Quercetin and fisetin enhanced the small intestine cellular uptake and plasma levels of epi-catechins in in vitro and in vivo models
Jin-Oh
Chung
 ab,
Seon-Bong
Lee
ab,
Seon-Bong
Lee
 b,
Kang-Hyun
Jeong
b,
Kang-Hyun
Jeong
 b,
Ji-Hoon
Song
b,
Su-Kyung
Kim
b,
Ji-Hoon
Song
b,
Su-Kyung
Kim
 a,
Kyung-Mi
Joo
a,
Kyung-Mi
Joo
 a,
Hyun-Woo
Jeong
a,
Hyun-Woo
Jeong
 a,
Jin-Kyu
Choi
a,
Jin-Kyu
Choi
 c,
Jeong-Kee
Kim
c,
Jeong-Kee
Kim
 a,
Wan-Gi
Kim
a,
Wan-Gi
Kim
 a,
Song-Seok
Shin
a and
Soon-Mi
Shim
a,
Song-Seok
Shin
a and
Soon-Mi
Shim
 *b
*b
aVital Beautie Research Institute, Amorepacific R&D Center, Yongin-si, Gyeonggi-do 17074, Republic of Korea
bDepartment of Food Science and Biotechnology, Sejong University, 98 Gunja-dong, Gwangjin-gu, Seoul 143-747, Republic of Korea. E-mail: soonmishim@sejong.ac.kr; Fax: +82-2-3408-4319; Tel: +82-2-3408-3229
cQuality Control Team, Aestura Corporation, 36, Gongdan 1-Ro, Anseong-si, Gyeonggi-do 17573, Republic of Korea
First published on 6th November 2017
Abstract
Quercetin and fisetin, known as catechol-containing flavonoids, could positively affect the absorption of catechins due to their strong affinity for catechol-O-methyl transferase (COMT), which can methylate and cause the excretion of catechins. The current study examined the effect of quercetin and fisetin on the absorption of epi-catechins (ECs) by using a Caco-2 cell line and an in vivo model. The intestinal transport of total catechins by Caco-2 cells was enhanced from 1.3- to 1.6-fold and 1.4- to 1.7-fold by adding quercetin and fisetin, respectively, compared to the control. It was even higher in the treatment with a mixture of quercetin and fisetin. While EC had the highest value of intestinal transport (169% of the control) in 10% quercetin treatment, EGC (235%), EGCG (244%), and ECG (242%) were significantly transported in the treatment with a 5% mixture of quercetin and fisetin (p < 0.05). In an in vivo pharmacokinetic study, the values of the area under the plasma concentration–time curve (AUC, ng h mL−1) were also higher in rats orally administered EGCG with 10% quercetin (365.5 ± 25.5) or 10% fisetin (825.3 ± 46.7) than in those administered EGCG only (111.3 ± 13.1). Methylated quercetin and methylated fisetin were determined to be m/z 317.24 and m/z 301.25 [M + H]+ with their own product ions, respectively. The results indicate that quercetin or fisetin is superior to ECs for methylation by COMT.
Introduction
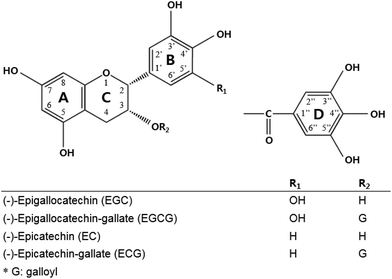
Green tea is a category of the processed tea plant (Camellia sinensis) that involves different manners and degrees of oxidation of the leaves.1 Systematic research from in vitro to clinical studies has ascribed the biological activities of green tea to green tea catechins. Catechins, including (−)-epigallocatechin gallate (EGCG), (−)-epicatechin gallate (ECG), (−)-epicatechin (EC), and (−)-epigallocatechin (EGC), are the most abundant components in green tea,2 and each is assigned a ring A, B, C, and D in the galloylated catechin group (Fig. 1).Many studies have pointed out that green tea catechins have low bioavailability in the human body.3–6 For example, the level of plasma catechins that exerts pharmacological activities was maximally 50 times lower in the human body than that in the in vitro system. One of the reasons for this is that green tea catechins are readily oxidized and unstable under neutral or marginally alkaline conditions during upper gastrointestinal (GI) tract digestion.7–9 Several studies modulated the formulation of green tea catechins with acidic components and sugars. For instance, co-formulating green tea catechins with ascorbic acid and xylitol enhanced the bioavailability of catechins by increasing the digestive stability.1,9–11 Once green tea catechins are taken up by epithelial cells, particularly in the gallated form, they are quickly metabolized to an inactive form by the gut microbiota.12,13 Some of the catechins such as EC are effluxed by MRP1/MRP2 or P-glycoprotein localized in the apical membrane of intestinal cells because they act as a substrate.14–16 Galloylated catechins such EGCG and ECG were also transported by MRP1/MRP2 in a Caco-2 cell monolayer study.17 Previous studies attempted to increase the transepithelial absorption of catechins with the co-presence of inhibitors of efflux transporters such as MK-571 or verapamil.5,18,19 It was revealed that the ingestion of green tea catechins along with vitamin C enhanced the cellular uptake of catechins by modulating the transport mechanism by inhibiting the gene expression of the efflux transporter.1,9
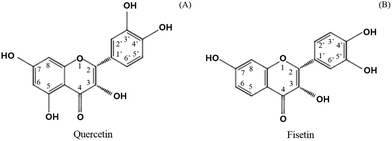
Catechol-O-methyl transferase (COMT) is another factor to consider in limiting the absorption of green tea catechins.20 Several studies reported that some of the phytochemicals found in fruits and vegetables can be a substrate for COMT.21 In particular, catechol-containing flavonoids such as green tea catechins are substrates of COMT, resulting in the methylation and excretion of green tea catechins.13,22 It was found that flavonoids containing a catechol structure (Fig. 2), including quercetin, dose-dependently inhibited the COMT activity in contrast to flavonoids without a catechol structure such as genistein, chrysin, baicalein, and flavone.21 It was recently recognized that the co-administration of polyphenols including hesperitin, isorhamnetin, kaempferol, diosmetin, chrysin, nevadensin, equol, and genistein provided more affinity for the efflux transporters, increasing the amount of EC metabolites.23 In addition, it was found that the extent of the chemotherapy activity of EGCG in human breast cancer cell lines apparently increased with the suppression of the COMT activity when a commercial COMT inhibitor (3,5-dinitrocatechol, DNC) was co-treated.24 A clinical study also found that the high activity related to the COMT genotype retained more green tea polyphenols in the body and provided greater biological activities in cancer treatment.25 It is expected that green tea catechins combined with other catechol-containing flavonoids could be effectively absorbed by replacing them with others as a substrate for COMT. The current study investigated the effect of adding quercetin and fisetin, known as COMT inhibitors, on the absorption of catechins by using human intestinal Caco-2 cells.
