Specific protein supplementation using soya, casein or whey differentially affects regional gut growth and luminal growth factor bioactivity in rats; implications for the treatment of gut injury and stimulating repair†
Tania
Marchbank
 ab,
Nikki
Mandir
c,
Denis
Calnan
d,
Robert A.
Goodlad
c,
Theo
Podas
d and
Raymond J.
Playford
ab,
Nikki
Mandir
c,
Denis
Calnan
d,
Robert A.
Goodlad
c,
Theo
Podas
d and
Raymond J.
Playford
 *a
*a
aPlymouth University Peninsula Schools of Medicine and Dentistry, Plymouth, UK. E-mail: raymond.playford@plymouth.ac.uk; Tel: +44 (0)1752582002
bCentre or Immunobiology, Blizard Institute, Barts and The London School of Medicine, Queen Mary, University of London, UK
cHistopathology, CRUK, Lincoln's Inn Fields Laboratory, 44 Lincoln's Inn Fields, London, UK
dUniversity Division of Gastroenterology, Leicester General Hospital, Gwendolen Road, Leicester, UK
First published on 17th November 2017
Abstract
Modulation of regional growth within specific segments of the bowel may have clinical value for several gastrointestinal conditions. We therefore examined the effects of different dietary protein sources on regional gut growth and luminal growth factor bioactivity as potential therapies. Rats were fed for 14 days on isonitrogenous and isocaloric diets comprising elemental diet (ED) alone (which is known to cause gut atrophy), ED supplemented with casein or whey or a soya protein-rich feed. Effects on regional gut growth and intraluminal growth factor activity were then determined. Despite calorie intake being similar in all groups, soya rich feed caused 20% extra total body weight gain. Stomach weight was highest on soya and casein diets. Soya enhanced diet caused greatest increase in small intestinal weight and preserved luminal growth factor activity at levels sufficient to increase proliferation in vitro. Regional small intestinal proliferation was highest in proximal segment in ED fed animals whereas distal small intestine proliferation was greater in soya fed animals. Colonic weight and proliferation throughout the colon was higher in animals receiving soya or whey supplemented feeds. We conclude that specific protein supplementation with either soya, casein or whey may be beneficial to rest or increase growth in different regions of the bowel through mechanisms that include differentially affecting luminal growth factor bioactivity. These results have implications for targeting specific regions of the bowel for conditions such as Crohn's disease and chemotherapy.
Introduction
Many intrinsic inflammatory and ingested injurious agents cause regional damage to the gut. These include Crohn's disease (mainly affecting distal small intestine), ulcerative colitis (affecting colon) and non-steroidal inflammatory drugs (mainly affecting stomach and small intestine). Therapeutic targeting of drugs may occur through site specific release formulations or topical therapy (such as colonic enemas). As an alternative to pharmacological approaches, there is currently interest in using nutritional manipulation to both reduce the risk of relapse and for treatment when an injury is present. For example, elemental diets (ED), consisting of amino acids, mono/disaccharides and short/medium chain fatty acids, are of proven value in the treatment of inflammatory bowel disease, especially in children.1,2 The mechanism(s) of action of EDs are unclear but might include reducing systemic antigenic load and “resting” the bowel. More recently, polymeric diets have been shown to be equally efficacious to ED,3 establishing the principal that selective additions to feeds may enhance efficacy without losing the beneficial effects of ED. Prolonged use of ED causes intestinal atrophy, particularly of the distal bowel, potentially increasing the risk of bacterial translocation and bacteraemia.4 Dietary formulations which reduce gut luminal antigenic load whilst minimizing the associated gut atrophy could, therefore, have therapeutic advantages. Similarly, because the gut is second only to the bone marrow in cell turnover, damage to the intestine is a common issue in patients undergoing chemotherapy (chemotherapy induced mucositis) and diets which reduce turnover while chemotherapy is administered or enhancing growth after treatment may also be useful.The physiological control of gut growth is complex and poorly understood. Possible controlling mechanisms include alteration in circulating trophic hormones, local nutrition and luminal growth factors. We have previously suggested that ingestion of specific food proteins could influence gut growth by reducing the destruction of luminal growth factors, such as epidermal growth factor (EGF), through acting as competitive substrates for pancreatic proteases.5 In this model, the efficacy of the various proteins to stimulate gut growth would be dependent on their ability to act as substrate for pancreatic proteases, relative to the affinity of the luminal growth factors for the same enzymes. Support for this idea comes from an in vitro study that showed soya bean trypsin inhibitor or casein were better substrates than whey protein (“lactalbumin”) for human pancreatic proteases. Furthermore, the addition of soya protein or casein to human duodenal juice reduced its ability to digest EGF, whereas addition of whey protein or elemental diets did not.5 Soya protein or casein supplementation of feeds such as elemental diets might, therefore, be a clinically useful method of decreasing gut hypoplasia, while having a relatively minor effect on “antigenic load”.
