On the effect of wearing personal nanoparticle monitors on the comparability of personal exposure measurements
Christof
Asbach
*a,
Volker
Neumann
b,
Christian
Monz
b,
Dirk
Dahmann
b,
Martie
van Tongeren
c,
Carla
Alexander
c,
Laura
MacCalman
c and
Ana Maria
Todea
a
aInstitut für Energie- und Umwelttechnik e. V. (IUTA), Air Quality & Filtration Unit, Bliersheimer Straße 58-60, 47229 Duisburg, Germany. E-mail: asbach@iuta.de
bInstitute for the Research on Hazardous Substances (IGF), Waldring 97, 44789 Bochum, Germany
cInstitute of Occupational Medicine (IOM), Research Avenue North Riccarton, Edinburgh EH14 4AP, UK
First published on 14th November 2016
Abstract
Personal inhalation exposures to airborne agents, including nanomaterials, are ideally measured in the breathing zone, using personal monitors or samplers. It is known from previous studies that the available personal monitors can measure airborne nanomaterial concentrations under laboratory conditions with an accuracy and comparability of ±30% or better. However, it is unclear whether this level of accuracy and comparability can also be achieved when these instruments are used as personal monitoring devices by individuals carrying out a wide variety of activities. In the present study, we investigated the reliability of DiSCmini and Partector during simulated exposure measurements. Two individuals were equipped with two identical instruments each, one mounted near the left and the other near the right collarbone. Both individuals went through a sequence of pre-determined and controlled activities, while simultaneously being exposed to well-defined NaCl aerosols within a 23 m3 chamber. A third specimen of both instruments was placed on a table in the middle of the chamber. The results of the Partector, mounted directly on the left or right side lapel within the personal breathing zone, agreed very well with each other and with the results from the third Partector on the table. The deviations were typically within ±10%. The scatter of the data was found to be larger when the individuals were walking than when they were sitting but the average concentrations remained unaffected by the activities. It can hence be concluded that the positioning of the sampling inlet within the breathing zone does not affect the measurement result, independent of personal activities and whether the carrying person is left- or right-handed. In contrast, the DiSCmini results showed very large deviations of up to a factor of three. However, this was caused by the use of silicone tubes in order to sample air from the personal breathing zone and transport to the belt mounted instruments. Siloxanes degas from the tubes into the airflow and are ionized in the unipolar diffusion charger of the DiSCmini and hence change the charging characteristics significantly affecting the measurement results.
Environmental significanceHuman exposure to ultrafine and nanoparticles needs to be measured directly in the breathing zone of an individual. Nanospecific personal samplers have recently become available and their accuracy and comparability under laboratory conditions have been published elsewhere. However, the reliability of the measurement results from personal measurements in the field were so far unknown and investigated in the present study. The study revealed that the monitors can be reliably used under field conditions and that the results are not significantly affected by the wearing of the instruments by an individual. These results are of utmost importance for both ambient and workplace exposure assessment. |
Introduction
The production and use of engineered nanomaterials has risen at a constant pace over the recent years,1 leading to an increased likelihood of human exposure to nanoparticles. Inhalation exposure is seen as the most critical route for human nanomaterial intake as airborne nanoparticles have a high mobility and, upon inhalation, the probability that they remain in the lung is high.2,3 It was found that the deposition of nanoscale particles in the lung can cause more severe health effects than larger particles for the same mass dose.4 Exposure to airborne nanomaterials may, in principle, occur during any stage of their life cycle, i.e. during manufacture, handling, use (and misuse) and disposal/recycling.5 However, workers producing or handling these materials are likely to have the highest exposure to engineered nanomaterials. Worker exposure therefore needs to be assessed in view of risk assessment. Various measurement strategies for exposure assessment have been developed and published in the past6–10 and were recently harmonized in an OECD initiative.11 The proposed strategy describes a tiered approach. In the first tier, information is gathered concerning the workplace, nanomaterials used and activities with the nanomaterials to judge whether a release of and subsequent exposure to the nanomaterials is possible. If exposure to the nanomaterials cannot reasonably be excluded, a limited measurement survey is carried out in tier 2, mainly using battery operated handheld instruments. If the survey in tier 2 does not yield a unanimous result, or if it reveals that particle concentrations in the workplace are significantly elevated compared to the background concentration, a detailed analysis of the workplace aerosol is conducted in tier 3, using more precise and mainly stationary instrumentation. Most tier 3 or similar workplace exposure measurement surveys reported in the literature used stationary measurement equipment.12It is generally accepted that inhalation exposure to airborne pollutants can best be assessed by measuring directly in the breathing zone of an individual (according to EN 1540:2012-3 defined as a 30 cm hemisphere around the mouth and nose13). However, personal exposure measurements are not explicitly covered by the aforementioned proposed approaches for nanomaterials. The reason for this is that small instruments capable of measuring or collecting concentrations of airborne nanomaterials in the breathing zone have only recently become available. These instruments can be grouped into personal samplers and personal monitors. A comprehensive overview of the existing measurement techniques and their possibilities and limitations can be found in the nanoIndEx Guidance Document.14
Personal samplers collect particles over a given time for subsequent analysis of, for example, the mass concentration or chemical composition, to provide time (mostly shift) averaged measurement results. Personal monitors are direct reading instruments that measure the airborne concentration with high time resolution. Despite a fairly large number of developments published in the scientific literature, the number of mature and commercially available personal instruments for measuring the exposure to engineered nanomaterials is still rather small. Commercially available personal nanomaterial samplers include the Nanoparticle Respiratory Deposition sampler (NRD, Zefon International, Ocala, Fl, USA),15 the NANOBADGE (ALCEN – NanoInspect, Paris, France),16 and the Thermal Precipitator Sampler (TPS, RJ Lee Group, Monroeville, PA, USA).17
Available personal monitors include the Partector (naneos particle solutions GmbH, Windisch, Switzerland),18 the DiSCmini (Testo AG, Titisee-Neustadt, Germany, essentially identical with its original prototype miniDiSC)19 and the nanoTracer (oxility B.V., Eindhoven, the Netherlands).20 The Partector is the smallest of the instruments and can be worn directly in the breathing zone, whereas the other two instruments need to be carried on a belt and sample from the breathing zone through (flexible) tubes. All three instrument types initially charge the incoming particles in a unipolar diffusion charger and eventually measure a particle charge induced current. The unipolar chargers of all instruments have very similar charging characteristics.