Materials and methods
Chemicals and reagents
(−)-Epigallocatechin (EGC), (−)-epigallocatechin gallate (EGCG), (−)-epicatechin (EC), and (−)-epicatechin gallate (ECG) standards were purchased from Wako (Osaka, Japan). Quercetin, fisetin, and acetic acid of liquid chromatography (LC) grade were purchased from Sigma-Aldrich (St Louis, MO, USA), and both acetonitrile and water were purchased from J. T. Baker (Phillipsburg, NJ, USA). Methanol and ethyl acetate were purchased from Burdick & Jackson (Muskegon, MI). Formic acid, ascorbic acid, Na2EDTA, ethyl gallate (EG), sodium valproate, 2,2′-dithiodipyridin (DTDP), 2-picolylamine (PA) and triphenylphosphine (TPP) were purchased from Aldrich (St Louis, MO). VPA-d6 (IS) was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Acetonitrile, methanol and dichloromethane were purchased from Burdick & Jackson (Muskegon, MI). Ammonium formate, formic acid and hydrochloric acid were purchased from Fluka (Burchs, Switzerland). The water used was ultra-pure deionized water (18.2 MΩ cm) produced from a Millipore Milli-Q Gradient system (Millipore, Bedford, MA). All other reagents used were of the highest grade available.Intestinal transport of catechins by Caco-2 cells
Each catechin standard solubilized in 50% methanol was combined as a standard mixture according to the ratio in the green tea extract obtained from the AmorePacific R&D Center (Yongin-si, South Korea). Quercetin and fisetin were solubilized in dimethyl sulfoxide (DMSO) and diluted with Dulbecco's modified Eagle's medium (DMEM; Corning Costar Corp., Corning, NY), respectively. The final concentration of DMSO was below 0.1% (v/v).The Caco-2 cells from the Korean Cell Line Bank (Seoul, Republic of Korea) were cultured in DMEM with 10% fetal bovine serum (FBS; Biotechnics Research), 1% penicillin/streptomycin (Corning Costar Corp., Corning, NY), 1% non-essential amino acids (Sigma-Aldrich), and 0.1% gentamycin (Sigma-Aldrich) and were grown under a humidified atmosphere of 5% CO2 at 37 °C and 95% air. For transport study, Caco-2 cells were seeded in collagen coated Transwell® inserts (12 mm i.d. PTFE membrane, 0.4 μm pore size, Corning Costar Corp., Corning, NY) in 12-well plates at a density of 105 cells per cm2. Caco-2 cells in Transwell at passages 35–42 were used for experiments 14–21 days post-seeding. The apical and basal culture media were changed every other day. Trans-epithelial electrical resistance (TEER) values across the cell monolayers were measured using a Millicell ERS-2 system (Millipore Corp., New Bedford, MA). Cells that had TEER values over 250 Ω cm2 were selected for the transport experiment. The medium was removed from the well, and cell layers were washed once with phosphate buffered saline (PBS; pH 7.4) before adding the treatments. The catechin standard mixture mixed with quercetin or fisetin (2.5%, 5%, 10%, and 20% of the catechin standard mixture) and the quercetin/fisetin mixture (5%, 10% and 20% of the catechin standard mixture) were added to the apical side. Cells were incubated at 37 °C with 5% CO2 for 2 h. After incubation, methanol was added (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, V
1, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V) into the collected basal media and sonicated for 10 s, followed by vortexing. It was filtered through a PTFE syringe filter (Advantec, pore size 0.45 μm) prior to UPLC-ESI-MS/MS analysis.
V) into the collected basal media and sonicated for 10 s, followed by vortexing. It was filtered through a PTFE syringe filter (Advantec, pore size 0.45 μm) prior to UPLC-ESI-MS/MS analysis.
Pharmacokinetics by animal experiments
The animal experiments were approved by the AmorePacific Institutional Animal Care and Use Committee (PP16-R017) and adhered to the OECD guidelines. 7-week-old male Sprague-Dawley (SD) rats were purchased from Samtako (Osan-si, Korea) and maintained on a 12 h dark/light cycle at a controlled temperature of 22–25 °C and humidity of 40–50%. For adaptation, animals were fed normal chow ad libitum for one week. After adaptation, the SD rats were divided into three groups (n = 5 per group): EGCG (100 mg per kg body weight), EGCG (100 mg per kg body weight) + quercetin (10 mg per kg body weight), and EGCG (100 mg per kg body weight) + fisetin (10 mg per kg body weight). Before the animal experiment, the SD rats were fasted overnight and orally administered with EGCG, quercetin, or fisetin. At 5, 15, 30, 60, 120, 240, and 720 minutes after administration, 200 μL of blood was collected from the retro-orbital plexus, to measure the plasma EGCG level.Standard stock solutions of EGCG and EG (IS) were prepared in methanol at a concentration level of 1 mg mL−1 and stored at −20 °C. The working standard solution of EGCG was serially diluted with methanol to obtain the concentrations for calibration curve standards. The working solution for the internal standard was diluted with methanol to achieve a final concentration of 200 ng mL−1. Calibration standards were prepared by spiking an appropriate amount of the working standard solution into a pool of drug-free rat plasma to yield calibration concentrations of 1.0, 2.0, 5.0, 10.0, 20.0, 50.0, 100, 200, 500 and 1000 ng mL−1 for EGCG. The quality control samples were spiked at 5.0, 100, and 500 ng mL−1 for EGCG. The standard spiked plasma samples were aliquoted (100 μL) into polypropylene tubes and stored at −20 °C until further analysis.
