420–434 CrossRef CAS PubMed; (c) T. Lind, L. Rydberg, A. Kyleback, A. Jonsson, T. Andersson, T. Hasselgren, J. Holmberg and K. Rohss, Aliment. Pharmacol. Ther., 2000, 14, 861–867 CrossRef CAS PubMed; (d) M. K. Unnikrishnan and M. N. A. Rao, Agents Actions, 1990, 31, 110–112 CrossRef CAS PubMed; (e) W. J. Parsons, V. Ramkumar and G. L. Stiles, Mol. Pharmacol., 1988, 33, 441–448 CAS.
- D. Parker, J. Bussink, H. T. van de Grampel, G. W. Wheatley, E.-U. Dorf, E. Ostlinning and K. Reinking, Polymers, High-Temperature, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2000 Search PubMed.
- (a) J. A. Ellman, T. D. Owens and T. P. Tang, Acc. Chem. Res., 2002, 35, 984–995 CrossRef CAS PubMed; (b) B. Hyean Kim and D. P. Curran, Tetrahedron, 1993, 49, 293–318 CrossRef.
- (a) B. M. Trost and M. Rao, Angew. Chem., Int. Ed., 2015, 54, 5026–5043 CrossRef CAS PubMed; (b) I. Fernandez and N. Khiar, Chem. Rev., 2003, 103, 3651–3706 CrossRef CAS PubMed.
- (a) M. Kazemi, L. Shiri and H. Kohzadi, Phosphorus, Sulfur Silicon Relat. Elem., 2015, 190, 978–1003 CrossRef CAS; (b) J.-E. Bäckvall, in Modern Oxidation Methods, Wiley-VCH Verlag GmbH & Co. KGaA, 2010 Search PubMed.
- (a) R. Trivedi and P. Lalitha, Synth. Commun., 2006, 36, 3777–3782 CrossRef CAS; (b) M. Matteucci, G. Bhalay and M. Bradley, Org. Lett., 2003, 5, 235–237 CrossRef CAS PubMed.
- S. S. Shin, Y. Byun, K. M. Lim, J. K. Choi, K.-w. Lee, J. H. Moh, J. K. Kim, Y. S. Jeong, J. Y. Kim, Y. H. Choi, H.-j. Koh, Y.-h. Park, Y. I. Oh, M.-S. Noh and S. Chung, J. Med. Chem., 2004, 47, 792–804 CrossRef CAS PubMed.
- R. G. Hiskey and M. A. Harpold, J. Org. Chem., 1967, 32, 3191–3194 CrossRef CAS.
- S. Lai and D. G. Lee, Tetrahedron, 2002, 58, 9879–9887 CrossRef CAS.
- S. Colonna and N. Gaggero, Tetrahedron Lett., 1989, 30, 6233–6236 CrossRef CAS.
- C. Samanta, Appl. Catal., A, 2008, 350, 133–149 CrossRef CAS.
- (a) N. Kockmann, P. Thenée, C. Fleischer-Trebes, G. Laudadio and T. Noël, React. Chem. Eng., 2017, 2, 258–280 RSC; (b) H. P. L. Gemoets, Y. Su, M. Shang, V. Hessel, R. Luque and T. Noël, Chem. Soc. Rev., 2016, 45, 83–117 RSC.
- For some selected reviews on photochemistry, see: (a) D. Cambié, C. Bottecchia, N. J. W. Straathof, V. Hessel and T. Noël, Chem. Rev., 2016, 116, 10276–10341 CrossRef PubMed; (b) I. Ghosh, L. Marzo, A. Das, R. Shaikh and B. König, Acc. Chem. Res., 2016, 49, 1566–1577 CrossRef CAS PubMed; (c) M. H. Shaw, J. Twilton and D. W. C. MacMillan, J. Org. Chem., 2016, 81, 6898–6926 CrossRef CAS PubMed.
- For some selected reviews on electrochemistry, see: (a) C. Sambiagio, H. Sterckx and B. U. W. Maes, ACS Cent. Sci., 2017, 3, 686–688 CrossRef CAS PubMed; (b) J.-I. Yoshida, Electrochem. Soc. Interface, 2009, 18, 40–45 CAS; (c) J.-i. Yoshida, K. Kataoka, R. Horcajada and A. Nagaki, Chem. Rev., 2008, 108, 2265–2299 CrossRef CAS PubMed; (d) J.-i. Yoshida, Chem. Commun., 2005, 4509 RSC.
- E. J. Horn, B. R. Rosen and P. S. Baran, ACS Cent. Sci., 2016, 2, 302–308 CrossRef CAS PubMed.
- (a) M. B. Plutschack, B. u. Pieber, K. Gilmore and P. H. Seeberger, Chem. Rev., 2017 DOI:10.1021/acs.chemrev.7b00183; (b) K. Watts, A. Baker and T. Wirth, J. Flow Chem., 2015, 4, 2–11 CrossRef.