We, therefore, examined the effect of isocaloric and isonitrogenous diets comprising standard elemental diet (E028, Nutricia, Liverpool, UK), E028 supplemented with either casein (ED + C) or whey protein (ED + W), and a commercially available soya-protein rich diet (SP, containing human food grade soya bean concentrate) on regional gut growth in rats and also assessed total “growth factor” bioactivity within the lumen of the small intestine.
Materials and methods
Diets
Three of the four test diets comprised standard elemental E028 (‘ED’, Nutricia Liverpool, UK) or variants of E028 containing whey protein (‘ED + W’, 12.5 g per 100 g, the two major components of whey being α-lactoglobin and β-lactoglobin.)6 or casein (‘ED + C’, 10.5 g per 100 g). Both protein supplemented elemental diets where specifically produced for the studies by Nutricia Liverpool. The fourth group consisted of a commercially available soya protein rich diet (‘SP’, produced by SDS, Essex, UK). All four diets were similar in terms of digestible protein equivalence and amino-acid content but the SP diet had a higher carbohydrate content, lower added fat and contained some crude fibre (5.3%). Details of the various diets are shown in ESI Table 1.†Protease inhibitory activity of feeds
To determine trypsin inhibitory activity within the feeds, a standard BAPNA substrate assay7 was undertaken with concentrations of trypsin (0–50 μg) added to generate a standard curve. Additional tubes, each containing 20 μg trypsin had varying concentrations of the feeds added and any effect on reduction of tryptic activity (due to competitive inhibition) determined. In addition, to reproduce the situation found in vivo, where the feeds would be exposed to gastric juice prior to entering the small intestine, additional samples were pre-incubated with pepsin (1 mg ml−1) for 1 h at pH 2, 37 °C before neutralization and subsequent BAPNA analyses.Animal experiments
All experiments were performed in compliance with relevant United Kingdom laws and guidelines. All animal experiments were approved by the Local Institutional Animals Ethics Committees (Imperial College and Cancer Research UK) and performed under institutional guidelines. Studies were covered by the appropriate licences under the United Kingdom Home Office Animals Procedures Acts, 1986 and performed under the UK home office (Animal Research: Reporting of In Vivo Experiments) guidelines (ARRIVE).Food was given in 2-ring concentric aluminium containers designed to minimize food spillage. The food consumption of each cage with determined each day by back weighing, including the small amount of spilled food beneath the cages.
Autopsy procedure and collection of samples
At the end of the 14 day study, rats were given vincristine sulphate by intraperitoneal injection (1 mg kg−1, Eli Lilly, Basingstoke, UK) and killed 2 h later. Analyses of tissue was performed using the methods published by our group previously.8 The weight and unstretched length of the various portions of the gastrointestinal tract were measured. To assess the effect of these various diets on luminal growth factor activity, 1 ml of iced, normal saline was then lavaged through the small intestine, collected into Eppendorf tubes, snap frozen using frozen CO2 (dry ice) and stored at −70 °C until assay for growth factor activity.The stomach and samples of the small intestine and colon were then fixed in Carnoy's fluid, and stored in 70% (volume/volume) ethanol. For the intestinal tissues, the position of the various samples was defined by expressing its harvest site as a percentage of that organs total length at 1.5, 50 and 90% small intestinal distance and can be considered as equivalent to duodenum, jejunum and ileum respectively. Similarly, 10, 50 and 90% colon can be considered as proximal, mid and distal colon.