To 100 μL aliquots of biological samples (blank, calibration standards or unknown rat plasma samples), 10 μL of a preservation solution (20% ascorbic acid and 0.05% Na2EDTA) was added. The plasma samples were extracted with 1000 μL of ethyl acetate (containing EG, 2 ng mL−1) in 1.5 mL polypropylene tubes by vortexing for 5 min and were centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, at 4 °C for 10 min. The upper organic layer was transferred into another tube and evaporated to dryness using a speedvac (EZ-2 Plus, Genevac, Swiss) at 40 °C. The residues were reconstituted in 100 μL of methanol and vortexed for 5 min, followed by centrifugation at 14
000 rpm, at 4 °C for 10 min. The upper organic layer was transferred into another tube and evaporated to dryness using a speedvac (EZ-2 Plus, Genevac, Swiss) at 40 °C. The residues were reconstituted in 100 μL of methanol and vortexed for 5 min, followed by centrifugation at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, at 4 °C for 5 min. 2 μL of the sample solutions was injected into an ACQUITY UPLC-MS/MS system.
000 rpm, at 4 °C for 5 min. 2 μL of the sample solutions was injected into an ACQUITY UPLC-MS/MS system.
Analysis of plasma samples by ACQUITY UPLC-MS/MS
Chromatographic separation was carried out using an ACQUITY ultra-high performance liquid chromatography (UPLC) system (Waters Co., Milford, MA). The column was an ACQUITY UPLC HSS T3 column (1.8 μm, 2.1 × 100 mm). The column temperature and auto-sampler tray temperature were maintained at 40 °C and 4 °C, respectively. The mobile phase consisted of 0.01% formic acid in water (solvent A) and acetonitrile (solvent B). Gradient elution was as follows: isocratic elution with 15% B for 0.5 min, followed by a 1.5 min gradient to 70% B, 3.0 min to 100% B, isocratic elution with 100% B for 5.0 min, then returned to 15% B in 5.5 min. The flow rate was 0.35 mL min−1 and the injection volume was 2 μL. Liquid chromatographic and mass spectrometric (LC-MS) analysis was performed using a Waters TQ-S triple quadrupole mass spectrometer. The mass spectrometer was operated in the negative electrospray ionization (ESI) mode under the following operation conditions: capillary voltage, 2.2 kV; ion source temperature, 150 °C; desolvation temperature, 350 °C; desolvation gas flow rate, 550 L h−1; and cone gas flow rate, 50 L h−1. The multiple reaction monitoring (MRM) was set at m/z 456.9 → 169.0 for EGCG and m/z 197.0 → 124.0 for EG (I.S.). The optimum cone voltages for EGCG and EG were set at 30 and 28 eV, respectively. The collision energy for the multiple reaction monitoring mode (MRM) was optimized for each analyte. We controlled the data acquiring process with MassLynx Version 4.1 (Waters Co., Milford, MA, USA).Analysis of epi-catechins and metabolites of quercetin and fisetin by UPLC-PDA-ESI-MS/MS
The amount of catechins was quantified by using a UPLC with a photodiode array (PDA) detector and an LCQ-Fleet ESI-MS (Thermo Fischer Scientific, Wathman, MA, USA) for the transport from apical to basolateral. Chromatographic separation was performed on an Agilent zorbax Eclipse XDB C18 (4.6 × 250 mm, 5 μm) column with mobile phases of solvents A and B (A: 0.1% acetic acid in water, B: acetonitrile). A gradient elution was performed by varying the proportion of solvents A and B at a flow rate of 1 mL min−1 with an initial phase of 10% solvent B. The gradient increased linearly to 15% of solvent B in 30 min, increased linearly to 20% in the next 12 min, and held at 20% for 2 min. The gradient decreased to 10% of solvent B in 44.1 min and held at 10% for 50 min until the injection of the next sample. The injection volume was 20 μL. The wavelength of the UV spectrum was set at 280 nm.For the confirmation of the transport from apical to basolateral, the following ESI-MS conditions were used in the experiments: negative-ion mode; capillary temperature, 200 °C; heater temperature, 50 °C, sheath gas, 44 arb; auxiliary gas, 2 arb; and spray voltage, 3.97 kV. The analysis of the samples was initially carried out using a full scan mode, data-dependent MS scanning from m/z 80 to 600. Chromatographic peaks and mass spectra in the samples were identified using comparative retention times and molecular weights of pure standards. The quantitative analysis was conducted using a standard curve.