- For selected reports on electrochemistry in flow, see: (a) C. Gütz, A. Stenglein and S. R. Waldvogel, Org. Process Res. Dev., 2017, 21, 771–778 CrossRef; (b) R. A. Green, K. E. Jolley, A. A. M. Al-Hadedi, D. Pletcher, D. C. Harrowven, O. De Frutos, C. Mateos, D. J. Klauber, J. A. Rincón and R. C. D. Brown, Org. Lett., 2017, 19, 2050–2053 CrossRef CAS PubMed; (c) R. Green, R. Brown and D. Pletcher, J. Flow Chem., 2015, 5, 31–36 CrossRef CAS; (d) K. Arai, K. Watts and T. Wirth, ChemistryOpen, 2014, 3, 23–28 CrossRef CAS PubMed; (e) K. Arai and T. Wirth, Org. Process Res. Dev., 2014, 18, 1377–1381 CrossRef CAS; (f) M. A. Kabeshov, B. Musio, P. R. D. Murray, D. L. Browne and S. V. Ley, Org. Lett., 2014, 16, 4618–4621 CrossRef CAS PubMed; (g) G. P. Roth, R. Stalder, T. R. Long, D. R. Sauer and S. W. Djuric, J. Flow Chem., 2013, 3, 34–40 CrossRef CAS; (h) R. Stalder and G. P. Roth, ACS Med. Chem. Lett., 2013, 4, 1119–1123 CrossRef CAS PubMed; (i) K. Watts, W. Gattrell and T. Wirth, Beilstein J. Org. Chem., 2011, 7, 1108–1114 CrossRef CAS PubMed; (j) B. Ahmed-Omer, J. C. Brandt and T. Wirth, Org. Biomol. Chem., 2007, 5, 733–740 RSC; (k) M. Küpper, V. Hessel, H. Löwe, W. Stark, J. Kinkel, M. Michel and H. Schmidt-Traub, Electrochim. Acta, 2003, 48, 2889–2896 CrossRef.
- For the oxidation of sulfides using hydrogen peroxide or ozonolysis in flow, see: (a) S. Doherty, J. G. Knight, M. A. Carroll, J. R. Ellison, S. J. Hobson, S. Stevens, C. Hardacre and P. Goodrich, Green Chem., 2015, 17, 1559–1571 RSC; (b) M. Irfan, T. N. Glasnov and C. O. Kappe, Org. Lett., 2011, 13, 984–987 CrossRef CAS PubMed; (c) R. Maggi, S. Chitsaz, S. Loebbecke, C. G. Piscopo, G. Sartori and M. Schwarzer, Green Chem., 2011, 13, 1121 RSC; (d) T. Noguchi, Y. Hirai and M. Kirihara, Chem. Commun., 2008, 3040 RSC.
- For some selected papers concerning electrochemical oxidations, see: (a) Y. Kawamata, M. Yan, Z. Liu, D.-H. Bao, J. Chen, J. T. Starr and P. S. Baran, J. Am. Chem. Soc., 2017, 139, 7448–7451 CrossRef CAS PubMed; (b) E. J. Horn, B. R. Rosen, Y. Chen, J. Tang, K. Chen, M. D. Eastgate and P. S. Baran, Nature, 2016, 533, 77–81 CrossRef CAS PubMed; (c) B. R. Rosen, E. W. Werner, A. G. O'Brien and P. S. Baran, J. Am. Chem. Soc., 2014, 136, 5571–5574 CrossRef CAS PubMed; (d) A. G. O'Brien, A. Maruyama, Y. Inokuma, M. Fujita, P. S. Baran and D. G. Blackmond, Angew. Chem., Int. Ed., 2014, 53, 11868–11871 CrossRef PubMed; (e) C. Edinger and S. R. Waldvogel, Eur. J. Org. Chem., 2014, 5144–5148 CrossRef CAS; (f) A. de León, J. García-Antón, J. Ros, G. Guirado, I. Gallardo and J. Pons, New J. Chem., 2013, 37, 1889 RSC; (g) R. Horcajada, M. Okajima, S. Suga and J.-i. Yoshida, Chem. Commun., 2005, 1303 RSC; (h) D. Vasudevan, J. Appl. Electrochem., 2000, 30, 1299–1302 CrossRef CAS; (i) R. C. Duty, I. Sahni and R. Roark, Phosphorus, Sulfur Silicon Relat. Elem., 1996, 116, 9–14 CrossRef CAS; (j) J. P. Desmarquest, France Pat., FR 208670019720204, 1971 Search PubMed.
- B. Beverskog and I. Puigdomenech, Corros. Sci., 1996, 38, 2121–2135 CrossRef CAS.
- M. Fleischmann and D. Pletcher, RIC Rev., 1969, 2, 87 RSC.
- (a) K. P. L. Kuijpers, M. A. H. van Dijk, Q. G. Rumeur, V. Hessel, Y. Su and T. Noël, React. Chem. Eng., 2017, 2, 109–115 RSC; (b) J. Zhang, K. Wang, A. R. Teixeira, K. F. Jensen and G. Luo, Annu. Rev. Chem. Biomol. Eng., 2017, 8, 285–305 CrossRef PubMed; (c) Y. Su, K. Kuijpers, V. Hessel and T. Noël, React. Chem. Eng., 2016, 1, 73–81 RSC.
- A. Guenther, K.-H. Mohrmann, M. Stubbe and H. Ziemann, Germany Pat., DE3516630, 1986 Search PubMed.
- W. S. Jenks, N. Matsunaga and M. Gordon, J. Org. Chem., 1996, 61, 1275–1283 CrossRef CAS.
- (a) A. Talla, B. Driessen, N. J. W. Straathof, L.-G. Milroy, L. Brunsveld, V. Hessel and T. Noël, Adv. Synth. Catal., 2015, 357, 2180–2186 CrossRef CAS; (b) C. Bottecchia, N. Erdmann, P. M. A. Tijssen, L. G. Milroy, L. Brunsveld, V. Hessel and T. Noël, ChemSusChem, 2016, 9, 1781–1785 CrossRef CAS PubMed.
- C. Adouama, R. Keyrouz, G. Pilet, C. Monnereau, D. Gueyrard, T. Noël and M. Médebielle, Chem. Commun., 2017, 53, 5653–5656 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7gc01973d |
| This journal is © The Royal Society of Chemistry 2017 |