Analysis of tissue samples
Assessment of metaphase accumulation was performed on micro-dissected tissue using our well validated, previously published methods8 by a person blinded to the experiment. Briefly, tissues were sequentially rehydrated, hydrolyzed and stained with Schiff's reagent (the Feulgen reaction) and transferred to 45% (volume/volume) acetic acid. The crypts were then teased apart under a dissecting microscope, transferred to a glass microscope slide, flattened gently beneath a coverslip and examined using a compound microscope. The number of blocked mitoses per crypt was counted and the mean for 20 crypts per animal per site determined and used in the subsequent one way analysis of variance (ANOVA).Analysis of luminal growth factor activity
Analyses of luminal growth factor activity were determined using thymidine incorporation into primary rat hepatocytes as a bioassay and an EGF-standard curve as described previously by us.9,10 Briefly, to prepare primary rat hepatocytes for the in vitro assay of luminal growth factor activity, male Sprague-Dawley rats were anaesthetized using Hypnorm (Janssen Pharmaceutica-Crown Chemicals Ltd, Lamberhurst, Kent, UK) and hepatocytes isolated by in situ collagenase perfusion. The basic protocol consists of a two-step perfusion of the liver in situ, via the portal vein, first with calcium-free buffer followed by a calcium supplemented buffer containing collagenases. The digested liver was removed, the cells dispersed, filtered, collected by centrifugation and resuspended in a plating medium. For all studies, hepatocytes were grown in Williams E medium without L-glutamine (Gibco BRL, Paisley, UK) containing 5% fetal calf serum. Cell viability, determined by the ability to exclude 0.2% trypan blue, was greater than 80% in all experiments.The increase in thymidine uptake was then expressed in terms of EGF-like bioactivity by determining the equivalent amount of EGF that was needed to be added to the hepatocytes, to stimulate a similar increase in thymidine uptake. Measurements of EGF-like bioactivity in intestinal juice samples were performed in quadruplicate in 4 separate wells.
Statistical analysis
Data were analysed using one-way ANOVA with the different diets as the factor. Where a significant effect of diet was seen, individual comparisons were performed comparing against ED, using t-tests based on the residual and degrees of freedom obtained from the ANOVA, a method equivalent to repeated measures analysis. All data is expressed as mean ± SEM unless stated.Results
Protease inhibitory activity of feeds
In vitro assay of feeds showed higher trypsin inhibitory activity (136 mg trypsin inhibition per 100 g feed) in SP feed, moderate amount in ED + C (42 mg trypsin inhibition per 100 g feed) but much lower amounts in ED (13 mg per 100 g) or ED + W (15 mg per 100 g). Samples which had been pre-incubated with pepsin at pH 2 to reproduce prior exposure to gastric contents gave similar results (SP 120 mg trypsin inhibition per 100 g feed, ED + C 38 mg per 100 g, ED 13 mg per 100 g and ED + W 12 mg per 100 g).Animal study
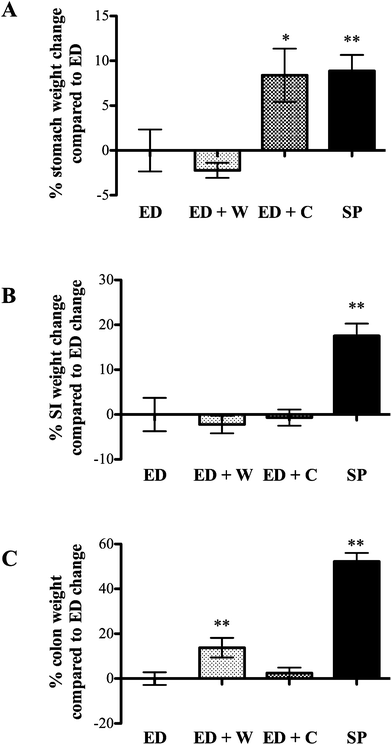
Small intestine (SI) weight
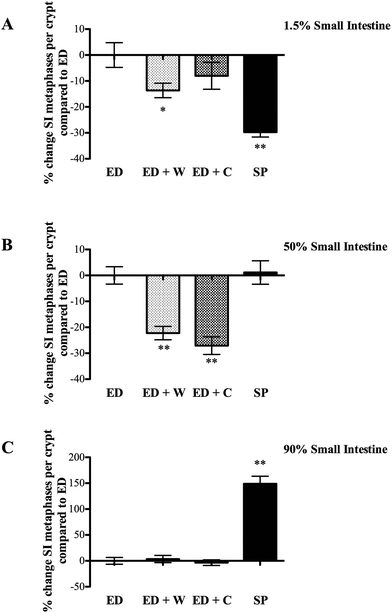
Small intestinal regional proliferation varied dependent on site with the different diets. Proximal small intestinal proliferation was greatest in the ED, with the other three groups being significantly lower (Fig. 2A). In contrast, SP group had the highest proliferation rate in the mid small intestine (significantly greater than ED + W and ED + C, Fig. 3B) and this difference was particularly marked in the distal small intestine with SP having proliferation rate of 150% of the other diets (Fig. 2C).