Statistical analysis
Values were reported as the mean ± standard deviation (SD) of three samples or five animals. A one-way analysis of variance (ANOVA) and Tukey's post hoc test were performed to measure the significant differences among the groups at the significant value of p < 0.05 by using Graphpad Prism 3.0 software (Graphpad, CA, USA).Results
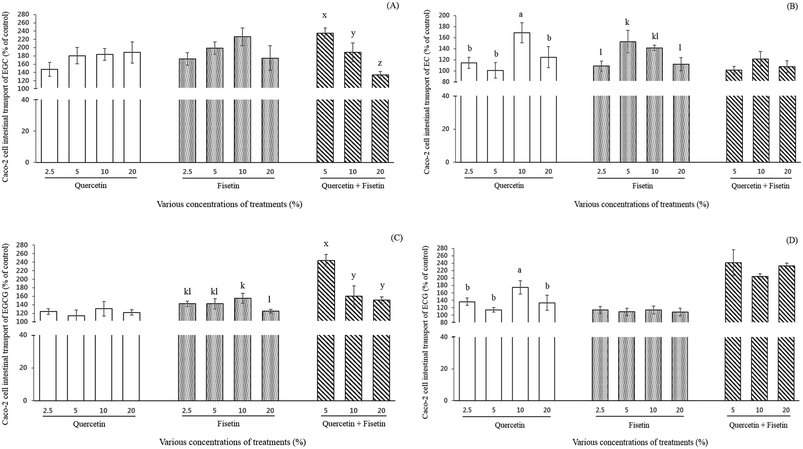
Effect of quercetin and fisetin on the intestinal transport of catechins by Caco-2 cells
The effect of quercetin or fisetin at the concentrations of 2.5, 5, 10, and 20% of ECs, and a mixture of these two compounds at the concentrations of 5, 10, and 20% on the intestinal transport of ECs by Caco-2 cells was examined after 2 h of incubation (Fig. 3). As shown in Fig. 3A, the relative intestinal transport of EGC with the addition of 2.5%, 5%, 10%, and 20% quercetin were 147%, 180%, 183%, and 188% of the control, respectively. When EGC was mixed with 2.5%, 5%, 10%, and 20% fisetin, its intestinal transport was 173%, 198%, 226%, and 175% of the control, respectively. However, there was no significant difference among the concentrations in the treatment of both quercetin and fisetin only (p > 0.05). Interestingly enough, the mixture of quercetin and fisetin at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (V
1 ratio (V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V) showed a decreasing pattern (235%, 189%, and 134% of the control) in the EGC intestinal transport at 5%, 10%, and 20%, respectively (p < 0.05).
V) showed a decreasing pattern (235%, 189%, and 134% of the control) in the EGC intestinal transport at 5%, 10%, and 20%, respectively (p < 0.05).
The intestinal transport of EC was 114%, 101%, 169%, and 125% of the control when EC was mixed with 2.5%, 5%, 10%, and 20% quercetin, respectively (Fig. 3B). In the treatment of 2.5%, 5%, 10%, and 20% fisetin, the intestinal transport of EC was 109%, 153%, 141%, and 112% of the control, respectively (Fig. 3B). In the case of the mixture of quercetin and fisetin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, V
1, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V), the intestinal transport of EC was 101%, 122%, and 108% of the control at 5%, 10%, and 20%, respectively. It is noteworthy that the addition of 10% quercetin significantly increased the intestinal transport of EC (p < 0.05).
V), the intestinal transport of EC was 101%, 122%, and 108% of the control at 5%, 10%, and 20%, respectively. It is noteworthy that the addition of 10% quercetin significantly increased the intestinal transport of EC (p < 0.05).
As shown in Fig. 3C, the intestinal transport of EGCG by Caco-2 cells (% of control) was enhanced from 1.1- to 1.3-fold (122–131%) without any significant difference among the various concentrations of quercetin. It was also 1.2- to 1.6-fold (125–155%) higher in the fisetin treatment compared to EGCG only. With a mixture of quercetin and fisetin of 5, 10 and 20%, the intestinal transport of EGCG ranged from 151% to 244%, indicating a 1.5- to 2.6-fold increase. The intestinal transport of EGCG was observed to decrease as the concentration of a mixture of quercetin and fisetin increased showing the highest value of 244% of the control with a 5% mixture of quercetin and fisetin (p < 0.05), which is comparable with the result of the intestinal transport of EGC.
The intestinal transport of ECG was significantly 1.8-fold higher (175%) with the addition of 10% quercetin than the control and approximately 1.1-fold higher without any significant difference among the concentrations of fisetin (Fig. 3D). In a mixture of quercetin and fisetin, the ECG transport increased up to approximately 240% of the control without any significant difference among all the concentrations of the mixture.
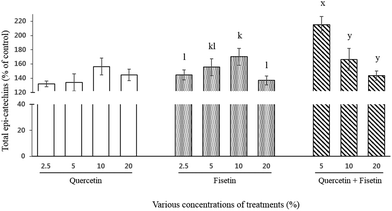
For total catechins, their intestinal transport was 132%, 134%, 156%, and 145% for quercetin and 145%, 155%, 170%, and 137% for fisetin at 2.5, 5, 10, and 20%, respectively (Fig. 4). In the case of the quercetin and fisetin mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, V
1, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V), the intestinal transport of total catechins was 215%, 166%, and 144% of the control at 5%, 10%, and 20% of the mixture, respectively. In contrast to a single treatment of quercetin or fisetin, the mixture of quercetin and fisetin showed the highest value of the intestinal transport of total catechins at the lowest concentration of 5% mixture.
V), the intestinal transport of total catechins was 215%, 166%, and 144% of the control at 5%, 10%, and 20% of the mixture, respectively. In contrast to a single treatment of quercetin or fisetin, the mixture of quercetin and fisetin showed the highest value of the intestinal transport of total catechins at the lowest concentration of 5% mixture.