It was found that the electrical current, stemming from unipolarly diffusion charged particles, is coincidentally proportional to the fraction of the airborne particle surface area concentration that would deposit in the alveolar or tracheobronchial region of the human lung,21,22 as long as the particle size range is within 20 nm ≤ dp ≤ 400 nm.23,24 This metric is referred to as the lung deposited surface area (LDSA) concentration and is of increasing interest, because health effects of inhaled particles have been reported to correlate better with the lung deposited surface area dose than with the mass dose.4,25,26
DiSCmini and nanoTracer also provide the mean particle size and the particle number concentration, based on several assumptions. In previous studies we have shown that the lung deposited surface area concentration and mean particle size can be measured with an accuracy and comparability of ±30%,23,27,28 whereas the comparability of number concentration measurements is lower and usually around ±50%.29 This result is in good agreement with data published by Bau et al.,30 who investigated the response of a DiSCmini. Meier et al.31 reported that in roadside measurements, number concentrations measured with a miniDiSC were between 30% and 60% higher than those measured in parallel with a handheld condensation particle counter (TSI P-Trak).
The aforementioned studies looked at the accuracy or comparability of the static instruments. None of the studies investigated whether wearing of the instruments as personal monitors has an effect on the measurement result. Potential influences may stem from personal activities, vibration, convection due to local temperature differences between the body and the environment, changing relative humidity (e.g. triggered by respiration) as well as local air flows and relative velocities between the person carrying the instrument and the aerosol to be measured. Salmanzadeh et al.32 reported, based on numerical simulations, that under certain circumstances the thermal plume around a human body can have a significant effect on the particle concentration in the breathing zone of the individual. Nilsson et al.33 conducted measurements of personal hairdresser exposure to airborne micron sized particles during hair bleaching. They found significant differences between the concentrations concurrently measured near the left and right collarbone during manual activities.
The aim of the present study was therefore to investigate whether the reported accuracy and comparability of the monitors remain valid when the instruments are person-carried as in personal exposure measurements. The study focused only on the personal monitors, and not the samplers, because with their high time resolution, potential influences on the measurement results can better be identified and linked to their origins like certain activities, concentration changes, etc., which is impossible with samplers that only provide the average result over the sampling time. It is, however, expected that any systematic sampling influence detected with the monitors would similarly apply to samplers as long as their positioning on the individual, the location of the sampling inlet and the sample flow rate are similar. Other potential influences of monitors caused, for example, by a vibration or temperature sensitive sensing principle, would not apply to samplers.
Methods
Personal instruments
The personal monitors used in this study were the Partector and DiSCmini. Three specimens of each instrument type were used. While all three Partectors were identical, two of the three DiSCminis were the original prototype miniDiSC, manufactured and distributed through the University of Applied Sciences Northwestern Switzerland. However, miniDiSC and DiSCmini are essentially the same instrument with only cosmetic changes and a different battery charger. Both have no effect on the instruments' performance and therefore miniDiSC and DiSCmini are considered as identical instruments here. The terms DiSCmini and miniDiSC are used synonymously in this paper.DiSCmini
The DiSCmini uses an impactor to remove particles larger than 700 nm (aerodynamic diameter) and operates at a flow rate of 1 l min−1. Aerosols then pass through a unipolar diffusion charger which generates ions that attach to the particles by diffusion. Excess ions are removed in an ion trap before the charged particles are collected on two subsequent stages. The first stage consists of a stack of stainless steel grids, where preferentially small particles are deposited. The particles not collected in the first stage are deposited in a downstream high efficiency filter.19 The currents resulting from the deposition of the particles in the two stages are measured simultaneously with highly sensitive Faraday cup electrometers. The total current, i.e. the sum of the two currents from both stages, is a function of the charging efficiency of the unipolar charger, which is known to be proportional to the LDSA.21 Due to the size dependence of the particle deposition in the first stage, the mean particle size can be determined from the ratio of the current in the first stage and the total current, by assuming a lognormal size distribution with a geometric standard deviation of σg = 1.9. With the LDSA concentration and mean particle size, the number concentration is estimated using the same assumptions concerning the particle size distribution. The size range in which the DiSCmini can measure according to specifications is limited to particles smaller than approximately 400 nm.Since the LDSA concentration is determined by the use of a simple calibration factor with no further assumptions, it is measured with the highest accuracy23,27,28 within the aforementioned size range, whereas the accuracy of the number concentration results is typically lower.29 Only the LDSA concentration was therefore used in this study.
The DiSCmini has a size of 180 mm × 90 mm × 45 mm and weighs 700 g. According to the feedback from field studies, especially because of its size, the DiSCmini was often considered to be too large to be mounted directly in the breathing zone, but it can be conveniently carried on a belt to sample from the breathing zone through a flexible tube. An overview of the manufacturer information on the DiSCmini is given in Table 1. Note that the information in the table including accuracies is taken from the manufacturer spec sheets and does not necessarily match those reported in the scientific literature.