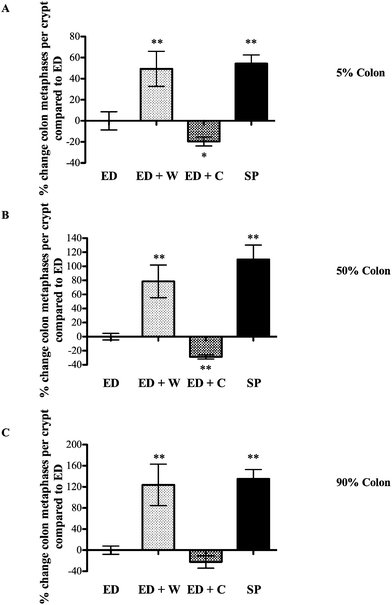 | ||
| Fig. 3 Effects of diets on colon mitosis. Colonic tissue from the same animals as Fig. 3 was collected and the number of mitosis per crypt assessed. The regions analyzed were located according their position as a percentage of total colonic length; 5% site of colon (proximal colon, A), 50% site of colon (mid-colon, B), 90% site of colon (distal colon, C). * and ** signifies p < 0.05 and p < 0.01, respectively compared with ED. | ||
Discussion
We have shown different dietary protein sources cause differential regional effects on gut growth and proliferation and that diets containing soya protein result in increased small intestinal luminal growth factor activity.Care was taken to ensure caloric intake of the groups were comparable to remove a potential confounding factor. We used fourteen days dietary intervention to reflect what would likely be used in a clinical setting in situations such as pre- or post-chemotherapy intervention. We used a rat hepatocyte system to assay intraluminal growth factor activity as this is a robust, reproducible method to determine relative bioactivity of EGF-R type ligands in the gut lumen and has the advantage over most cell lines of maintaining viability in the presence of bile and other luminal contents.9,10 The in vitro trypsin (inhibition) assay is a simple robust system to examine differential effects of food substances and builds on our earlier in vitro work5 showing that soya-bean trypsin inhibitor causes marked trypsin inhibition, casein causes moderate trypsin inhibition (probably acting as a competitive substrate), whereas ED or whey have very limited inhibitory activity.
The trophic effects of the various diets differed dependent on which region of the gut was analyzed. Compared to ED diet, stomach weight was better maintained in ED + C and SP rats. The effect of ED + C may be due to casein being precipitated in stomach acid, delaying gastric emptying, whereas rapid transit into the small intestine is seen in ED or ED + W animals.11
The most notable finding of the ED diet was the much higher proliferation rate seen in the proximal small intestine compared to the other diets. This is probably as a result of rapid transit into the proximal intestine, where the ED constituents are rapidly adsorbed. This finding could be explained by the luminal workload hypothesis that suggests that regions actively involved in digestion/absorption have increased proliferation. This would also explain the low mitotic level seen in more distal regions of the small bowel as the majority of the feed would have been adsorbed more proximally.
The most notable effects of ED + W were the increased weight and proliferation of the colon. Several potential mechanisms might have relevance in explaining these findings. Changes in circulating trophic factors such as glucagon-like peptide-2 (GLP-2) may have relevance although it is noteworthy that the trophic effect was restricted to the colon and not the small intestine. GLP-2 is produced by the ileum and colon, is trophic to the small intestine and colon and is rapidly degraded by tissue DPP-IV (dipeptidyl peptidase 4). Liu and co-workers showed oral whey supplementation decreased colonic tissue DPP-IV in rats receiving continuous intravenous GLP-2, possibly increasing local colonic tissue GLP-2 levels.12 Therefore, even if circulating levels of GLP-2 remain stable, local changes in DPP-IV as a result of dietary manipulation may alter local concentrations. A further mechanism that would explain our results is our finding that whey proteins are poor substrates for luminal proteases when compared against casein or soya. The amount of nutrient reaching the terminal bowel is, therefore, likely to be higher in the ED + W compared to the other groups. Support for this idea comes from recent studies showing whey supplementation increased colonic short chain fatty acids (SCFAs) concentrations, a known substrate for colonocytes.13 Further studies to determine the importance of colonic bacteria in maintaining the growth of the colon in these animals could include measurement of colonic SCFAs or use of ‘germ free’ animals.
The most notable effects of the SP diet in the current studies were the increased total body weight gain, the increased small and large bowel growth and the finding that bioactive growth factor activity was present in small intestinal washings. The additional increase in body weight in the SP-fed group was not due to differences in total calorie intake as these were well matched across the groups although it should be noted that the SP group received a slightly higher percentage of their calories from protein. We have previously suggested that control of intestinal growth might be mediated by changes in intraluminal growth factor concentrations, with the trophic effect dependent on the relative affinity of the luminal proteases for the ingested food and the luminal growth factors.5 This idea was supported by our findings that pancreatic diversion away from the gut lumen increases luminal growth factor concentrations and caused associated regional hypertrophy.5 The current studies extend these findings showing that ingestion of soy based proteins (which includes soya bean trypsin inhibitor) are effective competitive substrates for luminal proteases in vivo, allowing luminal growth factors such as EGF to survive better than when animals are fed whey or casein. These findings may have relevance to the trophic effect on the small and large intestine although it must be noted that the SP diet contained some fibre which may have relevance for colonic growth through acting as a fuel source for colonic bacteria, increasing colonic SCFAs levels in a similar way to that discussed for the whey fed animals. Further studies examining ED supplemented with pure soya protein which has had the fibre further reduced, combined with measurement of colonic SCFAs and possibly the use of germ-free animals could help address this question.
There is currently much interest in the use of dietary proteins for the prevention and treatment of ill health.14 Our findings that dietary manipulation can cause regional changes in gut growth and also modulates luminal growth factor levels has potential clinical relevance. ED or polymeric diets are of proven benefit for Crohn's disease, a condition that predominantly affects the small intestine. Specific protein supplementation of ED with whey may have advantages in “resting” the small intestine while preserving colonic growth, which among other advantages should reduce the risk of bacterial translocation. Supplementation of ED with soy protein may have the advantage of locally increasing luminal EGF levels, facilitating repair in a similar way to that shown by us in treating ulcerative colitis with topical therapy.15 Further studies examining the effect of these proteins on intraluminal growth factor concentrations and injury and repair in animal models of inflammatory bowel disease appear warranted but go beyond the scope of this paper. Similarly, chemotherapy induced injury to the gut is a common side effect of high dose chemotherapy and systemic administration of several different growth factors to promote repair have been tested or are being assessed.16 Dietary manipulation to reduce gut cellular turnover prior to treatment of non-gut tumours using systemic chemotherapy (to reduce gut side effects) and/or to increase gut proliferation post treatment, therefore, has potential therapeutic advantage by locally increasing luminal growth factor concentrations without the risk of systemic administration. Further studies appear justified.
Abbreviations
| ANOVA | Analysis of variance |
| C | Casein |
| ED | Elemental diet |
| EGF | Epidermal growth factor |
| GLP-2 | Glucagon-like peptide-2 |
| SCFAs | Short chain fatty acids |
| SI | Small intestine |
| SP | Soya protein |
| W | Whey protein |
Funding
Funded by institutional funding.Conflicts of interest
All authors declare no conflict of interest.References
- J. Lee, R. Allen, S. Ashley, S. Becker, P. Cummins, A. Gbadamosi, O. Gooding, J. Huston, J. Le Couteur, D. O'Sullivan, S. Wilson and M. C. Lomer, Dietetic Association evidence-based guidelines for the dietary management of Crohn's disease in adults, J. Hum. Nutr. Diet., 2014, 27, 207–218 CrossRef CAS PubMed.
- A. K. Akobeng, V. Miller, J. Stanton, A. M. Elbadri and A. G. Thomas, Double-blind randomized controlled trial of glutamine-enriched polymeric diet in the treatment of active Crohn's disease, J. Pediatr. Gastroenterol. Nutr., 2000, 30, 78–84 CrossRef CAS PubMed.
- S. Verma, S. Brown, B. Kirkwood and M. H. Giaffer, Polymeric versus elemental diet as primary treatment in active Crohn's disease: a randomized, double-blind trial Polymeric vs Elemental Diet in Crohn's Disease, Am. J. Gastroenterol., 2000, 95, 735–739 CrossRef CAS PubMed.
- E. A. Deitch, D. Xu, M. B. Naruhn, D. C. Deitch, Q. Lu and A. A. Marino, Elemental diet and IV-TPN-induced bacterial translocation is associated with loss of intestinal mucosal barrier function against bacteria, Ann. Surg., 1995, 221, 299–307 CrossRef CAS PubMed.
- R. J. Playford, A. C. Woodman, P. Clark, P. Watanapa, D. Vesey, P. Deprez, R. C. Williamson and J. Calam, Effect of luminal growth factor preservation on intestinal growth, Lancet, 1993, 341, 843–848 CrossRef CAS.
- D. P. Mohanty, S. Mohapatra, S. Misra and P. S. Sahu, Milk derived bioactive peptides and their impact on human health - A review, Saudi J. Biol. Sci., 2016, 23, 577–583 CrossRef CAS PubMed.
- T. Marchbank, A. Mahmood and R. J. Playford, Pancreatic secretory trypsin inhibitor causes autocrine-mediated migration and invasion in bladder cancer and phosphorylates the EGF receptor, Akt2 and Akt3, and ERK1 and ERK2, Am. J. Physiol.: Renal, Fluid Electrolyte Physiol., 2013, 305, F382–F389 CrossRef CAS PubMed.
- P. A. Kitchen, R. A. Goodlad, A. J. FitzGerald, N. Mandir, M. A. Ghatei, S. R. Bloom, J. Berlanga-Acosta, R. J. Playford, A. Forbes and J. R. Walters, Intestinal growth in parenterally-fed rats induced by the combined effects of glucagon-like peptide 2 and epidermal growth factor, J. Parenter. Enteral Nutr., 2005, 29, 248–254 CrossRef CAS PubMed.
- R. J. Playford, T. Marchbank, D. P. Calnan, J. Calam, P. Royston, J. J. Batten and H. F. Hansen, Epidermal growth factor is digested to smaller, less active forms in acidic gastric juice, Gastroenterology, 1995, 108, 92–101 CrossRef CAS.
- D. P. Calnan, A. Fagbemi, J. Berlanga-Acosta, T. Marchbank, T. Sizer, K. Lakhoo, A. D. Edwards and R. J. Playford, Potency and stability of C terminal truncated human epidermal growth factor, Gut, 2000, 47, 622–627 CrossRef CAS PubMed.
- M. Dangin, Y. Boirie, C. Garcia-Rodenas, P. Gachon, J. Fauquant, P. Callier, O. Ballèvre and B. Beaufrère, The digestion rate of protein is an independent regulating factor of postprandial protein retention, Am. J. Physiol.: Endocrinol. Metab., 2001, 280, E340–E348 CAS.
- X. Liu, S. G. Murali, J. J. Holst and D. M. Ney, Whey protein potentiates the intestinotrophic action of glucagon-like peptide-2 in parenterally fed rats, Am. J. Physiol.: Regul., Integr. Comp. Physiol., 2009, 297, R1554–R1562 CrossRef CAS PubMed.
- K. Tomoda, K. Kubo, K. Dairiki, T. Yamaji, Y. Yamamoto, Y. Nishii, A. Nakamura, M. Yoshikawa, K. Hamada and H. Kimura, Whey peptide-based enteral diet attenuated elastase-induced emphysema with increase in short chain fatty acids in mice, BMC Pulm. Med., 2015, 15, 64 CrossRef PubMed.
- A. Pihlanto, P. Mattila, S. Mäkinen and A. M. Pajari, Bioactivities of alternative protein sources and their potential health benefits, Food Funct., 2017, 8, 3443–3458 CAS.
- A. Sinha, J. Nightingale, K. P. West, J. Berlanga-Acosta and R. J. Playford, Epidermal Growth Factor Enemas with Oral Mesalamine for Mild-to-Moderate Left-Sided Ulcerative Colitis or Proctitis, N. Engl. J. Med., 2003, 349, 350–357 CrossRef CAS PubMed.
- C. J. Xian, Roles of growth factors in chemotherapy-induced intestinal mucosal damage repair, Curr. Pharm. Biotechnol., 2003, 4, 260–269 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7fo01251a |
| This journal is © The Royal Society of Chemistry 2018 |