Effect of quercetin and fisetin on the pharmacokinetic profile of EGCG
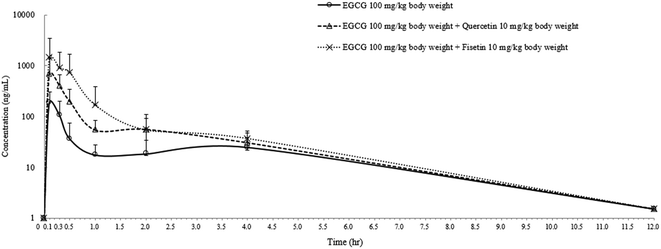
In order to confirm the enhancing effect of quercetin or fisetin on the intestinal absorption of epi-catechins, we further conducted an in vivo pharmacokinetic study with EGCG which is one of the major epi-catechins found in green tea. After the oral administration of EGCG only (100 mg per kg body weight), EGCG with 10% quercetin (10 mg per kg body weight), or EGCG with 10% fisetin (10 mg per kg body weight) to SD rats, the levels of EGCG in the plasma were measured for pharmacokinetic analysis (Fig. 5). The peak plasma concentration of EGCG was reached within 0.14–0.18 h in all of the SD rats and gradually reduced to undetectable levels in 12 h. The calculated pharmacokinetic parameters are summarized in Table 1. The peak plasma concentration (Cmax) of EGCG was 186.6 ± 15.1 ng mL−1. This value was 3.6 or 7.7 times lower than the values of the co-treatment of EGCG with quercetin (672.5 ± 7.3 ng mL−1) or fisetin (1450.8 ± 11.2 ng mL−1), respectively. The time to reach the Cmax (Tmax) values for EGCG, EGCG + quercetin, and EC + fisetin was in the range of 0.14–0.18 h. The values of EGCG of the area under the plasma concentration–time curve (AUC) were also higher in animals administered EGCG with quercetin (365.5 ± 25.5 ng h mL−1) or fisetin (825.3 ± 46.7 ng h mL−1) than in those administered with EGCG only (111.3 ± 13.1 ng h mL−1). The co-consumption of fisetin showed an approximately 2.3 times higher AUC values of EGCG than that of quercetin; however, the average rate of EGCG absorption as determined by the Cmax/AUC values from the co-administration of quercetin (1.83) was slightly higher than that of fisetin (1.75). The elimination rate of 4 h post-dose was similar among treatments. The results showed that EGCG has a higher amount and a longer half-life in the blood when administered with quercetin or fisetin than with no co-treatment, indicating that the co-consumption of quercetin or fisetin remarkably improved EGCG absorption.| Treatment | Dose (mg per kg body weight) | C max (ng mL−1) | T max (h) | AUC (ng h mL−1) |
|---|---|---|---|---|
| EGCG | 100 mg | 186.6 ± 15.1a* | 0.14 | 111.3 ± 13.1a |
| EGCG + quercetin | 100 mg + 10 mg | 672.5 ± 7.3b | 0.18 | 365.5 ± 25.5b |
| EGCG + fisetin | 100 mg + 10 mg | 1450.8 ± 11.2c | 0.14 | 825.3 ± 46.7c |
Identification of methylated quercetin and fisetin by UPLC-PDA-ESI-MS/MS
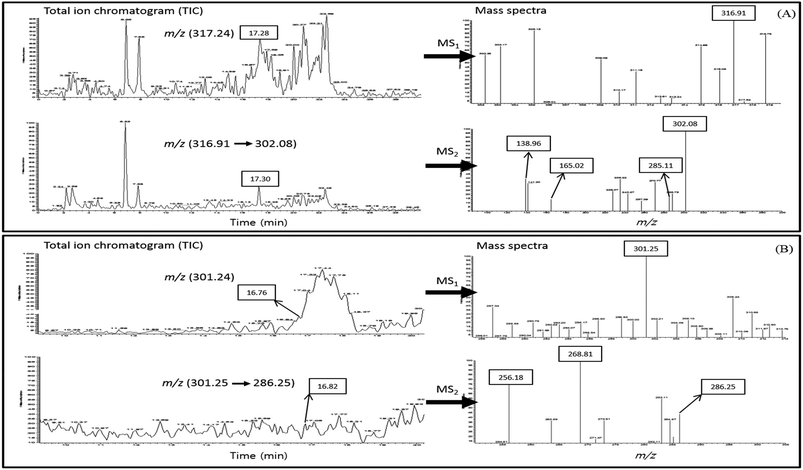
We hypothesized that the higher binding capacity of quercetin or fisetin with COMT than epi-catechins may enhance the absorption of epi-catechins. In order to elucidate this mechanism, the UPLC-PDA-ESI-MS/MS method with full scan in positive ion modes (m/z 50 → m/z 400) was adopted to identify methylated quercetin or methylated fisetin in the basal media after the transport of Caco-2 cells. The total ion chromatograms (TIC) of methylated quercetin or methylated fisetin and the mass spectra of its product ions are presented in Fig. 6. The methylated quercetin was determined by the base peak with a mass to charge ratio (m/z) of 317.24 [M + H]+ eluted at 17.28 min of retention time and UPLC-MS2 was used to identify the methylated quercetin, and it revealed a product ion at m/z 316.91 (Fig. 6A). Further fragmentation of methyl quercetin produced ions at m/z 302.08, 285.11, 165.02, and 138.96 in MS2. For the identification of methylated fisetin, the base peak at m/z 301.25 [M + H]+ was determined at 16.76 min of the retention time and UPLC-MS2 was used (Fig. 6B). It revealed a product ion at m/z 286.25 in MS2. Further fragmentation produced ions at m/z 268.81 and 256.18 as shown in Fig. 6B.Discussion
Although many in vitro/in vivo studies have shown the various health benefits of green tea catechins, the biological activities of green tea catechins in humans can be limited by several factors such as the intestinal membrane barrier and biotransformation.26 The cellular uptake of catechins, particularly EC, was regulated by the activities of P-glycoprotein and multidrug resistance proteins (MRPs) located at the apical and basal membrane, respectively. In the case of EC, it was mostly effluxed in the intestinal membrane1 and the current study found that the addition of quercetin significantly increased the intestinal transport of EC, indicating that quercetin provided a higher affinity for either the efflux transporter or COMT. Sanchez-Bridge et al.23 screened specific polyphenols such as quercetin and luteolin that can change the metabolite profiles of EC in a Caco-2 cell monolayer by competing for the transporters and metabolic enzymes. Regarding the biotransformation reaction of catechins, extensive studies have revealed that the methylation of catechins is one of the major metabolic pathways in a wide range of species such as rats and humans. Methylation mainly occurs at the 4′- and 3′ positions in the catechol-O structure (B ring), forming 4′-o-methyl EGC, 4′′-o-methyl ECG, and 4′′-o-methyl EGCG and 4′,4′′-o-dimethyl EGCG from EGC, ECG, and EGCG, respectively (Fig. 1).27,28 It was found that EGCG was metabolized by COMT to dimethylated or monomethylated EGCG according to its concentration.29 Previous in vivo studies suggested that methylated catechins were less active in preventing several cancers due to their low bioavailability.26,30 COMT is known to induce catechin methylation, suggesting that the COMT activity is responsible for the bioavailability of catechins as well as the biological activities of catechins.21,30,31 A previous study concluded that