| Monitor | Particle size range (nm) | LDSA conc. range (μm2 cm−3) | Accuracy | Time resolution (s) | Flow rate (l/min) | Weight (g) | Size (mm3) | Battery time (h) |
|---|---|---|---|---|---|---|---|---|
| DiSCmini | 10–300 | Not specified | ±30% | 1 | 1 | 700 | 180 × 90 × 45 | 6–8 |
| Partector | 10–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0–2 × 104 | ±20% | 1 | 0.5 | 400 | 134 × 78 × 29 | 15 |
Partector
The Partector is smaller than the DiSCmini with a size of 134 mm × 78 mm × 29 mm and a weight of 400 g. In previous field studies it has been reported to be small and light enough to be conveniently carried directly in the breathing zone of an individual and sample without the need for any flexible tubes. The aerosol is drawn into the instrument at a flow rate of 0.5 l min−1 and the particles are charged in a unipolar diffusion charger. Although the geometry of the charger is very similar to the DiSCmini charger, its operation is different.18 The high voltage applied to the corona electrode is switched on and off with a frequency of 0.5 Hz, thereby generating parcels of charged particles. After removal of excess ions in an ion trap, the aerosol enters an induction tube. In case of a temporal charge gradient within the tube, a current is induced with a magnitude proportional to the particle borne charges. A negative current peak is induced when the parcel enters and a positive peak when it leaves the induction tube. The peak to peak value of the induced current is measured and yields the LDSA concentration by the application of a calibration factor. This measurement principle has several advantages over the conventional filter based measurement. Since only the peak-to-peak value of the induced current is measured, the system is self-referencing, i.e. no potential electrometer drift needs to be taken into account. The Partector therefore needs no frequent electrometer zeroing, whereas the DiSCmini automatically interrupts the measurement once per hour for one minute to zero the electrometers. Another main advantage of the Partector's measurement principle is that it does not require the particles to be collected and hence upon detection they are still available in the airborne state for collection on a substrate for subsequent offline analyses. An upgraded version of the Partector hence includes an electrostatic precipitator to collect particles onto TEM grids. A downside of the self-referencing principle is that it uses the average between the positive and the negative peak as “zero”. In the case of pre-charged particles, the mean value differs from zero and the measurement is therefore systematically biased. Manufacturer information for the Partector is also given in Table 1.Study design
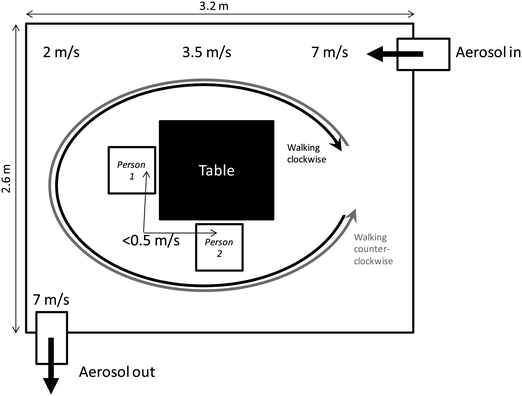
During the experiments, two individuals were simultaneously exposed to NaCl test aerosols inside a 23 m3 (3.2 m × 2.6 m, height: 2.75 m) chamber (see Fig. 1). Two different sodium chloride test aerosols were used on two subsequent days. Sodium chloride was chosen as the test aerosol as it can be easily and cheaply produced and is known to be harmless to human health. Ethics approval for these measurements was received from the ethics committee of the Medical Faculty at Ruhr University Bochum (register No.: 5198-15). Informed consent to participate in the study and be exposed to NaCl aerosols was obtained from both individuals. The chamber was furnished with a table in the centre and two chairs.Each individual simultaneously wore two identical instruments, while the third specimen of both instrument types was placed on the table. The person carried instruments were positioned on the left and right side of the individual's breathing zones to investigate whether the positioning has an effect, particularly during manual activities. Both individuals were right-handed. The Partectors were directly mounted on the chest within the breathing zone, whereas the DiSCminis were carried on a belt on the back of the individual, each one sampling from the breathing zone through new, 75 cm long, conductive silicone tubes (purchased through TSI GmbH, Aachen, Germany). These tubes are usually considered as the best choice for aerosol sampling due to the avoidance of electrostatic particle losses.
In addition, the number size distributions of the test aerosols were measured with a Scanning Mobility Particle Sizer (SMPS, TSI model 3936 (ref. 34)), equipped with a long DMA (TSI model 3080L) and a CPC (TSI model 3772), while the number concentration was simultaneously monitored with a butanol based ultrafine condensation particle counter (UCPC, TSI model 3776) in high flow mode, i.e. sampling the air at a flow rate of 1.5 L min−1. Due to space restrictions on the table in the chamber, both SMPS and CPC were placed outside of the chamber and sampled through a lance from the centre of the chamber where the wind speed was close to zero. In several previous studies it has been shown that under undisturbed aerosol conditions the particle concentrations within the chamber can be considered as homogenous and that the aerosol sampled through the lance is representative of the aerosol in the chamber.27,28
The two individuals conducted a set of identical activities during each experimental run. Initially, they sat quietly on the chairs for 5 minutes, followed by 5 minutes of clockwise walking. After the walking, the individuals rested on the chairs for 2 minutes in order to calm down the air in case of any disturbances, before a 5 minute period of walking counterclockwise. Due to the flow direction inside the chamber, walking clockwise meant walking with headwind towards the aerosol inlet, whereas the individuals walked with tailwind away from the aerosol inlet during counterclockwise walking. After another 2 minute period of sitting, the two individuals finally assembled, disassembled and inspected a clean MPG II dust sampler35 for 5 minutes to mimic manual activity. This set of activities was repeated three times to complete one experimental run.
After the first experimental run, the instruments were swapped between the left and the right side of the breathing zone and the whole activity sequence was repeated in order to check whether the positioning had an effect on the measurement results. For the third experimental run, the instruments were exchanged between the individuals. The left/right positioning on the two individuals during the third run was identical with that of the first run. For the fourth experimental run the instruments were again swapped between left and right positioning. The distribution and the positioning of the monitors are summarized in Table 2. The exact same sequence of experimental runs was carried out for both test aerosols. During sitting periods, the individuals always used the same chairs.
| 1st run | 2nd run | 3rd run | 4th run | ||
|---|---|---|---|---|---|
| Partector | Individual | 1 | 1 | 2 | 2 |
| Left | Partector 1 | Partector 2 | Partector 1 | Partector 2 | |
| Right | Partector 2 | Partector 1 | Partector 2 | Partector 1 | |
| Table | Partector 3 | Partector 3 | Partector 3 | Partector 3 | |
| miniDiSC | Individual | 2 | 2 | 1 | 1 |
| Left | miniDiSC 1 | miniDiSC 2 | miniDiSC 1 | miniDiSC 2 | |
| Right | miniDiSC 2 | miniDiSC 1 | miniDiSC 2 | miniDiSC 1 | |
| Table | miniDiSC 3 | miniDiSC 3 | miniDiSC 3 | miniDiSC 3 |
Experimental
Two different NaCl test aerosols were produced. The test aerosol with a larger modal diameter was produced using a homebuilt atomizer by dispersing a solution of 30 g NaCl in 1 L deionized (DI) water at an atomizer pressure of 2.2 bar. The NaCl aerosols were subsequently dried in a homebuilt silica gel dryer and brought to charge equilibrium with an 85Kr neutralizer (TSI model 3054A with an initial activity of 740 MBq, approximately 3 years old). Particles were neutralized to establish a well-defined charge state, which represents the typical charge state of airborne particles.36 If particles carry an (unknown) unipolar pre-charge, the charge may bias the measurement with diffusion charger based instruments.37The test aerosol with the smaller modal diameter was produced with a flame generator (FG2, MoTec Konzepte, Bochum, Germany38,39). In the flame generator, a 5 g L−1 solution of sodium chloride in DI water was dispersed into a hydrogen–oxygen flame, where the NaCl evaporated. The feed rate of the solution was adjusted to 20 mL h−1. Upon cooling downstream of the flame, the NaCl vapor supersaturated and new particles were formed by nucleation and condensation. Neutralization of the flame generated particles was not possible due to the high temperature. Previous measurements using the same setup have shown, however, that the flame generated NaCl aerosols are mostly electrically neutral.
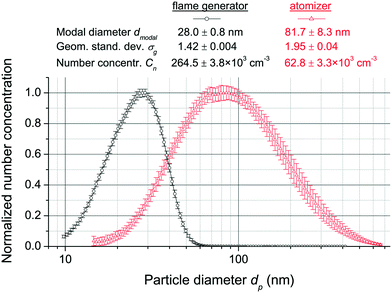
The flame generated test aerosol had a modal diameter of (28 ± 0.8) nm, a geometric standard deviation of 1.42 ± 0.004 and a number concentration of (2.65 ± 0.038) × 105 cm−3, while the atomizer generated test aerosol had a modal diameter of (81.7 ± 8.3) nm, a geometric standard deviation of 1.95 ± 0.04 and a number concentration of (6.28 ± 0.33) × 104 cm−3. The particle number size distributions, normalized with respect to the maximum concentration, are shown in Fig. 2. The associated standard deviations (averaged over 291 minutes and 257 minutes for the flame and atomizer generated aerosols, respectively) indicate the very high temporal stability of the test aerosols.
The produced test aerosols from both generators were fed into a ca. 20 m long wind tunnel with a diameter of 0.7 m, where it was mixed with approximately 1500 m3 h−1 clean dilution air. The end of the wind tunnel was connected to the chamber shown in Fig. 1. A detailed description of the setup at the nano Test Center can be found in ref. 27. Potentially harmful gases, that may for example be generated as a by-product by the flame generator, were monitored with a multi gas detector (Dräger x-am® 7000). CH4, CO, NO and NO2 levels were found to be below the detection limits of the monitor. CO2 concentrations remained constant at around 0.05% and therefore within the normal range for an indoor environment.
The test aerosol was constantly fed into the exposure chamber from one corner at an average height of approximately 1.2 m while air was extracted from the chamber from the opposite corner at an average height of approximately 0.5 m as shown in Fig. 1. While this setup assures a high spatial and temporal homogeneity of the test aerosol inside the chamber,28 it does generate a flow profile. Flow velocities were measured with a rotating vane anemometer (Airflow™ model AV-2) and are given in Fig. 1. Flow velocities within the room given in the figure were measured at the height of the instruments' sampling inlets within the breathing zone, i.e. approximately 1 m above the ground near the table (sitting subjects) and 1.5 m elsewhere (standing/walking subjects). The maximum flow velocities were 7 m s−1 near the air inlet and outlet, with quickly reducing velocities away from the inlet and outlet. The flow velocity in the centre of the chamber near the table and chairs was well below 0.5 m s−1.
Results and discussion
For the evaluation of the DiSCmini and Partector data measured with 1 s time resolution, the results from the instruments resting on the table in the centre of the chamber were used as a reference for the respective person carried instruments. Three ratios of the LDSA concentrations were calculated for each instrument type and experimental run, i.e. (1) the ratio of the personal LDSA concentrations measured left and right (black in Fig. 3 and 5, (2) the ratio of the personal concentration measured on the left and the stationary concentration measured on the table (red) and (3) the ratio of the personal concentration measured on the right and the stationary concentration measured on the table (blue). Due to the large number of data points (n ≥ 900) per experimental run, they are represented as boxplots, in which the box represents the range between the 25th and the 75th precentile with a centered line for the median. The arithmetic means are shown as square symbols. The whiskers give the range between the 5th and the 95th and the asterisks between the 1st and the 99th percentiles. Horizontal lines show the maximum and minimum ratios.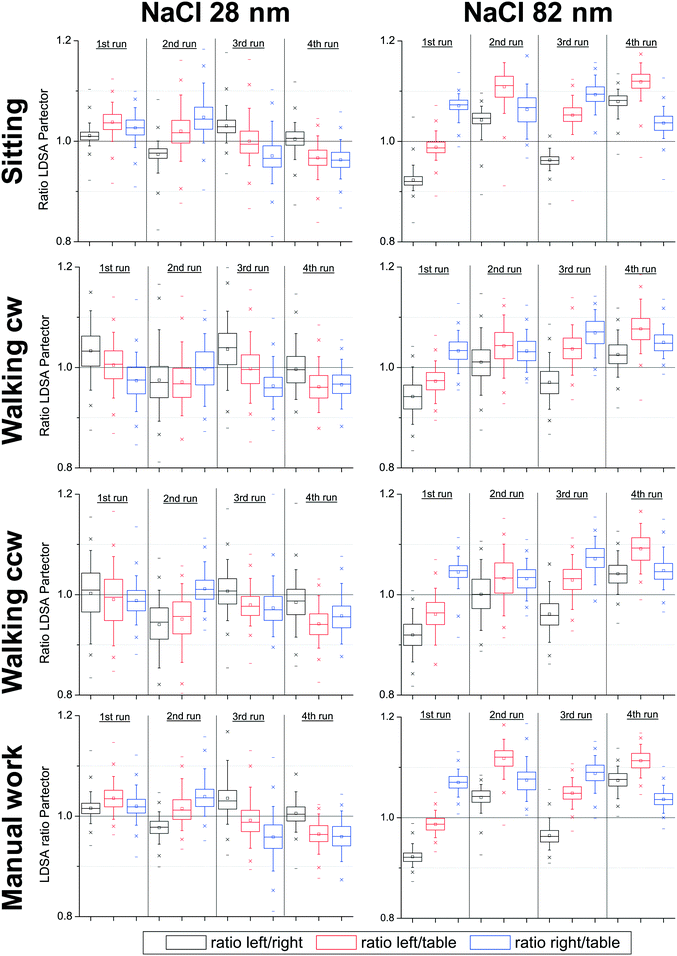 | ||
| Fig. 3 Boxplots of the ratios of the LDSA concentrations measured with Partectors on the left and right side of the breathing zone and stationary on the table in the middle of the chamber during the different activities; refer to Table 2 for the distribution of instruments. | ||
Partector results
Fig. 3 shows the boxplots for the ratios of the LDSA concentrations measured with the Partectors for both test aerosols and separately for the different activities of the two individuals within the chamber. The upper row presents the ratios of the LDSA concentrations measured with the three instruments while the individuals were sitting at the table. It can be seen that the ratios are generally within ±10%, although perhaps somewhat larger for the aerosol with the larger particle size. This agreement is better compared with the usually assumed ±30% comparability of diffusion charger instruments.27,29,40The second row in Fig. 3 shows the ratios measured while the individuals were walking clockwise within the chamber. The mean and median ratios are well within ±10% and hence comparable with the ones measured while the individuals were sitting. The scatter of the ratios is larger compared to the results when the individuals were sitting, as represented by the wider boxplots. However, the 5th and 95th percentile are still >0.85 and <1.15, respectively, i.e. within ±15%. The larger scatter is not unexpected, as a human walking within an aerosol will always induce local turbulences and hence disturb the local concentration distribution. The results obtained while the individuals were walking counterclockwise are shown in the third row of Fig. 3. The results are qualitatively and quantitatively comparable to the ones obtained while the individuals were walking clockwise, showing that the larger scatter of the data during walking in general stems from local concentration fluctuations and is induced by the personal movement itself rather than by the wind speed and wind direction.
The bottom row in Fig. 3 shows the ratios measured while the individuals were sitting at a table and carrying out manual activities (assembly, disassembly and inspection of clean MPG II dust samplers). The average ratios were very comparable with the ones measured during the first run, showing that the manual activity carried out did not noticeably affect the measurement.
In conclusion, the comparability of the average personal exposure concentrations measured in the left and the right side of the breathing zone with Partectors was very high whether the individuals were sitting or walking in either direction. The scatter increased somewhat when walking and with the larger particle size. However, all measured concentrations are well within the expected comparability range of ±30% and the deviations are hence small enough to be neglected. The results obtained with the sitting individuals show that the thermal plume around the individuals, generated by body heat, did not seem to noticeably affect the personal exposure measurement.
Comparing the different runs, there did appear to be a minor difference in measurement results between the two instruments used for personal monitoring. This can be observed by the apparent systematic difference between the ratios observed in the 1st and 3rd runs on the one hand and the 2nd and 4th runs on the other hand. The position of the instrument was changed between the runs, meaning that the same instrument was on the left side in runs 1 and 3 and on the right side during runs 2 and 4. Consequently, measurements during run 1 and run 3 as well as during run 2 and run 4 were essentially identical with the only difference being the individual wearing the instruments. When using the smaller sized NaCl aerosols the ratios were consistently higher during the 1st and 3rd runs. This was reversed when using the larger NaCl particles, with higher ratios for the 2nd and 4th runs. Since the two test aerosols were measured on two consecutive days, it is possible that the observed difference originates from a day to day variability of the measurement devices. This result is somewhat surprising, because previous studies did not show a major difference in the instrument accuracy23 and comparability40 of the Partector in this size range.
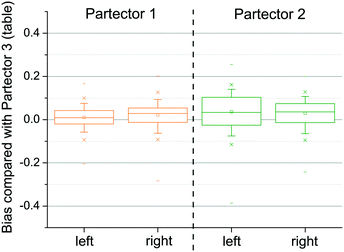
In order to better demonstrate the agreement of the concentrations, the bias of the personal and the stationary concentrations was calculated. If CP1 and CP2 are the personal concentrations measured with Partector 1 and Partector 2, respectively, and CP3 is the stationary concentration (considered as the “true” concentration) measured with Partector 3, the bias is defined as
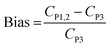 | (1) |
Bias is a measure of how close the personal concentrations CP1 and CP2 are to the stationary concentration CP3. For the analysis, all data for measurements on the left side and the right side were grouped for all activities during all experimental runs, but individually for both person carried Partectors. The results are shown in Fig. 4. The graph clearly shows that the average bias of Partector 1 and the data scatter were very small and almost identical for measurements on the left and the right side (left: 0.0099 ± 0.0421; right: 0.0215 ± 0.0487). The bias and scatter of Partector 2 were slightly larger (left: 0.0359 ± 0.0731; right: 0.0291 ± 0.0556), but still quite small.
miniDiSC results
Fig. 5 shows the boxplots of the ratios of the LDSA concentrations measured with the miniDiSCs. It is obvious that the deviations between the results are much larger (note the different and logarithmic scale in Fig. 5) than in the case of the Partectors shown in Fig. 3. In particular, the ratios of the concentrations measured with the person carried instruments and with the stationary miniDiSC deviate strongly from unity. In all cases, miniDiSC 2 was affected more strongly than miniDiSC 1 and the deviations were larger with the 28 nm aerosol than with the 82 nm aerosol. For example, during quiet sitting, the average ratios of miniDiSC 1 concentrations during all four runs with 28 nm aerosol were 0.577 ± 0.047 and 0.360 ± 0.022 for miniDiSC 2, i.e. miniDiSC 1 and miniDiSC 2 underestimated the LDSA concentration by a factor of 1.73 and 2.78, respectively. The mean ratios when using the 82 nm test aerosol were 0.670 ± 0.087 and 0.554 ± 0.077, respectively, for miniDiSC 1 and 2. The LDSA concentrations were hence underestimated by factors of 1.49 and 1.81, respectively. The average ratios are comparable for all other activities.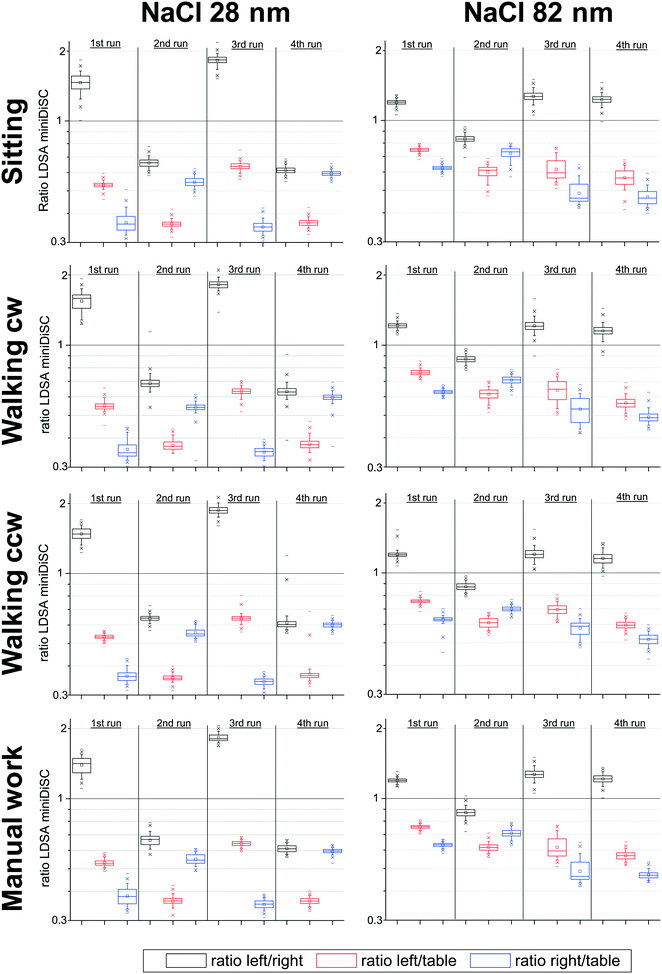 | ||
| Fig. 5 Boxplots of the ratios of the LDSA concentrations measured with miniDiSCs on the left and right side of the breathing zone and stationary on the table in the middle of the chamber during the different activities; refer to Table 2 for distribution of instruments. | ||
The LDSA concentrations dropped instantly when a sampling tube was connected and went back to normal when the tube was detached. During overnight measurements without a sampling tube, the LDSA concentrations measured with the miniDiSC and the DiSCmini agreed very well and within ±2.5%. To determine if particle losses in the flexible tubes could explain these results, the theoretical particles losses were calculated using equations provided by Gormley and Kennedy41 and Soderholm.42 The losses were estimated to be only 4.0% in terms of LDSA concentration (4.5% for number concentration) in the case of the 28 nm aerosol and 3.1% for LDSA concentration (3.2% for number concentration) for the 82 nm aerosol.
It was furthermore recognized that the corona voltage of the miniDiSC increased instantly by 10 to 15% when a tube was attached and dropped back close to its original value when the tube was detached. A literature study revealed that degassing of siloxanes is a known problem with this type of tubing.43,44 Siloxanes are organometallic molecules that can be easily ionized.45 As a result, the ion atmosphere in the unipolar diffusion charger is altered by the presence of siloxanes, resulting in a reduced charging efficiency. Consequently, the measured currents are lower than they would be in the absence of siloxanes and are hence misinterpreted as lower LDSA concentrations. The results shown in Fig. 5 are hence too strongly affected by these artifacts to be evaluated towards the effect of wearing miniDiSCs. Instead, they led to a thorough study on the effect of sampling tubes on unipolar diffusion chargers, which is published elsewhere.46 The key finding of this study was that silicone tubing should be avoided as sampling tubes for unipolar diffusion chargers, whereas Tygon® tubes are recommended, as they were found to have only a minimal effect on the measurement.
Summary and conclusions
The effect of wearing Partectors and DiSCminis for the measurement of the personal exposure to airborne nanomaterials in the breathing zone has been investigated. Two individuals were simultaneously exposed to NaCl test aerosols with modal diameters of 28 nm and 82 nm, respectively, inside a 23 m3 chamber. Each individual carried two identical instruments, one sampling from the left side, the other one from the right side in the personal breathing zone. The two individuals repeatedly carried out a sequence of different activities inside the chamber, including sitting, walking and manual activities. While the Partectors are small enough to be mounted directly in the breathing zone, the miniDiSCs were carried on a belt and sampled through 75 cm long conductive silicone tubes. The results from the Partectors, measuring on the left and right side of the breathing zone, agreed well with each other as well as with those of a third Partector placed on a table in the chamber. Average deviations between the measured concentrations were within ±10% during all activities. During walking, the scatter of the measured concentrations was marginally higher than during sitting and during manual activity; however, even during walking with headwind and tailwind, the deviations of the single data points were still within ±20%. It can hence be concluded that the Partector can be used to measure the personal exposure directly in the breathing zone with high accuracy. The measurements did not appear to be affected by the fact that the instruments were person carried. The left vs. right positioning of the instruments did not have an effect on the measurement.The DiSCmini measurement results were drastically biased, due to the use of inappropriate sampling tubes. Although the conductive silicone tubes used are usually considered as the best choice for aerosol sampling, we found that they can strongly affect the measurement with instruments based on unipolar diffusion charging due to the degassing of siloxanes. These gaseous siloxanes negatively affect the charging efficiency in the instruments and hence cause the measured concentrations to be too low. Observed discrepancies reached a factor of up to three. It is therefore recommended not to use silicone tubes along with the personal samplers, whereas Tygon® tubing currently seems to be the best compromise. Although this effect has not been directly investigated in this study, it can safely be assumed that miniDiSC monitors equipped with Tygon® tubing are applicable in personal air monitoring of nanoparticles.
Acknowledgements
The work presented here was conducted as part of the nanoIndEx project. Funding for this project was provided by the French National Funding Agency for Research (ANR), the German Federal Ministry of Education and Research (BMBF, grant no. 03X0127), the British Technology Strategy Board (TSB) and the Swiss TEMAS AG, under the frame of SIINN, the ERA-NET for a Safe Implementation of Innovative Nanoscience and Nanotechnology. The authors declare no conflict of interest relating to the material presented in this article. Its contents, including any opinions and/or conclusions expressed, are solely those of the authors.References
- M. Vance, T. Kuiken, E. Vejerano, S. McGinnis, M. Hochella Jr., D. Rejeski and M. Hull, Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory, Beilstein J. Nanotechnol., 2015, 6, 1769–1780 CrossRef CAS PubMed.
- J. Vincent, Health-related aerosol measurement: a review of existing sampling criteria and proposals for new ones, J. Environ. Monit., 2005, 7, 1037–1053 RSC.
- H.-C. Yeh and G. Schum, Models of human lung airways and their application to inhaled particle deposition, Bull. Math. Biol., 1980, 42, 461–480 CrossRef CAS PubMed.
- G. Oberdörster, Toxicology of ultrafine particles: in vivo studies, Philos. Trans. R. Soc., A, 2000, 358, 2719–2740 CrossRef.
- L. Abbott and A. Maynard, Exposure assessment approaches for engineered nanomaterials, Risk Anal., 2010, 30, 1634–1644 CrossRef PubMed.
- C. Asbach, T. Kuhlbusch, B. Stahlmecke, H. Kaminski, H. Kiesling, M. Voetz, D. Dahmann, U. Götz, N. Dziurowitz and S. Plitzko, Measurement and monitoring strategy for assessing workplace exposure to airborne nanomaterials, in Safety of nanomaterials along their lifecycle: Release, Exposure and Human Hazard, ed. W. Wohlleben, T. Kuhlbusch, C. Lehr and J. Schnekenburger, CRC Press, Taylor & Franics Group, London, 2014, pp. 233–245 Search PubMed.
- VCI, Tiered approach to an exposure measurement and assessment of nanoscale aerosols released from engineered nanomaterials in workplace operations, August 2001. [Online]. Available: https://www.vci.de/vci/downloads-vci/tiered-approach.pdf [Accessed 03 11 2015].
- M. Methner, L. Hodson and C. Geraci, Nanoparticle Emission Assessment Technique (NEAT) for the identification and Measurement of Potential Inhalation Exposure to Engineered nanomaterials – Part A, J. Occup. Environ. Hyg., 2010, 7, 127–132 CrossRef CAS PubMed.
- O. Witschger, O. Le-Bihan, M. Reynier, C. Durand and D. Charpenter, Préconisation en matière de caractérisation et d'exposition des potentiels d'emission et d'exposition professionnelle aux aerosols lors d'operations nanomateriaux, Hygiène et sécurité du travail - 1er trimestre, 2012, vol. 226, pp. 41–55, http://www.inrs.fr/media.html?refINRS=ND%202355 [accessed November 30th, 2016] Search PubMed.
- G. Ramachandran, M. Ostraat, D. Evans, M. Methner, P. O'Shaughnessy, J. D'Arcy, C. Geraci, E. Stevenson, A. Maynard and K. Rickabaugh, A strategy for assessing workplace exposures to nanomaterials, J. Occup. Environ. Hyg., 2011, 8, 673–685 CrossRef PubMed.
- OECD, Harmonized tiered approach to measure and assess the potential exposure to airborne emissions of engineered nano-objects and their agglomerates and aggregates at workplaces, Paris, 2015.
- T. Kuhlbusch, C. Asbach, H. Fissan, D. Göhler and M. Stintz, Nanoparticle Exposure at nanotechnology workplaces: A review, Part. Fibre Toxicol., 2011, 8, 22 CrossRef PubMed.
- EN, EN 1540:2012–03: Workplace Exposure - Terminology, Beuth Verlag, 2012 Search PubMed.
- nanoIndEx, Assessment of Personal Exposure to Airborne Nanomaterials - A Guidance Document, 2016, [Online]. Available: http://www.nanoindex.eu/wp-content/uploads/2016/06/Nano_Brosch%C3%BCre.pdf [Accessed 22 August 2016].
- L. Cena, T. Anthony and T. Peters, A personal nanoparticle respiratory deposition (NRD) sampler, Environ. Sci. Technol., 2011, 45, 6483–6490 CrossRef CAS PubMed.
- B. Faure, H. Dozol, C. Brouard, C. Guiot and S. Clavaguera, Evaluation of a personal sampelr for the assessment of mass-based exposure to airborne nanoparticles, J. Aerosol Sci. Search PubMed , (submitted).
- D. Leith, D. Miller-Lionberg, G. Casuccio, T. Lersch, H. Lentz, A. Marchese and J. Volckens, Development of a transfer function for a personal, thermophoretic nanoparticle sampler, Aerosol Sci. Technol., 2014, 48, 81–89 CrossRef CAS.
- M. Fierz, D. Meier, P. Steigmeier and H. Burtscher, Aerosol measurement by induced currents, Aerosol Sci. Technol., 2014, 48, 350–357 CrossRef CAS.
- M. Fierz, C. Houle, P. Steigmeier and H. Burtscher, Design, Calibration, and Field Performance of a Miniature Diffusion Size Classifier, Aerosol Sci. Technol., 2011, 45, 1–10 CrossRef CAS.
- J. Marra, M. Voetz and H. J. Kiesling, Monitor for detecting and assessing exposure to airborne nanoparticles, J. Nanopart. Res., 2010, 12, 21–37 CrossRef CAS.
- H. Fissan, S. Neumann, A. Trampe, D. Pui and W. G. Shin, Rationale and principle of an instrument measuring lung deposited nanoparticle surface area, J. Nanopart. Res., 2007, 9, 53–59 CrossRef.
- W. Shin, D. Pui, H. Fissan, S. Neumann and A. Trampe, Calibration and numerical simulation of nanoparticle surface area monitor (TSI model 3550 NSAM), J. Nanopart. Res., 2007, 9, 61–69 CrossRef CAS.
- A. Todea, S. Beckmann, H. Kaminski and C. Asbach, Accuracy of electrical aerosol sensors measuring lung deposited surface area, J. Aerosol Sci., 2015, 89, 96–109 CrossRef CAS.
- C. Asbach, H. Fissan, B. Stahlmecke, T. Kuhlbusch and D. Pui, Conceptual limitations and extensions of lung-deposited Nanoparticle Surface Area Monitor (NSAM), J. Nanopart. Res., 2009, 11, 101–109 CrossRef.
- K. Driscoll, Role of inflammation in the development of rat lung tumor in response to chronic particle exposure, Inhalation Toxicol., 1996, 8(Suppl.), 139–153 Search PubMed.
- O. Schmid and T. Stoeger, Surface area is the biologically most effective dose metric for acute nanoparticle toxicity in the lung, J. Aerosol Sci., 2016, 99, 133–143 CrossRef CAS.
- H. Kaminski, T. Kuhlbusch, S. Rath, U. Götz, M. Sprenger, D. Wels, J. Polloczek, V. Bachmann, K. H. J. Dziurowitz, A. Schwiegelshohn, C. Monz, D. Dahmann and C. Asbach, Comparability of mobility particle sizers and diffusion chargers, J. Aerosol Sci., 2013, 57, 156–178 CrossRef CAS.
- C. Asbach, H. Kaminski, D. von Barany, T. A. J. Kuhlbusch, C. Monz, N. Dziurowitz, J. Pelzer, K. Vossen, K. Berlin, S. Dietrich, U. Götz, H. J. Kiesling, R. Schierl and D. Dahmann, Comparability of Portable Nanoparticle Exposure Monitors, Ann. Occup. Hyg., 2012, 56, 606–621 CAS.
- C. Asbach, H. Kaminski, D. Dahmann, C. Monz, V. Neumann, S. Plitzko, A. Meyer-Plath, N. Dziurowitz, B. Simonow, M. Fierz and A. Todea, Genauigkeit, Vergleichbarkeit und Feldtauglichkeit personengebundener Monitore zur Bestimmung der Exposition gegenüber Nanopartikeln, Gefahrstoffe - Reinhalt. Luft, 2015, 29, 469–478 Search PubMed.
- S. Bau, B. Zimmermann, R. Payet and O. Witschger, A laboratory study of the performance of the handheld diffusion size classifier (DiSCmini) for various aerosols in the 15-400 nm range, Environ. Sci.: Processes Impacts, 2015, 17, 261–269 CAS.
- R. Meier, K. Clark and M. Riediker, Comparative testing of Miniature Diffusion Size Classifier to assess airborne ultrafine particles under field conditions, Aerosol Sci. Technol., 2013, 47, 22–28 CrossRef CAS.
- M. Salmanzadeh, G. Zahedi, G. Ahmadi, D. Marr and M. Glauser, Computational modeling of effects of thermal plume adjacent to the body on the indoor air flow and particle transport, J. Aerosol Sci., 2012, 53, 29–39 CrossRef CAS.
- P. Nilsson, S. Marini, A. Wierzbicka, M. Karedal, E. Blomgren, J. Nielsen, G. Buonanno and A. Gudmundsson, Characterization of Hairdresser Exposure to Airborne Particles during Hair Bleaching, Ann. Occup. Hyg., 2016, 60, 90–100 Search PubMed.
- S. Wang and R. Flagan, Scanning Mobility Particle Sizer, Aerosol Sci. Technol., 1990, 13, 230–240 CrossRef CAS.
- H.-D. Bauer and E. Bruckmann, Neues gravimetrisches Staubsammelgerät zur Messung des atembaren Feinstaubes nach MAK-Werten, Kompaß, 1974, 84, 96–102 Search PubMed.
- W. Hinds, Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles, Wiley, New York, 1999 Search PubMed.
- C. Qi, C. Asbach, W. Shin, H. Fissan and D. Pui, The effect of particle pre-existing charge on unipolar charging and it implication on electrical aerosol measurements, Aerosol Sci. Technol., 2009, 43, 232–240 CrossRef CAS.
- C. Monsé, C. Monz, D. Dahmann, C. Asbach, B. Stahlmecke, N. Lichtenstein, K.-E. Buchwald, R. Merget, J. Bünger and T. Brüning, Vorbereitung zur Untersuchung gesundheitlicher Effekte von Zinkoxidpartikeln, Gefahrstoffe - Reinhalt. Luft, 2013, 73, 144–148 Search PubMed.
- C. Monsé, C. Monz, D. Dahmann, C. Asbach, B. Stahlmecke, N. Lichtenstein, K.-E. Buchwald, R. Merget, J. Bünger and T. Brüning, Development and Evaluation of a Nanoparticle Generator for Human Inhalation Studies with Airborne Zinc Oxide, Aerosol Sci. Technol., 2014, 48, 418–426 CrossRef.
- A. Todea, S. Beckmann, H. Kaminski, C. Monz, D. Dahmann, V. Neumann, B. Simonow, N. Dziurowitz, A. Meyer-Plath, M. Fierz, H. Dozol, S. Clavaguera, G. Lidén, I. Tuinman, J. Pelzer, D. Bard, P. Thali, S. Bau, A. van der Vleuten, H. Vroomen and C. Asbach, Inter-comparison or personal monitors for nanoparticle exposure at workplaces and in the environment, Sci. Total Environ., 2016 Search PubMed , (submitted).
- P. Gormley and M. Kennedy, Diffusion from a stream flowing through a cylindrical tube, Proc. R. Ir. Acad., Sect. A, 1949, 52, 163–169 Search PubMed.
- S. Soderholm, Analysis of diffusion battery data, J. Aerosol Sci., 1979, 10, 163–175 CrossRef.
- M. Timko, Z. Yu, J. Kroll, J. Jayne, D. Worsnop, R. Miake-Lye, T. Onasch, D. Liscinsky, T. Kirchstetter, H. Destaillats, A. Holder, J. Smith and K. Wilson, Sampling artifacts from conducitve silicone tubing, Aerosol Sci. Technol., 2009, 43, 855–865 CrossRef CAS.
- Y. Yu, M. Alexander, V. Perraud, E. Brins, S. Johnson, M. Ezell and B. Finlayson-Pitts, Contamination from electrically conductive silicone tubing during aerosol chemical analysis, Atmos. Environ., 2009, 43, 2836–2839 CrossRef CAS.
- A. Maißer, J. Thomas, C. Larriba-Andaluz, S. He and C. Hogan, The mass-mobility distributions of ions produced by a Po-210 source in air, J. Aerosol Sci., 2015, 90, 36–50 CrossRef.
- C. Asbach, H. Kaminski, Y. Lamboy, U. Schneiderwind, M. Fierz and A. Todea, Silicone sampling tubes can cause drastic artifacts in measurements with aerosol instrumentation based on unipolar diffusion charging, Aerosol Sci. Technol., 2016, 50, 1375–1384 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |