Comparative cradle-to-gate energy assessment of indium phosphide and cadmium selenide quantum dot displays
Shauhrat S.
Chopra
 * and
Thomas L.
Theis
* and
Thomas L.
Theis
Institute for Environmental Science and Policy, University of Illinois at Chicago, 2121 West Taylor SPHW (MC 673), Chicago, IL 60612, USA. E-mail: chopras@uic.edu; Fax: +1 312 3550760; Tel: +1 312 9527622
First published on 7th November 2016
Abstract
Quantum dots (QDs) are semiconductor nanocrystals (2–10 nm) with tunable band gaps and desirable luminescence properties. QDs have created great interest in consumer electronics, photovoltaics, and chemical and/or biological sensor applications. For example, QDs absorb short wavelength light and emit light with a desired wavelength, making them ideal for LCD displays. QDs are just beginning to be used in mass-produced displays, including products such as televisions, tablets, and smartphones. The most widely applied QD composition—a CdSe core metalloid complex (CdQD)—faces increasing environmental and regulatory scrutiny because cadmium and selenium nanomaterials pose uncertain human health and environmental risks. As a result, some manufacturers have introduced InP-based (InQD) displays, which have been shown to be less toxic than CdQDs. However, instead of basing environmental decisions on material hazard alone, it is important to take a more holistic approach to avoid unintended consequences. This paper presents a comparative cradle-to-gate life cycle environmental assessment of CdQD- and InQD-enabled displays. In this study, we compare the cumulative energy demand (CED), also referred to as the primary energy consumption, of colloidally synthesized CdSe and InP multishell QDs, and their subsequent incorporation in different display types. Additionally, we compare the CED for the two competing commercially available QD display technologies: on-surface and on-edge. Lastly, we estimate the energy repercussion of large-scale adoption of each of the production pathways for QD display manufacture. The results show that InP QD-enabled displays are far more energy intensive than the CdSe QD displays, and this difference becomes further amplified when large-scale adoption is considered. Our study highlights that life cycle thinking is essential for recognizing opportunities to advance QD production along environmentally sustainable pathways, which is critical information for researchers, QD display manufacturers, and regulatory agencies.
Environmental significanceCadmium-based QDs represent the current state of the art for commercial quantum dot (QD)-enabled display; however their toxicity has driven the commercialization of comparable non-heavy metal alternatives. Rather than basing decisions only on material hazard, a systematic approach considering various life cycle stages of QD enabled-displays is necessary to avoid unintended shifting of environmental burden. We perform a comparative cradle-to-gate life cycle energy assessment between CdSe and InP QDs enabled-displays. We consider the synthesis of competing QD materials, deployment of competing QD embedding technologies, production of different display device types, and the implication of their large-scale adoption. The results highlight the importance of QD synthesis and product assembly life cycle stages in the creation of the environmentally preferable QD display. |
1. Introduction
Quantum dots (QDs) are semiconductor nanomaterials (2–10 nm) that exhibit unique optical and electrical properties that have been known for decades.1,2 The advent of colloidal core–shell QDs, multicomponent nanocrystals capped with surfactant molecules and dispersed in solution, and solution-processed optoelectronic device structures, has allowed development of promising QD applications that include lighting, displays, solar cells, photocatalysis, photodetectors, lasers, bioimaging, and diagnosis.3–6 Among these, QD-enabled lighting and display products are already commercially available, and are expected to drive the demand for emerging nanomaterials in the near future. As per latest research estimates, the market for QD displays is projected to have a compound annual growth rate (CAGR) of around 64% for the period of 2016–2021.7 QD displays are expected to overtake the organic LED market; prominent display manufacturers like Samsung have already begun this transition.4,8QDs are capable of fine-tuning photoluminescence and electroluminescence emissions over a wider energy range in comparison to similar bulk semiconductor materials. This tunable narrow emission width from QDs is due to the quantum confinement effect exhibited at the nano-scale.9 By controlling the average size and size distribution of nanocrystals, researchers and nanomaterial manufacturers can produce QDs that emit a particular wavelength of light. The smaller the nanoparticle, the smaller the emitted photoluminescence wavelength: for example, smaller dots appear blue while larger ones emit red. This feature makes them an ideal candidate for display technology as such displays not only have high color accuracy, which is the ability to produce precise colored lights, but also exhibit wider color gamut, which means these nanocrystals emit a wider array of more saturated colors.10 Another advantage of utilizing QDs for displays is their compatibility with the existing Liquid Crystal Display (LCD) technology. QDs embedded in LCDs are excited by blue-emitting backlight Light Emitting Diodes (LEDs), which in turn convert the blue spectrum to sharp green and red peaks through photoluminescence.11 These peaks can then be clearly separated by commonly used color filters, which significantly enhances display efficiency as well as color gamut in comparison to a traditional LCD display.12
In addition to the influence of particle size on QD properties and performance, the semiconductor alloy composition of a QD core and shell also plays a role.13 Tuning the composition of the core and adding shells of semiconducting alloy materials with different band gaps are known to improve brightness of QD materials and energy efficiency of displays incorporating them.14 For instance, the brightness of light-emitted by CdSe/ZnS (core–shell) QD may differ from that produced by CdSe/ZnSSe or InP/ZnS core–shell QDs of the same size. As the number of products using QD display technology in the market and their adoption is increasing, the possibility exists that considerable amounts of QDs will be produced and eventually released into the environment. Even though QD-enabled displays are more resource efficient than the previous display technologies, like cathode ray tube displays, it is critical to understand environmental and toxicity tradeoffs of QD materials with differing compositions in order to identify the preferable option for incorporation in next-generation displays.
QD nanocrystals are generally composed of group II–VI (zinc sulfide (ZnS), zinc selenide (ZnSe), cadmium selenide (CdSe), and cadmium telluride (CdTe) cores) or III–V (indium phosphide (InP), indium arsenide (InAs), gallium arsenide (GaAs) and gallium nitride (GaN) cores) semiconductor compounds. Cadmium-based QDs (CdQDs) have played a major role in the advancement of nano-enabled display technologies.4 In particular, CdSe QDs, the first to be synthesized through the colloidal approach, are the most widely used constituent nanomaterial for QD displays since their emission can be tuned throughout the visible spectrum.15 For this reason, CdSe QDs are the most efficient down-converting material (i.e. medium that tunes high frequency light to low frequency) in terms of energy efficiency, stability, and broad-spectrum color performance used by display manufacturers.16 While QDs are known to be cytotoxic due to the production of reactive oxygen species that cause DNA fragmentation, mitochondrial malfunction and neurodegeneration,17 the toxicity of CdSe QDs is especially concerning because cadmium is a poisonous metal.18,19 Dissolution of a CdSe core is particularly toxic since the Cd(II) ion is a probable carcinogen, and is known to bioaccumulate in humans.20 It can cross the blood–brain barrier and placenta, distribute to all bodily tissues, with liver and kidney being target organs of toxicity, and has a biological half-life of 15–20 years.21 Furthermore, selenium is also known to have considerable human health and environmental concerns. For example, the role of elevated environmental concentrations of selenium on ecosystem destruction of the Kesterson Reservoir, California, and Belews Lake, North Carolina is well documented.22
Given the danger of acute and chronic toxicities associated with CdSe QDs, there has been an incentive to create non-toxic metal QDs with relatively lower toxicity but high photoluminescence properties for application in displays. Recently, the use of CuInS2/ZnS, ZnSe/ZnS, ZnS (doped Mn), Si, InP-based QDs in displays has become an active area of reasearch.16,23–27 However, in comparison to CdSe QDs, they are characterized by greatly reduced color accuracy and efficiency. It is challenging to synthesize high quality Cd-free QDs with controlled nanoparticle size and size distribution for facile color tuning and high color saturation.28 Among the various Cd-free QD types being developed for display technologies, InP-based QDs are emerging as the most viable alternative.24,29 Nanosys, a QD producer, and Samsung, an electronics manufacturer, have partnered to create InP QD-enabled displays that are the only Cd-free option that is presently commercially available.30 Despite their low photoluminescence performance, in vitro and in vivo toxicity tests have revealed that InP QDs are considerably less toxic than Cd-containing QDs.18 This study showed that although the amounts of In(III) ions and Cd(II) ions released from the QD core were comparable, the lower toxicity of indium made the InP-based QDs inherently less toxic.
Until 2014, CdQD-enabled displays were exempt from the European Union's (EU) regulation on cadmium in consumer products under the Restriction of Hazardous Substances (RoHS) and Registration, Evaluation, Authorisation and Restriction of Chemical substances (REACH) guidelines. However, later, the EU parliament restricted the use of CdQDs and directed the commission to reevaluate the exemption of CdQDs.31 Instead of basing environmental decisions on material hazard alone, a systematic environmental assessment between the latest CdQDs and Cd-free QDs display technologies is required to avoid any unintended consequences. Especially since resource efficiency in terms energy and material consumption for synthesizing QD materials composed of different semiconductor compounds may differ significantly.
Life Cycle Assessment (LCA) is the most extensively developed and standardized methodology for assessing the possible environmental and human health impacts throughout a product's life cycle, i.e. from raw materials, manufacturing, assembly, distribution, use, and final disposition.32 This methodology has been frequently adopted for development and evaluation of emerging technologies and products.33–35 However, the life cycle paradigm has been infrequently used for assessing environmental emissions associated with nanoparticles.36–38 Since impacts for any given ENM is a function of the application and the product it is incorporated in, lately, studies are moving beyond impact assessment on material basis for comprehensive investigation of nano-enabled applications.33,39–41 In the case of QDs, only a handful of studies have adopted such a holistic approach for identifying materials, processes, or components that contribute the most to environmental impacts.42–45 These studies were a good first step as they focused on a single QD material type, but more studies are needed to understand the nature of tradeoffs associated with CdQDs and Cd-free QD material options for applications.
In addition to understand the implications of incorporating one QD material over another, the choice of a technique of embedding them in displays may be a critical point for decision-making. Since QDs are incorporated in the LCD assembly either in a diffuser film (on-surface technology) or in a glass capillary at the display edge (on-edge), the amount of QD material required for a display varies.46 While the difference between the two technologies might be insignificant at the lab-scale, large-scale production and subsequent widespread adoption of QD displays in diverse product types can considerably amplify the environmental inefficiencies. The majority of studies exploring the life cycle impacts of emerging nanotechnologies exclude analysis of implications of large-scale production. In order to bridge the above-mentioned gaps in the literature, we undertake a comparative, prospective cradle-to-gate life cycle assessment of QD display technologies with different nanomaterials, various designs, and in diverse device types.
The overall goal of this research is to compare alternative production pathways for QD displays and identify opportunities for reducing the life cycle energy demand from their manufacture. There are four specific objectives: 1) to quantify the life cycle energetics of colloidal synthesis of CdSe and InP multishells QDs; 2) to explore energy implications of embedding QDs in different display types such as TVs, monitors, notebooks, tablets, and smartphones; 3) to compare the cradle-to-gate energy demand for the two competing QD display technologies that are commercially available, i.e. on-surface and on-edge technologies; and 4) to estimate the energy repercussion of large-scale adoption of each of the production pathways for QD display manufacture. This work is of interest to researchers and companies involved in production of QDs and development of QD displays. Additionally, since investments in nanomanufacturing technologies involve significant risks, findings from this study will support decision-making for early adopters.47 Lastly, with the growing concern for environmental impacts of emerging nano-enabled products, this study may also interest a wider audience, particularly policy makers.
The rest of the paper is organized in four sections. Section 2 presents the methodology adopted to compare impacts associated with alternative QD display manufacture pathways. Section 3 contains the results and discusses the implications of the findings for various stakeholders. Section 4 summarizes the study, discloses limitations of the analysis at hand, and presents a plan for future work.
2. Methods and materials
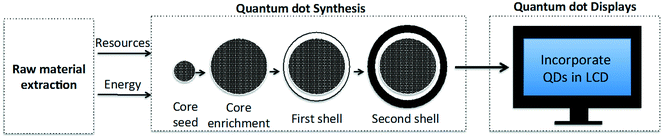
We apply LCA in order to compare the environmental impacts for utilizing CdSe and InP QDs in the manufacture of QD-enabled displays. The scope of this LCA is cradle-to-gate (Fig. 1), as we aim to use this analysis to compare the environmental performance of alternate QD material production and the devices in which these nanomaterials may be subsequently used. It must be noted that this analysis neither takes into account the use and the end-of-life phases of a display device's life cycle, nor attempts to characterize human and ecotoxicity of different QDs. To address these concerns we plan to complement this work with gate-to-grave analysis in the future. LCA quantifies environmental impacts in terms of impact categories for a functional unit. Descriptions of the impact category and the functional unit chosen for our cradle-to-gate analysis are presented next.2.1. Impact category
In consideration of the dynamically changing landscape of the energy sector in the US, cumulative energy demand (CED), also referred to as the primary energy consumption, was chosen as an impact category in this study. CED is known to be a good overall indicator for other environmental impact categories.48,49 We compute the life cycle energy use associated with the synthesis of CdSe and InP QDs, manufacture of different QD-enabled displays made of competing technologies, and large-scale adoption of a QD display technology for each device market segment.2.2. Functional unit
Three different functional units are chosen for the different stages of the manufacturing of QD-enabled displays. First, for the initial energy assessment of colloidal synthesis of red and green QD types, our functional unit is 25 mg of QD core/shell/shell material. Second, for comparing the impacts of CdSe and InP QD enabled-displays in different device types (TVs, monitors, laptops, tablets and smartphones), the chosen functional unit is mg of QDs embedded per mm2 of display area. This functional unit allows us to meet the goal of this analysis, because the amount of QD material embedded in a display varies depending on several factors: the type of QDs, the technology of incorporating the QDs in displays, as well as the type and the size of a display device. Third, for comparing the energy consumption attributed to QDs for large-scale adoption of QD-enabled displays of different types, we choose the entire market segment as the functional unit (considering the annual production of the consumer display devices).2.3. Data sources and sensitivity
In order to perform a cradle-to-gate life cycle energy comparison between the two QD types, we obtained process data for state-of-the-art colloidal synthesis of CdSe and InP-multishell QDs from the most recent patent literature: patents by QD Vision Inc. were used as the main data sources for the CdSe QD synthesis system, whereas patents from Nanosys Inc. were the data source for InP QD synthesis system.50–56 The patent literature is the most detailed source of data available on large-scale production of quantum dots, especially for display application, since patents contain production processes that are believed to be technically feasible and considered to have economic value.57 Data for modeling QDs embedded in various display devices and comparing competing QD display technologies were taken from documents submitted by multiple manufacturers as part of a request for exemption of cadmium QD technology from the European Union Restriction of Hazardous Substances (RoHS) directive.58Comprehensive LCA database Ecoinvent version 3.0 was used as the source for life cycle inventory data for established products in the analysis.59 Since process data for the production of proprietary products and chemicals are not available in Ecoinvent, stoichiometric reaction equations are employed for LCI (Life Cycle Inventory) compilation. For raw materials whose stoichiometry of the synthesis process was unknown, we used estimates of environmental impacts (specifically cumulative energy demand) from tools based on their molecular structure.60,61 Since this analysis is intended for comparative purposes, we estimate the energy requirement for each step in the synthesis process for both CdSe and InP QDs according to the following equation:
| E = m × c × ΔT | (1) |
Details of the inventory data for CdSe and InP mutishell QD synthesis are provided in the ESI.64
2.4. Description of colloidal synthesis of competing QD types for displays: CdSe and InP QDs
A set synthesis process is used to create QDs with a particular composition, structure, and size in order to achieve the desired wavelength of light (∼600 nm for red and ∼450 nm for green) on being stimulated by an excitation source. This process information provided in the patent literature establishes the reaction yield for QD production. While the reaction stoichiometry for creating red and green-emitting QDs may differ, the steps associated with the colloidal synthesis process are quite similar. In general, the methodology for synthesizing CdSe and InP multishell QDs can be divided into three generalizable stages: the process begins with the synthesis of the core seed, followed by growing the core to the desired size, and finally the core is coated with shells. In the interest of space, a detailed description for the colloidal synthesis of CdSe/ZnS/CdZnS and InP/ZnSSe/ZnS QDs is provided in the ESI.2.5. Description of competing technology pathways for manufacturing QD displays
There are two modes to utilize QDs in displays: first, quantum-dot light emitting diodes (QLEDs) that exploit electroluminescence properties, and second, LCD backlight units (BLUs) with photoluminescence QDs. While the former approach is still in the early stages of research and development, straightforward incorporation of QDs into existing LCD manufacturing based on the latter approach has allowed rapid commercialization of displays.46 For this reason, we compare the implications of utilizing two competing LCD compatible QD technologies: 1) on-edge technology, and 2) on-surface technology. On-edge technology, also known as edge-lit technology, developed by QD Vision Inc., packages red and green QDs in a glass tube that mounts on the edge of the display. On-surface technology, or quantum dot enhanced film (QDEF) technology by Nanosys, involves an optically clear sheet with red and green QDs placed on top of backlight blue GaN LED. Although both these technologies can be found in display devices currently available in the market, the on-edge is known to utilize considerably less QD material than the QD film alternative. As per information provided by display manufacturers in their request for exemption from ROHS, the QD film alternative requires 40 times more QD material than on-edge displays of the same size.58The size of the displays also influences the amount of QD material embedded in the device. For this reason, it is critical to compare energy demand attributed to QDs in different display types. This study considers a wide range of consumer devices, including TVs, PC monitors, notebooks, tablets, and smartphones, that may utilize these two QD display technologies in the coming years. Moreover, we compare the environmental implications of large-scale adoption of alternate pathways for producing QD-enabled displays for consumer devices. While the difference between competing technologies or devices might be insignificant at the lab-scale or product-scale, large-scale production and subsequent widespread adoption of QD displays in diverse product types can considerably amplify the environmental inefficiencies. To quantify the implications of widespread adoption of QD-enabled display devices, probable consumption trajectories will be followed as QD-enabled displays continue to enter markets. Rather than focusing on modeling market demand for QD-enabled display technology, data on consumption of different consumer electronics that employ QD display technologies by market research firms and industry surveys will be used for scenario development.65 Additionally, since this analysis is meant to inform comparisons, the 35% adoption rate of QD-enabled displays is assumed for each of the display device markets. This information will help early stage manufacturers identify the overall trend in energy demand for each device market segment, and may inform regulatory benchmarking for alternate QD materials for manufacturing different device types.
The LCA results for primary energy consumption for QD synthesis presented in this study provide a conservative benchmark for comparing environmental implication of substituting Cd-based (CdSe) cores with Cd-free (InP in particular) variant in emerging QD displays. Moreover, the LCI generated from this study, especially regarding colloidal synthesis of QDs, can be adapted to analyze environmental profile of other emerging applications of QDs.
3. Results and discussion
The results are organized such that each sub-section focuses on one of the four research objectives stated in the introduction section.3.1. Colloidal synthesis of QDs
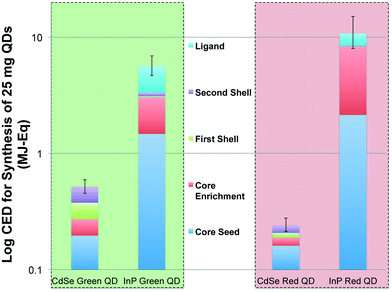
The primary energy consumption (in MJ-Eq.) associated with the colloidal synthesis of 25 mg of CdSe and InP QDs for next-generation display technology is presented in Fig. 2. It is clear that production of multishell InP QDs is more energy intensive in comparison to CdSe QDs. Based on the process flow modeling of QD synthesis, we estimate syntheses of both green and red-emitting QDs composed of CdSe cores (0.52 and 0.21 MJ-Eq. mean CED, respectively) to have a considerably lower cumulative energy demand in comparison to green and red-emitting QDs made of InP cores (5.67 and 10.78 MJ-Eq. mean CED, respectively). Uncertainty at the life cycle scale was captured through Monte Carlo simulations that randomly sample 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times from statistical distributions for each inventory item. The error bars represent the 2.5th and 97.5th percentile of CED for QD synthesis.
000 times from statistical distributions for each inventory item. The error bars represent the 2.5th and 97.5th percentile of CED for QD synthesis.
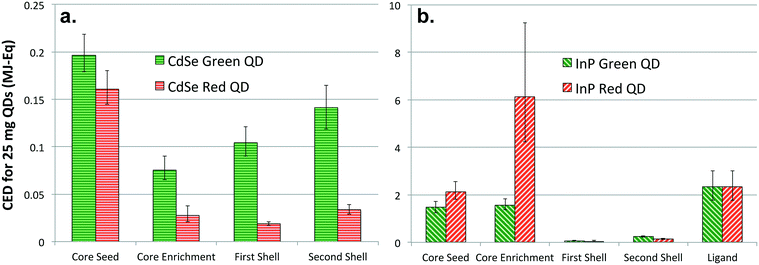
Fig. 3 presents CED results for each step of the colloidal synthesis process. It is important to compare the primary energy consumption at each step of red and green emitting CdSe and InP QD synthesis for identifying stages with higher energy and material use. While the first step of core seed generation is the most energy intensive process in CdSe-multishell QD synthesis, the core enrichment step is responsible for highest primary energy consumption for InP QD synthesis. Additionally, Fig. 3 highlights that synthesis of green-emitting CdSe QD cores, i.e. both core seed generation and core enrichment steps, has a greater primary energy consumption than the larger, red-emitting form. Interestingly, the opposite trend is observed for InP QD core synthesis, with higher CED for production of larger, red-emitting QD cores than smaller, green-emitting QDs. Augmentation of CdSe and InP QD cores with multiple shells exhibit similar trends. The process of addition of the first shell has a higher CED than the second shell, while the shelling process for green-emitting QDs having more primary energy than red-emitting QDs, irrespective of their composition. In addition, it is important to note that InP multishell QD synthesis requires an additional step of ligand attachment to the surface of the QD in order to have a performance more comparable to CdSe-based QDs.
From our evaluation of primary energy consumption for synthesis of QDs, it is apparent that high performance InP QD production for display technology continues to be more energy intensive despite the growing interest in recent times. The synthesis process for CdSe QDs has continually matured since its inception in the 1980s.1,2 Scrutiny of results for the QD fabrication process highlights relatively lower material efficiency for InP QD synthesis in comparison to the refined process of CdSe QD. InP QD synthesis requires more solvents and specialized chemicals that tend to be energy intensive. The embodied energy of the indium precursor (In(III)acetate) and phosphine precursor (tris(tri)methylsilyl phosphine) utilized for InP core generation is far greater than the cadmium precursor (Cd(II) acetate or oleate) and selenium precursor (DIBP-Se) employed in CdSe QD synthesis. Lastly, evident from the inventory presented in the SI, energy inputs associated with the InP QD synthesis technique are also considerably higher (9 MJ for 25 mg of green InP QDs in comparison to 0.7 MJ for synthesis of the same mass of green CdSe QDs). This can be partly attributed to the extra post-processing step involving ligand addition, required optimal performance of displays. Considering the growing demand of cadmium-free QDs for displays, it would be useful to establish lower energy techniques with higher material efficiencies and throughputs for InP QD production to stay competitive and relevant.
In addition, it should be noted that while nanofabrication of green-emitting CdSe QD cores is more energy consuming than red-emitting QD cores, the opposite is the case of green and red-emitting InP QDs. We speculate that there might be a connection between the color accuracy of QDs and the primary energy consumption for QD synthesis. Color accuracy, measured by FWHM, is a function of the size distribution of QDs generated. A narrow size distribution yields greater color accuracy and wider color gamut, which is the main advantage along with reduced energy consumption. Production of certain nanoparticles with narrow size distribution is known to require advanced techniques and additional resources.66 These supplementary steps in nanomaterial production may require additional material and energy inputs. A similar trend exists in the case for QDs, where the cumulative energy demand is higher for synthesizing a batch of nanocrystals with smaller FWHM. Red-emitting CdSe QDs analyzed in this study have a FWHM of 28 nm, and the mean CED associated with synthesis was 0.52 MJ. On the contrary, green-emitting CdSe QDs whose FWHM is 35 nm has a mean CED of 0.21 MJ. The same trend was noticed between FWHM and CED associated with the nanofabrication of InP QDs.
3.2. QD displays in electronic devices
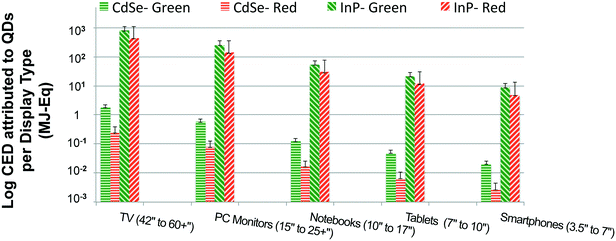
Fig. 4 presents the estimates for primary energy consumption attributed to incorporation of QDs for production of one consumer device with display (calculated in terms of mg of QD per mm2 of display area) such as TVs, monitors, notebooks, tablets and smartphones. It is the ratio of green to red-emitting QDs embedded in a display that is responsible for the desired color gamut. The green to red ratio is in the range of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the literature,51 which was used to develop a Monte-Carlo simulation (uniform distribution within the range) to estimate the quantity of green and red-emitting QDs embedded for each display type. While it is clear from Fig. 2 that syntheses of InP QDs have a higher CED than the cadmium-based alternate, this difference is further amplified when these QDs are embedded in displays as shown in Fig. 4. Lower performance of InP QDs in comparison with CdSe QDs as measured by color accuracy and quantum yield means greater amounts of the former are needed for a comparable picture quality. For our analysis, we assume that 40 times more InP QD is needed for a given amount of CdSe QD, a conservative estimate compared to the 400
1 in the literature,51 which was used to develop a Monte-Carlo simulation (uniform distribution within the range) to estimate the quantity of green and red-emitting QDs embedded for each display type. While it is clear from Fig. 2 that syntheses of InP QDs have a higher CED than the cadmium-based alternate, this difference is further amplified when these QDs are embedded in displays as shown in Fig. 4. Lower performance of InP QDs in comparison with CdSe QDs as measured by color accuracy and quantum yield means greater amounts of the former are needed for a comparable picture quality. For our analysis, we assume that 40 times more InP QD is needed for a given amount of CdSe QD, a conservative estimate compared to the 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio for InP QD to CdSe QD provided by QD display manufacturers themselves to the EU commission.58 Given the impact of the difference in QD performance on the overall primary energy consumption for QD displays, it is not only important to reduce the CED for syntheses of InP QDs, but also to improve their luminescent properties.
1 ratio for InP QD to CdSe QD provided by QD display manufacturers themselves to the EU commission.58 Given the impact of the difference in QD performance on the overall primary energy consumption for QD displays, it is not only important to reduce the CED for syntheses of InP QDs, but also to improve their luminescent properties.
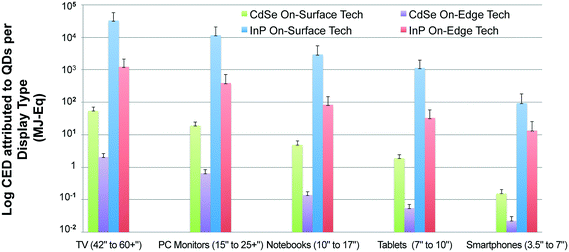
Additionally, Fig. 4 reveals that display types with larger screen sizes tend to have a substantially higher embodied energy, which is expected since they require more QD material. This trend was noticed for both InP and CdSe QD-enabled displays devices from a cradle-to-gate perspective. However, we might not observe the same trend for all display device types when the scope of our analysis is extended to include the use and the disposal phase of product's life cycle. Also, the results presented in Fig. 4 plot the primary energy consumption for displays types using on-edge QD display technology. A similar trend is noted for devices that use on-surface QD technology (presented in the ESI). The embodied energy implication for choosing one display technology over the other is discussed in the following section in more detail.
3.3. QD display technologies
Fig. 5 compares the cradle-to-gate CED for two competing commercially available QD display technologies-on-surface and on-edge. The functional unit here is a single display, which is expressed in terms of mg of QD per mm2 of display area. Based on the results, we observe that the CED for a display type that uses on-surface technology is about three orders of magnitude larger than the alternate on-edge technology. This is primarily because the on-edge display technology requires only a thin tube coated with quantum dots in comparison to the sheet of quantum dots needed to cover the entire display area for on-surface technology.58 For this reason, while the need for quantum dots increases proportionally to the perimeter of the on-edge technology-based displays, the amount of quantum dots required for on-surface technology increases with area Fig. 5 presents a comparison of primary energy consumption for incorporating CdSe and InP QDs in competing display technologies. As expected, the cradle-to-gate life cycle energy required for production of a CdSe QD-enabled on-edge display is consistently lower than for an InP QD-enabled on-surface display.3.4. Large-scale adoption of QD displays
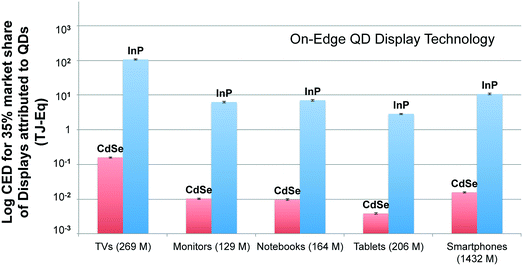
Fig. 6 compares the CED associated with market segment of each display type employing on-edge QD display technology for understanding the implications of their large-scale adoption. The result presented here compares the production of QD displays using CdSe and InP QDs as well. The functional unit for this analysis is market segment of each display types. Market segment is quantified in terms of the number of units shipped worldwide for each display type in 2015. For each display type, we consider the breakdown of units sold of different display sizes.65 The number of units shipped in 2015 for each display type is given in parenthesis next to the labels on the x-axis. The analysis computes the primary energy consumption for a scenario where 35% market share captured by QD displays is assumed. Fig. 6 shows that large-scale adoption of QD technology in the television market will have significant energy implications (106.5 and 0.16 TJ-Eq. using InP and CdSe QD, respectively). This finding is quite expected, as we know that TVs incorporate the largest quantities of QDs per device. However, it is surprising to see the smartphone market segment emerge as the second largest consumer of primary energy, instead of the monitor and notebook segments that have larger displays. This is due to the sheer number of smartphone devices produced, which is more than all other display devices put together.It is interesting to note that CED results for large-scale adoption of display devices employing on-surface technology (presented in ESI) are notably different from the findings from Fig. 6. On-edge technology based displays for the smartphone segment have the greatest CED after the television market segment despite having the smallest screen size. However, in the case of QD displays using on-surface technology, smartphone segment has the least CED among other market segments. The primary reason for divergence between CED for large-scale adoption of QD displays using the two technologies is the unequal amount of QDs incorporated into the same device type. It is known that while the amount of QDs used scale linearly for QD displays using on-edge technology, the quantity of QD increase exponentially with size for displays employing on-surface technology. This is the reason behind the varying CED trends observed for competing technologies despite the number of displays produced for each market segment are kept constant. It highlights that energy prudence of using one technology over the other is related to the display size: while there may not be much difference in the primary energy implications of using either on-edge or on-surface technologies of smaller display devices, on-edge technology is more suited for larger displays from an energy perspective. In addition, these results also reiterate what was noted previously, the adoption of on-edge technology is considerably less life cycle energy intensive than on-surface technology using either InP or CdSe-based QDs.
4. Conclusions
A prospective cradle-to-gate environmental assessment of state-of-the-art nanofabrication of CdSe QD and its most eligible substitute InP QD for application in displays is presented in this article. This comparative assessment in particular estimated primary energy consumption of competing QD synthesis pathways, commercially available competing QD display technologies, different display types, and their subsequent large-scale production. We found that CdSe QD synthesis and its incorporation in consumer display devices has a lower CED in comparison to the Indium-based alternative. Also, the choice of technology for QD incorporation into displays contributes substantially to the overall embodied energy of the device. Employing Cd-free QDs in displays is a promising solution for creating less toxic nano-enabled products, but is not an environmentally preferable option from a cradle-to-gate life cycle perspective considering their current synthesis efficiencies. There is a need to continue to advance synthesis techniques for producing high-performing QD material composed of elements with low toxicity and low cradle-to-gate primary energy consumption. From our analysis of QD enabled-display devices, it is clear that one must deliberate on both QD synthesis and product assembly life cycle stages in order to create the environmentally preferable product.Such an analysis is particularly useful for QD display manufacturers and researchers working on QD synthesis routes as it provides them with information regarding the environmental burdens of this emerging technology. The traditional approach to enhance environmental sustainability for nanomaterial production tends to focus on reducing toxic chemicals and increasing the use of renewable inputs. However, such an approach fails to provide QD manufacturers with comprehensive solutions to sustainable production of QDs and the products in which they are used. Our results show that life cycle thinking is essential for recognizing opportunities to advance QD production along environmentally sustainable pathways.
Material criticality and scarcity is not discussed in great detail in this study. The National Research Council (NRC) report on elemental scarcity identified indium as one of the critical metals limited by natural abundance and/or accessibility due to geopolitical circumstances.67 While cadmium did not make the same list, both metals are by-products of zinc production, implying that they have the same inherent supply risk. Cadmium concentration in Zn ore ranges from 200 to 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm, and indium content of Zn ore deposits range from 100 to 2730 ppm.68,69 Critics of In-based QD displays often cite rarity of indium as one of the major constraints to large-scale production, however as illustrated in this study, the “scarcity” of indium may have more to do with its higher material requirements (due to InP QD inefficiencies) than geopolitical availability. Addtionally, cradle-to-gate environmental burden for indium production (CED – 1170–2520 MJ kg−1) is greater than cadmium (CED – 28–118 MJ kg−1).70 As suggested for metals categorized as rare-earth elements, there is a need to close the loop for sustainable indium consumption, which mandates development of recycling infrastructure and designing final products for recyclability.71–73 Moreover, selenium is a far rarer element, especially in comparison to phosphorus. Since cradle-to-gate assessment of embodied energy considers energy consumption in the metal extraction phase, there is little reason to get into an extensive analysis of material criticality.
000 ppm, and indium content of Zn ore deposits range from 100 to 2730 ppm.68,69 Critics of In-based QD displays often cite rarity of indium as one of the major constraints to large-scale production, however as illustrated in this study, the “scarcity” of indium may have more to do with its higher material requirements (due to InP QD inefficiencies) than geopolitical availability. Addtionally, cradle-to-gate environmental burden for indium production (CED – 1170–2520 MJ kg−1) is greater than cadmium (CED – 28–118 MJ kg−1).70 As suggested for metals categorized as rare-earth elements, there is a need to close the loop for sustainable indium consumption, which mandates development of recycling infrastructure and designing final products for recyclability.71–73 Moreover, selenium is a far rarer element, especially in comparison to phosphorus. Since cradle-to-gate assessment of embodied energy considers energy consumption in the metal extraction phase, there is little reason to get into an extensive analysis of material criticality.
The lack of industrial-scale process data for nanomaterials makes it especially difficult to produce detailed life cycle inventories. In this study, this challenge is addressed using patent literature along with other secondary data, and making appropriate assumptions for comparative analysis of alternative QD display pathways. While our model makes certain unavoidable simplifications in the absence of proprietary manufacturing data, it provides critical information for directing data gathering exercises and future research efforts. The scope of this LCA is restricted to cradle-to-gate analysis of the system, and it is important to compare alternate QD pathways throughout the life cycle (i.e. cradle-to-grave), especially to evaluate environmental tradeoffs among different compositions, designs, and pathways. Insights based on standalone “cradle-to-gate” studies can be quite misleading, and regulatory decisions based on them may result in unintended consequences.74–76 We plan to extend this study, as part of future work, to consider effect of consumer behavior inclusive of rebound effects, product use, and final disposal strategies on safety and environmental impacts associated with alternate QD displays. It is especially critical to account for consumption patterns for nanoproducts that generate considerable share of environmental impact during the use phase.35,77 Devices using QD-enabled displays consume energy for operation, thus are examples of such products. In addition, we aim to develop QD-specific characterization factors that use information regarding their release, fate and transport, and exposure to understand the direct environmental and human health impacts. By incorporating these additional steps, we will be able to delineate the associated tradeoffs, and provide a more comprehensive scheme for sustainable manufacturing of QD-enabled displays.
Lastly, from a regulatory perspective, it is critical to understand risks and benefits surrounding large-scale production of nano-enabled products. Rather than creating regulations just based on toxicity of elements comprising QDs, our study shows that life cycle assessment backed regulations will be more capable of avoiding unintended consequences. In 2015, the European Parliament requested reevaluation of the exemption dealing with CdQD applications, including an assessment of life cycle implications. Although the latest report submitted by the Oeko-Institut did conduct a life cycle assessment, it did not account for impacts from the QD synthesis process.78 A systems approach can prevent unintentional displacement and amplification of environmental burden and/or human and ecological toxicity due to exemptions. This study exemplifies such an approach by evaluating production of competing QD nanomaterials, but also the manufacturing technologies being used to incorporate these nanocrystals into displays. Thus, identifying opportunities for pollution prevention and reduction of resource consumption for the development of scalable and sustainable nano-enabled products.
Acknowledgements
We are grateful to Benjamin A. Wender and Thomas P. Seager from Arizona State University for insightful discussions during the initial stages that contributed to the design of this research. This work is part of the project NCCLCs: Life Cycle of Nanomaterials (LCnano) supported by the U.S. Environmental Protection Agency (Grant No. RD835580). The views expressed in this document are solely those of the authors and do not necessarily reflect those of the Agency. EPA does not endorse any products or commercial services mentioned in this publication.References
- A. I. Ekimov and A. A. Onushchenko, JETP Lett., 1981, 34, 363 CAS.
- L. Brus, IEEE J. Quantum Electron., 1986, 22, 1909–1914 CrossRef.
- G. H. Carey, A. L. Abdelhady, Z. Ning, S. M. Thon, O. M. Bakr and E. H. Sargent, Chem. Rev., 2015, 115, 12732–12763 CrossRef CAS PubMed.
- Y. Shirasaki, G. J. Supran, M. G. Bawendi and V. Bulović, Nat. Photonics, 2013, 7, 13–23 CrossRef CAS.
- B. A. Kairdolf, A. M. Smith, T. H. Stokes, M. D. Wang, A. N. Young and S. Nie, Ann. Rev. Anal. Chem., 2013, 6, 143 CrossRef CAS PubMed.
- X. Dai, Z. Zhang, Y. Jin, Y. Niu, H. Cao, X. Liang, L. Chen, J. Wang and X. Peng, Nature, 2014, 515, 96–99 CrossRef CAS PubMed.
- K. Mathews, Quantum Dot Display Market to Grow 64% CAGR till 2021, TechSci Research, Manhattan, NY, 2016 Search PubMed.
- J. Happich, Quantum dots taking over OLED at Samsung, http://www.electronics-eetimes.com/news/quantum-dots-taking-over-oled-samsung, 2016 Search PubMed.
- A. P. Alivisatos, Science, 1996, 271, 933 CAS.
- S. Coe-Sullivan, Nat. Photonics, 2009, 3, 315–316 CrossRef CAS.
- K. Bourzac, Nature, 2013, 493, 283 CrossRef CAS PubMed.
- J. S. Steckel, J. Ho, C. Hamilton, J. Xi, C. Breen, W. Liu, P. Allen and S. Coe-Sullivan, J. Soc. Inf. Disp., 2015, 23, 294–305 CrossRef CAS.
- M. D. Regulacio and M.-Y. Han, Acc. Chem. Res., 2010, 43, 621–630 CrossRef CAS PubMed.
- B. O. Dabbousi, J. Rodriguez-Viejo, F. V. Mikulec, J. R. Heine, H. Mattoussi, R. Ober, K. F. Jensen and M. G. Bawendi, J. Phys. Chem. B, 1997, 101, 9463–9475 CrossRef CAS.
- C. Murray, D. J. Norris and M. G. Bawendi, J. Am. Chem. Soc., 1993, 115, 8706–8715 CrossRef CAS.
- Y. Yang, Y. Zheng, W. Cao, A. Titov, J. Hyvonen, J. R. Manders, J. Xue, P. H. Holloway and L. Qian, Nat. Photonics, 2015, 9, 259–266 CAS.
- K.-T. Yong, W.-C. Law, R. Hu, L. Ye, L. Liu, M. T. Swihart and P. N. Prasad, Chem. Soc. Rev., 2013, 42, 1236–1250 RSC.
- V. Brunetti, H. Chibli, R. Fiammengo, A. Galeone, M. A. Malvindi, G. Vecchio, R. Cingolani, J. L. Nadeau and P. P. Pompa, Nanoscale, 2013, 5, 307–317 RSC.
- A. M. Derfus, W. C. Chan and S. N. Bhatia, Nano Lett., 2004, 4, 11–18 CrossRef CAS.
- R. Hardman, Environ. Health Perspect., 2006, 165–172 CrossRef.
- B. Wang and Y. Du, Oxid. Med. Cell. Longevity, 2013, 2013, 898034 Search PubMed.
- A. D. Lemly, Ecotoxicol. Environ. Saf., 1993, 26, 181–204 CrossRef CAS PubMed.
- Z. Tan, Y. Zhang, C. Xie, H. Su, J. Liu, C. Zhang, N. Dellas, S. E. Mohney, Y. Wang and J. Wang, Adv. Mater., 2011, 23, 3553–3558 CrossRef CAS PubMed.
- J. Lim, M. Park, W. K. Bae, D. Lee, S. Lee, C. Lee and K. Char, ACS Nano, 2013, 7, 9019–9026 CrossRef CAS PubMed.
- F. Maier-Flaig, J. Rinck, M. Stephan, T. Bocksrocker, M. Bruns, C. Kübel, A. K. Powell, G. A. Ozin and U. Lemmer, Nano Lett., 2013, 13, 475–480 CrossRef CAS PubMed.
- C. Ippen, T. Greco, Y. Kim, J. Kim, M. S. Oh, C. J. Han and A. Wedel, Org. Electron., 2014, 15, 126–131 CrossRef CAS.
- C. Xiang, W. Koo, S. Chen, F. So, X. Liu, X. Kong and Y. Wang, Appl. Phys. Lett., 2012, 101, 053303 CrossRef.
- V. Wood and V. Bulovic, Colloidal Quantum Dot Optoelectronics and Photovoltaics (Cambridge University Press, 2013), 2013, p. 148 Search PubMed.
- C. Ippen, T. Greco and A. Wedel, J. Infect. Dis., 2012, 13, 91–95 CAS.
- G. Tarr, Nanosys Sheds Light On Next-Gen Quantum Dot Displays, http://hdguru.com/nanosys-sheds-light-on-next-gen-quantum-dot-displays/, (accessed June 15, 2016) Search PubMed.
- EP, Objection to a delegated act: exemption for cadmium in illumination and display lighting applications, European Parliament (EP), Strasbourg, France, 2016.
- ISO-14040, London: British Standards Institution, 2006.
- B. A. Wender, R. W. Foley, V. Prado-Lopez, D. Ravikumar, D. A. Eisenberg, T. A. Hottle, J. Sadowski, W. P. Flanagan, A. Fisher and L. Laurin, Environ. Sci. Technol., 2014, 48, 10531–10538 CrossRef CAS PubMed.
- L. M. Gilbertson, A. A. Busnaina, J. A. Isaacs, J. B. Zimmerman and M. J. Eckelman, Environ. Sci. Technol., 2014, 48, 11360–11368 CrossRef CAS PubMed.
- A. L. Hicks and T. L. Theis, Int. J. Life Cycle Assess., 2016, 1–10, DOI:10.1007/s11367-016-1145-2.
- M. J. Eckelman, J. B. Zimmerman and P. T. Anastas, J. Ind. Ecol., 2008, 12, 316–328 CrossRef CAS.
- T. L. Theis, B. R. Bakshi, D. Durham, V. M. Fthenakis, T. G. Gutowski, J. A. Isaacs, T. Seager and M. R. Wiesner, Phys. Status Solidi RRL, 2011, 5, 312–317 CrossRef CAS.
- A. A. Busnaina, J. Mead, J. Isaacs and S. Somu, J. Nanopart. Res., 2013, 15, 1–6 CrossRef.
- A. Hicks, R. Reed, T. Theis, D. Hanigan, H. Huling, T. Zaikova, J. Hutchison and J. Miller, Environ. Sci.: Nano, 2016, 3(5), 1124–1132 RSC.
- A. L. Hicks, L. M. Gilbertson, J. S. Yamani, T. L. Theis and J. B. Zimmerman, Environ. Sci. Technol., 2015, 49, 7529–7542 CrossRef CAS PubMed.
- P. Zhai, J. A. Isaacs and M. J. Eckelman, Appl. Energy, 2016, 173, 624–634 CrossRef CAS.
- H. Sengul and T. L. Theis, in Nanotechnology Applications for Clean Water, ed. N. S. D. D. S. Sustich, William Andrew Publishing, Boston, 2009, pp. 561–582, DOI:10.1016/B978-0-8155-1578-4.50047-0.
- H. Sengul and T. L. Theis, J. Cleaner Prod., 2011, 19, 21–31 CrossRef.
- H. Sengul, T. L. Theis and S. Ghosh, J. Ind. Ecol., 2008, 12, 329–359 CrossRef CAS.
- B. Azzopardi and J. Mutale, Renewable Sustainable Energy Rev., 2010, 14, 1130–1134 CrossRef CAS.
- T. Frecker, D. Bailey, X. Arzeta-Ferrer, J. McBride and S. J. Rosenthal, ECS J. Solid State Sci. Technol., 2016, 5, R3019–R3031 CrossRef CAS.
- I. Ribeiro, J. Kaufmann, A. Schmidt, P. Peças, E. Henriques and U. Götze, J. Cleaner Prod., 2016, 112, 3306–3319 CrossRef.
- M. A. Huijbregts, S. Hellweg, R. Frischknecht, H. W. Hendriks, K. Hungerbühler and A. J. Hendriks, Environ. Sci. Technol., 2010, 44, 2189–2196 CrossRef CAS PubMed.
- M. A. Huijbregts, L. J. Rombouts, S. Hellweg, R. Frischknecht, A. J. Hendriks, D. van de Meent, A. M. Ragas, L. Reijnders and J. Struijs, Environ. Sci. Technol., 2006, 40, 641–648 CrossRef CAS PubMed.
- K. Venkataraman, J. R. Linton, R. J. Nick and A. Gupta, U.S. Pat., 20150049491, 2015 Search PubMed.
- R. J. Nick and C. Breen, U.S. Pat., 20150021521 A1, 2015 Search PubMed.
- R. S. Dubrow, W. P. Freeman, E. Lee and P. Furuta, U.S. Pat., 20150300600, 2015 Search PubMed.
- E. W. Nelson, K. L. Eckert, W. B. Kolb, T. D. Nesvik and M. Tu, U.S. Pat., 20150368553, 2015 Search PubMed.
- M. Liu, R. S. Dubrow, W. P. Freeman, A. D. Kucma and J. W. Parce, U.S. Pat., 20150166342, 2015 Search PubMed.
- M. Liu, R. Dubrow, W. P. Freeman, A. Kucma and J. W. Parce, U.S. Pat., 20100276638, 2010 Search PubMed.
- W. Guo, J. Chen, R. Dubrow and W. P. Freeman, U.S. Pat., 20140001405, 2014 Search PubMed.
- A. B. Jaffe and M. Trajtenberg, Patents, citations, and innovations: A window on the knowledge economy, MIT press, 2002 Search PubMed.
- Oeko-Institut, Joint Revaluation of Two Requests for Exemption, First Reviewed in 2013–2014, Related to Cadmium Quantum Dot Applications, http://rohs.exemptions.oeko.info/index.php?id=265, (accessed June 20, 2016).
- B. Weidema, C. Bauer, R. Hischier, C. Mutel, T. Nemecek, J. Reinhard, C. Vadenbo and G. Wernet, Overview and methodology. Data quality guideline for the ecoinvent database version 3, Ecoinvent Report 1(v3). St. Gallen: The ecoinvent Centre, Swiss Centre for Life Cycle Inventories, 2013 Search PubMed.
- G. Wernet, S. Hellweg, U. Fischer, S. Papadokonstantakis and K. Hungerbühler, Environ. Sci. Technol., 2008, 42, 6717–6722 CrossRef CAS PubMed.
- G. Wernet, S. Papadokonstantakis, S. Hellweg and K. Hungerbühler, Green Chem., 2009, 11, 1826–1831 RSC.
- W. H. Seaton, E. Freedman and D. N. Treweek, Chetah: The ASTM Chemical Thermodynamic and Energy Release Evaluation Program, American Society for Testing and Materials, 1974 Search PubMed.
- S. W. Benson, F. Cruickshank, D. Golden, G. R. Haugen, H. O'neal, A. Rodgers, R. Shaw and R. Walsh, Chem. Rev., 1969, 69, 279–324 CrossRef CAS.
- B. Xing, C. D. Vecitis and N. Senesi, Engineered Nanoparticles and the Environment: Biophysicochemical Processes and Toxicity, John Wiley & Sons, 2016 Search PubMed.
- Statista, Statistics and Market Data on Consumer Electronics, https://www.statista.com/markets/418/topic/485/consumer-electronics/.
- A.-T. Le, P. Huy, P. D. Tam, T. Q. Huy, P. D. Cam, A. Kudrinskiy and Y. A. Krutyakov, Curr. Appl. Phys., 2010, 10, 910–916 CrossRef.
- R. G. Eggert, A. Carpenter, S. W. Freiman, T. E. Graedel, D. A. Meyer, T. P. McNulty, B. Moudgil, M. M. Poulton, L. J. Surges and E. A. Eide, Minerals, critical minerals and the US economy, 2008 Search PubMed.
- M. E. Cook and H. Morrow, Anthropogenic sources of cadmium in Canada. National workshop on cadmium transport into plants, Canadian Network of Toxicology Centres, Ottawa, 1995 Search PubMed.
- S. Ishihara, H. Murakami and M. F. Marquez-Zavalia, Resour. Geol., 2011, 61, 174–191 CrossRef CAS.
- P. Nuss and M. J. Eckelman, PLoS One, 2014, 9, e101298 Search PubMed.
- T. Werner, M. Gavin and J. Simon, Trans. Inst. Min. Metall., Sect. B, 2015, 124(4), 213–226 CAS.
- G. G. Zaimes, B. J. Hubler, S. Wang and V. Khanna, ACS Sustainable Chem. Eng., 2015, 3, 237–244 CrossRef CAS.
- T. E. Graedel and B. R. Allenby, Industrial ecology and sustainable engineering, Prentice Hall, 2010 Search PubMed.
- R. Hischier and T. Walser, Sci. Total Environ., 2012, 425, 271–282 CrossRef CAS PubMed.
- S. Gavankar, S. Suh and A. F. Keller, Int. J. Life Cycle Assess., 2012, 17, 295–303 CrossRef.
- W. C. Walker, C. J. Bosso, M. Eckelman, J. A. Isaacs and L. Pourzahedi, J. Nanopart. Res., 2015, 17, 1–16 CrossRef CAS.
- L. Pourzahedi and M. J. Eckelman, Environ. Sci.: Nano, 2015, 2, 361–369 RSC.
- C.-O. Gensch, Y. Baron and M. Blepp, https://circabc.europa.eu/sd/a/0444ed6b-65c5-4c6f-b777-20b871f4a428/20160602_Final Report_RoHS_Pack_10_Cd_QDs_amended.pdf, 2016.
| This journal is © The Royal Society of Chemistry 2017 |