individuals with high COMT activity excreted a greater amount of green tea catechins than those with low COMT activity.32 Recently, it was reported that quercetin predominantly distributed in fruits and vegetables such as onions and apples inhibited the activity of MRPs and COMT.33,34 Silberberg et al.35 found that catechins were 2.5 times less methylated than quercetin by COMT, suggesting that quercetin is a superior substrate for COMT compared to catechins. It has been reported that quercetin inhibits the COMT activity through both the formation of S-adenosyl-L-homocysteine via rapid o-methylation itself and competitive inhibition of COMT by serving as its substrate.36 Furthermore, Wang et al.37 also demonstrated that quercetin decreases EGCG methylation in prostate cancer cell lines by inhibiting COMT protein expression, resulting in a significantly decreased methylation of EGCG. It has been reported that the IC50 values for quercetin and fisetin in inhibiting the O-methylation of 2- and 4-hydroxyestradiol by COMT are lower than those of EC and C.33 These results imply that quercetin and fisetin could act as a stronger COMT inhibitor than EC and C, revealing that quercetin and fisetin have a higher affinity for COMT than EC and C. The results from the current study demonstrated that combined treatment with catechins and either quercetin or fisetin enhanced the intestinal transport of epi-catechins. In addition, a mixture of quercetin and fisetin at a certain concentration induced the Caco-2 human intestinal transport of epi-catechins. A previous study found that the co-treatment of EGCG with quercetin promoted the chemopreventive activity of EGCG in various types of cell lines by decreasing the methylation of green tea catechins.30 The study also indicated that quercetin provided a stronger inhibitory effect on COMT protein expression than EGCG, resulting in a decreased methylation of catechins. Our results indicated that quercetin and fisetin having the same number of –OH on the B-ring enhanced the intestinal transport of catechins, implying that they are rapidly methylated as competitive inhibitors for COMT. EGC and EGCG having 3′,4′,5′-OH on the B-ring showed a similar pattern of intestinal transport. In detail, a combination of both quercetin having 3′,4′-OH and fisetin having 4′,5′-OH on the B-ring is more significantly effective in enhancing intestinal transport. Meanwhile, the intestinal transport of EC having 3′,4′-OH on the B-ring was highly enhanced by only 10% of quercetin. Comparable to results from our study, a previous study indicated that luteolin, quercetin, and myricetin having 3′,4′-OH on the B-ring showed potent COMT inhibiting activity.38 As revealed by another previous study,1 our results indicated that EC is more preferably taken up and effluxed by intestinal cells than acting as a substrate for COMT.Various pharmacokinetic studies on EGCG discovered that the inhibition of the intestinal efflux and methylation via COMT played an important role in increasing the AUC values of EGCG.29 The current study further conducted a pharmacokinetic analysis to find the relevance of EGCG absorption with an in vitro model. Our study found that the extent of the absorption of EGCG by measuring the plasma level significantly increased with the co-administration of EGCG with quercetin or fisetin. It was comparable to the findings that a combination of quercetin with EGCG increased the plasma level of EGCG after administration in in vivo studies.39,40 Wang et al.30 suggested that the extensive methylation of quercetin was the main factor that limits the methylation of catechins, leading to an increase in the small intestinal uptake of catechins and subsequent plasma levels. Considering the fact that EGCG has a remarkable biological activity in vitro but a very low bioavailability in vivo, findings from the current study might provide valuable scientific information for promoting the health function by green tea catechins. Moreover, the current study further identified the methylated quercetin and methylated fisetin in Caco-2 cell media after intestinal transport to elucidate this mechanism. When compared to quercetin, reports on the mass profiling of fisetin metabolites is limited but the current study confirmed the methylated fisetin based on previous findings, reporting that the product ions of methylated fisetin were m/z 286.2, 269.1, and 257.3.41 Although no quantitative information about methylation is provided, one of the possible mechanisms of limiting the methylation of epi-catechins by quercetin or fisetin was elucidated by confirming methylated quercetin and methylated fisetin in the current study. However, other possible factors including transporters, the efflux mechanism, and other phase II enzymes in the intestinal cell that can affect the mechanism of the interaction between flavonols and epi-catechins should be further investigated. Taking all these findings together, this study demonstrates that catechol-containing flavonoids, particularly quercetin and fisetin, are potent substrates for COMT and thus competitively inhibit the methylation of catechins. The quantification of methylated quercetin and fisetin was not conducted in this study due to the lack of commercial standards. However, the quantification of the metabolite profiles of methylated quercetin or fisetin in the apical and basolateral compartment of Caco-2 cells and plasma is necessary. Additional gene expression of COMT should be tested to confirm the benefit of the combination of quercetin and fisetin on the bioavailability of epi-catechins.
Conclusion
In summary, we demonstrated that the intestinal transport of major epi-catechins by the human intestinal Caco-2 cells was enhanced by adding quercetin, fisetin, or a mixture of quercetin and fisetin. Among the treatments, co-incubation of a 5% mixture of quercetin and fisetin with catechins significantly enhanced the intestinal transport of four major epi-catechins (EC, ECG, EGC, and EGCG) in green tea via the human intestinal Caco-2 cells. A similar pattern was found in a pharmacokinetic study in which considerably high plasma levels of EGCG were observed in the co-administration of EGCG with 10% quercetin or fisetin, indicating that EGCG absorption was significantly affected by quercetin or fisetin at the optimum concentration. The analysis of methylated metabolites in the basal media shows that quercetin and fisetin were, to a certain extent, methylated.Conflicts of interest
The authors have declared no conflict of interest.Acknowledgements
This study was supported by the AmorePacific R&D Center.References
- J. H. Chung, S. Kim, S. J. Lee, J. O. Chung, Y. J. Oh and S. M. Shim, Green tea formulations with vitamin C and xylitol on enhanced intestinal transport of green tea catechins, J. Food Sci., 2013, 78, C685–C690 CrossRef CAS PubMed.
- M. J. Kim, J. H. Kim, J. H. Kim and Y. J. Kim, Comparative studies on the antioxidant capacities and catechin profiles of conventional and organic green tea, J. Korean Soc. Appl. Biol. Chem., 2015, 4, 475–480 CrossRef.
- D. Kumar, M. Poornima, R. Kushwaha, T.-J. Won, C. Ahn, C. G. Kumar, K. Jang and D.-S. Shin, Antimicrobial and docking studies of (−)-catechin derivatives, J. Korean Soc. Appl. Biol. Chem., 2015, 58, 581–585 CrossRef CAS.
- R. J. Green, A. S. Murphy, B. Schulz, B. A. Watkins and M. G. Ferruzzi, Common tea formulations modulate in vitro digestive recovery of green tea catechins, Mol. Nutr. Food Res., 2007, 51, 1152–1162 CAS.
- L. Zhang, Y. Zheng, M. S. Chow and Z. Zuo, Investigation of intestinal absorption and disposition of green tea catechins by Caco-2 monolayer model, Int. J. Pharm., 2004, 287, 1–12 CrossRef CAS PubMed.
- J. Hong, H. Lu, X. Meng, J.-H. Ryu, Y. Hara and C. S. Yang, Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (−)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells, Cancer Res., 2002, 62, 7241–7246 CAS.
- J. D. Lambert, S. Sang, A. Y. Lu and C. S. Yang, Metabolism of dietary polyphenols and possible interactions with drugs, Curr. Drug Metab., 2007, 8, 499–507 CrossRef CAS PubMed.
- I. R. Record and J. M. Lane, Simulated intestinal digestion of green and black teas, Food Chem., 2001, 73, 481–486 CrossRef CAS.
- C. M. Peters, R. J. Green, E. M. Janle and M. G. Ferruzzi, Formulation with ascorbic acid and sucrose modulates catechin bioavailability from green tea, Food Res. Int., 2010, 43, 95–102 CrossRef CAS PubMed.
- S.-M. Shim, S.-H. Yoo, C.-S. Ra, Y.-K. Kim, J.-O. Chung and S.-J. Lee, Digestive stability and absorption of green tea polyphenols: influence of acid and xylitol addition, Food Res. Int., 2012, 45, 204–210 CrossRef CAS.
- Z.-Y. Chen, Q. Y. Zhu, Y. F. Wong, Z. Zhang and H. Y. Chung, Stabilizing effect of ascorbic acid on green tea catechins, J. Agric. Food Chem., 1998, 46, 2512–2516 CrossRef CAS.
- T. Kohri, N. Matsumoto, M. Yamakawa, M. Suzuki, F. Nanjo, Y. Hara and N. Oku, Metabolic fate of (−)-[4-3H] epigallocatechin gallate in rats after oral administration, J. Agric. Food Chem., 2001, 49, 4102–4112 CrossRef CAS PubMed.
- H. S. Chow, I. A. Hakim, D. R. Vining, J. A. Crowell, J. Ranger-Moore, W. M. Chew, C. A. Celaya, S. R. Rodney, Y. Hara and D. S. Alberts, Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals, Clin. Cancer Res., 2005, 11, 4627–4633 CrossRef CAS PubMed.
- J. B. Vaidyanathan and T. Walle, Transport and metabolism of the tea flavonoid (–)-epicatechin by the human intestinal cell line Caco-2, Pharm. Res., 2001, 18, 1420–1425 CrossRef CAS.
- J. Jodoin, M. Demeule and R. Béliveau, Inhibition of the multidrug resistance P-glycoprotein activity by green tea polyphenols, Biochim. Biophys. Acta, 2002, 1542, 149–159 CrossRef CAS.
- T. Walle, R. A. Walgren, U. K. Walle, A. Galijatovic and J. B. Vaidyanathan, Flavonoids in health and disease, ed. C. A. Rice-Evans and L. Packer, CRC Press, New York, 2003 Search PubMed.
- J. B. Vaidyanathan and T. Walle, Cellular uptake and efflux of the tea flavonoid (-) epicatechin-3-gallate in the human intestinal cell line Caco-2, J. Pharmacol. Exp. Ther., 2003, 307, 745–752 CrossRef CAS PubMed.
- Q. Song, D. Li, Y. Zhou, J. Yang, W. Yang, G. Zhou and J. Wen, Enhanced uptake and transport of (+)-catechin and (−)-epigallocatechin gallate in niosomal formulation by human intestinal Caco-2 cells, Int. J. Nanomed., 2014, 9, 2157 CrossRef PubMed.
- J. Hong, J. D. Lambert, S.-H. Lee, P. J. Sinko and C. S. Yang, Involvement of multidrug resistance-associated proteins in regulating cellular levels of (−)-epigallocatechin-3-gallate and its methyl metabolites, Biochem. Biophys. Res. Commun., 2003, 310, 222–227 CrossRef CAS PubMed.
- C. Worda, M. Sator, C. Schneeberger, T. Jantschev, K. Ferlitsch and J. Huber, Influence of the catechol-O-methyltransferase (COMT) codon 158 polymorphism on estrogen levels in women, Hum. Reprod., 2003, 18, 262–266 CrossRef CAS PubMed.
- M. B. van Duursen, J. T. Sanderson, P. C. de Jong, M. Kraaij and M. van den Berg, Phytochemicals inhibit catechol-O-methyltransferase activity in cytosolic fractions from healthy human mammary tissues: implications for catechol estrogen-induced DNA damage, Toxicol. Sci., 2004, 81, 316–324 CrossRef CAS PubMed.
- B. T. Zhu, U. K. Patel, M. X. Cai and A. H. Conney, O-Methylation of tea polyphenols catalyzed by human placental cytosolic catechol-O-methyltransferase, Drug Metab. Dispos., 2000, 28, 1024–1030 CAS.
- B. Sanchez-Bridge, A. Lévèques, H. Li, E. Bertschy, A. Patin and L. Actis-Goretta, Modulation of (–)-Epicatechin Metabolism by Coadministration with Other Polyphenols in Caco-2 Cell Model, Drug Metab. Dispos., 2015, 43, 9–16 CrossRef PubMed.
- K. Landis-Piwowar, D. Chen, T. H. Chan and Q. P. Dou, Inhibition of catechol-O-methyltransferase activity in human breast cancer cells enhances the biological effect of the green tea polyphenol (-)-EGCG, Oncol. Rep., 2010, 24, 563–569 CAS.
- S. M. Henning, P. Wang, C. L. Carpenter and D. Heber, Epigenetic effects of green tea polyphenols in cancer, Epigenomics, 2013, 5, 729–741 CrossRef CAS PubMed.
- K. G. Daniel, K. R. Landis-Piwowar, D. Chen, S. B. Wan, T.-H. Chan and Q. P. Dou, Methylation of green tea polyphenols affects their binding to and inhibitory poses of the proteasome β5 subunit, Int. J. Mol. Med., 2006, 18, 625–632 CAS.
- K. Okushio, M. Suzuki, N. Matsumoto, F. Nanjo and Y. Hara, Methylation of tea catechins by rat liver homogenates, Biosci., Biotechnol., Biochem., 1999, 63, 430–432 CrossRef CAS PubMed.
- H. Lu, X. Meng and C. S. Yang, Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (−)-epigallocatechin gallate, Drug Metab. Dispos., 2003, 31, 572–579 CrossRef CAS.
- A. H. Perry, PhD Thesis, University of Minnesota, 2014.
- P. Wang, D. Heber and S. M. Henning, Quercetin increased bioavailability and decreased methylation of green tea polyphenols in vitro and in vivo, Food Funct., 2012, 3, 635–642 CAS.
- R. M. Weinshilboum, D. M. Otterness and C. L. Szumlanski, Methylation pharmacogenetics: catechol O-methyltransferase, thiopurine methyltransferase, and histamine N-methyltransferase, Annu. Rev. Pharmacol. Toxicol., 1999, 39, 19–52 CrossRef CAS PubMed.
- M. Inoue-Choi, J.-M. Yuan, C. S. Yang, D. J. Van Den Berg, M.-J. Lee, Y.-T. Gao and M. C. Yu, Genetic association between the COMT genotype and urinary levels of tea polyphenols and their metabolites among daily green tea drinkers, Int. J. Mol. Epidemiol. Genet., 2010, 1, 114–123 CAS.
- M. Nagai, A. H. Conney and B. T. Zhu, Strong inhibitory effects of common tea catechins and bioflavonoids on the O-methylation of catechol estrogens catalyzed by human liver cytosolic catechol-O-methyltransferase, Drug Metab. Dispos., 2004, 32, 497–504 CrossRef CAS PubMed.
- J. J. van Zanden, H. M. Wortelboer, S. Bijlsma, A. Punt, M. Usta, P. J. van Bladeren, I. M. Rietjens and N. H. Cnubben, Quantitative structure activity relationship studies on the flavonoid mediated inhibition of multidrug resistance proteins 1 and 2, Biochem. Pharmacol., 2005, 69, 699–708 CrossRef CAS PubMed.
- M. Silberberg, C. Morand, C. Manach, A. Scalbert and C. Remesy, Co-administration of quercetin and catechin in rats alters their absorption but not their metabolism, Life Sci., 2005, 77, 3156–3167 CrossRef CAS PubMed.
- B. T. Zhu and J. G. Liehr, Inhibition of catechol O-methyltransferase-catalyzed O-methylation of 2-and 4-hydroxyestradiol by quercetin possible role in estradiol-induced tumorigenesis, J. Biol. Chem., 1996, 271, 1357–1363 CrossRef CAS PubMed.
- P. Wang, D. Heber and S. M. Henning, Quercetin increased the antiproliferative activity of green tea polyphenol (-)-epigallocatechin gallate in prostate cancer cells, Nutr. Cancer, 2012, 64, 580–587 CrossRef CAS PubMed.
- M. Nomura, T. Takahashi, N. Nagata, K. Tsutsumi, S. Kobayashi, T. Akiba, K. Yokogawa, S. Moritani and K.-i. Miyamoto, Inhibitory mechanisms of flavonoids on insulin-stimulated glucose uptake in MC3 T3-G2/PA6 adipose cells, Biol. Pharm. Bull., 2008, 31, 1403–1409 CAS.
- A. Kale, S. Gawande, S. Kotwal, S. Netke, W. Roomi, V. Ivanov, A. Niedzwiecki and M. Rath, Studies on the effects of oral administration of nutrient mixture, quercetin and red onions on the bioavailability of epigallocatechin gallate from green tea extract, Phytother. Res., 2010, 24(Suppl 1), S48–S55 CrossRef PubMed.
- Y. J. Moon and M. E. Morris, Pharmacokinetics and bioavailability of the bioflavonoid biochanin A: effects of quercetin and EGCG on biochanin A disposition in rats, Mol. Pharm., 2007, 4, 865–872 CrossRef CAS PubMed.
- J. H. Jo, J. J. Jo, J.-M. Lee and S. Lee, Identification of absolute conversion to geraldol from fisetin and pharmacokinetics in mouse, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2016, 1038, 95–100 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |