Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Arsenic in residential soil and household dust in Cornwall, south west England: potential human exposure and the influence of historical mining†
Daniel R. S.
Middleton
abc,
Michael J.
Watts
 *b,
Darren J.
Beriro
b,
Elliott M.
Hamilton
b,
Giovanni S.
Leonardi
c,
Tony
Fletcher
c,
Rebecca M.
Close
c and
David A.
Polya
a
*b,
Darren J.
Beriro
b,
Elliott M.
Hamilton
b,
Giovanni S.
Leonardi
c,
Tony
Fletcher
c,
Rebecca M.
Close
c and
David A.
Polya
a
aSchool of Earth, Atmospheric and Environmental Sciences, Williamson Research Centre for Molecular Environmental Science, University of Manchester, Manchester, M13 9PL, UK
bInorganic Geochemistry, Centre for Environmental Geochemistry, British Geological Survey, Nottingham, NG12 5GG, UK. E-mail: mwatts@bgs.ac.uk
cEnvironmental Change Department, Centre for Radiation, Chemicals and Environmental Hazards (CRCE), Public Health England, Chilton, Didcot, Oxfordshire OX11 0RQ, UK
First published on 1st March 2017
Abstract
Exposure to arsenic (As) via residential soil and dust is a global concern, in regions affected by mining or with elevated concentrations present in underlying geology. Cornwall in south west England is one such area. Residential soil (n = 127) and household dust (n = 99) samples were collected from across Cornwall as part of a wider study assessing exposure to environmental As. Samples were analysed for total As (soil and dust samples) and human ingestion bioaccessible As (soil samples from properties with home-grown produce). Arsenic concentrations ranged from 12 to 992 mg kg−1 in soil and 3 to 1079 mg kg−1 in dust and were significantly higher in areas affected by metalliferous mineralisation. Sixty-nine percent of soils exceeded the 37 mg kg−1 Category 4 Screening Level (C4SL), a generic assessment criteria for As in residential soils in England, which assumes 100% bioavailability following ingestion. The proportion of exceedance was reduced to 13% when the bioavailability parameter in the CLEA model was changed to generate household specific bioaccessibility adjusted assessment criteria (ACBIO). These criteria were derived using bioaccessibility data for a sub-set of individual household vegetable patch soils (n = 68). Proximity to former As mining locations was found to be a significant predictor of soil As concentration. This study highlights the value of bioaccessibility measurements and their potential for adjusting generic assessment criteria.
Environmental impactArsenic is an environmentally ubiquitous element occurring both naturally and as a result of anthropogenic activities. It is an established carcinogen, categorised as “carcinogenic to humans” (Group 1) by the International Agency for Research on Cancer, as well as being an established cause of numerous other non-communicable diseases. Cornwall, South West England is an area of notable residential arsenic contamination resulting from the natural geochemical environment and extensive historical mining operations. This paper assesses the potential for human exposure to arsenic in residential soil and dust on a scale not previously attempted (127 households). Existing assessment criteria are employed as well as a modified approach incorporating measured bioaccessibility by reducing the parameter in the contaminated land exposure assessment model from 100% to that determined in vitro. Soil concentrations are also modelled in relation to historical mining site proximity. This paper addresses important considerations for human exposure assessment and reiterates a potentially important human exposure pathway in the study location using updated methodologies. |
1. Introduction
Chronic exposure to environmental inorganic arsenic (As) is a recognised risk-factor of numerous cancerous and non-cancerous human health effects.1,2 Globally, the most significant non-occupational exposure pathway is the ingestion of contaminated groundwater, notable examples include Bangladesh3 and West Bengal.4 Other sources include contaminated food, soil and dust.5 The latter two media form the focus of this paper.Arsenic exposure via soil and dust can occur as a result of ingestion, inhalation and dermal absorption.6 Specific pathways include: inhalation of soil dust; direct soil ingestion or dermal contact; plant uptake and subsequent ingestion; ingestion of soil adhered to vegetables and direct indoor dust ingestion or dermal contact.6 Depending on the scenario, and the behavioural patterns of a given ‘receptor’, the relative importance of these pathways varies. For example, children <6 years old are more likely to be exposed to soil/dust due to the frequency of hand-to-mouth behaviour and thus accidental ingestion.7 Gardeners and home-grown vegetable consumers are also likely to come into contact with soil more frequently.6
Category 4 Screening Levels (C4SL)8 are, health-based, generic assessment criteria for chemicals in soil for England. They were developed by contaminated land: applications in real environments on behalf of the UK Department for Environment, Food and Rural Affairs to support the implementation of UK Government Statutory Guidance for England in Part 2a of the Environmental Protection Act 1990. Two C4SLs for residential land use were developed for total As. They are 37 and 40 mg kg−1 for residential properties with and without home-grown produce, respectively. These C4SLs represent the concentration of a substance in soil that would result in an average daily exposure equal to the low level of toxicological concern8 given the generic model parameters, e.g. exposure rate for soil and dust ingestion (mg per day). For As, the oral low level of toxicological concern is 0.3 μg per kg of bodyweight (BW) per day, which aligns with the WHO drinking water guideline value9 of 10 As μg per L. The residential C4SLs were derived using the Contaminated Land Exposure Assessment (CLEA) model under generic exposure parameters, one of which is that As uptake from soil is equal to intake i.e. the chemical form once released from soil is 100% bioavailable in humans. The CLEA model permits the user to adjust the relative bioavailable fraction from the generic setting of 1.0 to a site specific value (i.e. bioaccessibility measured in vitro).
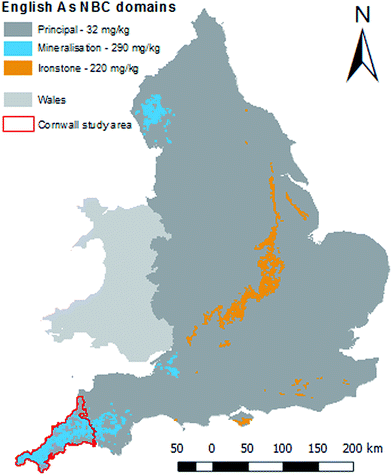
Normal Background Concentrations (NBCs) of contaminants (including As) in soils provide non-health based soil concentrations reflecting the observed variation in concentration attributable to underlying geology and diffuse pollution for a given geochemical domain.10 Like C4SLs, NBCs were produced to guide the implementation of Part 2a of the Environmental Protection Act 1990. NBCs are defined statistically as the upper 95% confidence limit of the 95th percentile of the measured soil concentrations. The underlying geology has been classified into three domains by BGS. Derivations are made for two pre-defined domains where soils exhibit significantly elevated contaminant concentrations, “ironstone” and “mineralised”, and the remainder of the country termed “principal”.10 The English NBCs for As are 32, 290 and 220 mg kg−1 for principal, mineralised and ironstone domains, respectively.10Fig. 1 shows the distribution of the different NBC domains for As across England. As can be seen, much of Cornwall is classified as a mineralised domain.
Under normal circumstances, soil parent material is the dominant determinant of As and other elemental concentrations.11 Anthropogenic activities including mining and mineral processing can lead to further enrichment of As in soils and household dusts.12 Being a constituent of many sulphide minerals, notably arsenopyrite (FeAsS), As contamination can result from the mining of numerous associated metalliferous ores.13 Global examples of mining related As contamination include: gold (Au) mining in many parts of Africa (e.g. artisanal mining in Ghana14,15), South America (e.g. Nicaragua16) and Oceania (e.g. Australia17,18); copper (Cu) mining in South America (e.g. Chile19) and Europe (e.g. France20 and Portugal21) and tin (Sn), Cu and As mining in south west England, UK.12 In some of these places there is evidence of human exposure such as elevated biomarker As concentrations (urine,14,18,20 hair22 and toe/fingernails16,23) and epidemiological evidence of human health risk.17 At least 74 countries worldwide are reported24 to be affected by mining (excluding coal mining) related As contamination, making human exposure to mining-related As contamination an issue of global importance.
Cornwall, in south west England, is an area of elevated environmental As. Although concentrations in this highly mineralised region would be expected to be naturally elevated, a history of extensive mining and mineral processing, predominantly of Sn, Cu and As, has resulted in further, widespread anthropogenic contamination.25 It has been estimated13 that an area of 722 km2 (7.9% of the region) is contaminated with As to some extent (>110 As mg per kg was applied as a cut-off in this particular study) in south west England. Contamination may arise from mine tailings of which As concentrations vary depending on ore grade, processing efficiency and the economic cut-off point at which the ore was worth processing (Mitchell and Barr, 1995). Measured As concentrations in tailings have been reported at up to 20% As.26 Cornwall has many such tailing heaps and the inability of all but the hardiest of plant species to inhabit them leaves them susceptible to wind erosion. One report27 describes a 100 m plume of dust when a former As mining site, Devon Great Consols, was used as a car racing circuit, a potential source of airborne exposure for local residents. Given Cornwall's former (mid-19th century) status as the world's leading As exporter, the region is highly appropriate for investigating the transport and fate of As in the environment and the implications for human exposure and public health.
Studies conducted in south west England have reported elevated As concentrations in residential soils (i.e. >100 mg kg−1),7,28,29 home/locally-grown vegetables (i.e. relative to control areas),29,30 household dust (i.e. >100 mg kg−1),7,31 private drinking water supplies (i.e. >10 As μg per L)32 and human biomarkers (e.g. relative to control volunteers or correlated with environmental concentrations) such as urine,33–36 toenails23,37 and hair.38 Exposure of infants (0–6 year olds) in the area has been specifically investigated, with modelled As intake estimates as high as 2.43 and 3.53 μg per kg BW per day for soil and dust, respectively.7
Many of the studies discussed have reported elevated As concentrations in media (e.g. soil and dust) collected at former mining areas relative to control locations.23,30,35,38 The relationship between environmental As concentrations (e.g. in soil and dust) and proximity to former mines has not been investigated before on a large (e.g. county-wide) scale. One study39 investigated ambient air particulate As concentrations in relation to proximity to, and surrounding density of former mining sites and found no significant correlation.
The present study was part of a wider investigation of human exposure to As in Cornwall which included biomonitoring36,37 and environmental sampling, comprising of water,32 soil and dust – forming the largest non-occupational focus on environmental As exposure in the UK to-date. This paper aimed to assess potential human exposure to As via residential soil and dust including the potential role that historical arsenic mining has played in its distribution in soils and dusts present in Cornwall. This aim was addressed with the following objectives:
(i) Measure total As concentrations in residential soils (with and without home-grown produce) and household dust, in a sample of Cornwall residents.
(ii) Measure the bioaccessible As concentration in soils used for home-grown produce.
(iii) Derive health-based household bioaccessibility adjusted assessment criteria (ACBIO) for the study households with home grown produce.
(iv) Investigate the relationship between proximity to mining sites and residential soil As concentrations.
2. Materials and methods
2.1. Ethical approval and household selection
Ethical approval for the overall study was granted by the University of Manchester Research Ethics Committee (ref. 13068) and the NHS Health Research Authority National Research Ethics Committee (ref. 13/EE/0234). Sampling units consisted of households selected for having a private water supply as per their participation in a wider study of As exposure in south west England.36 All householders provided written informed consent.2.2. Sample collection
The soil collection protocol was based on the British Geological Survey's Geochemical Baselines Survey of the Environment methodology.40 Composite (5 sub samples from different points within the plot) topsoil (15 cm) samples were collected from vegetable patches (‘vegetable patch soils’). Where no vegetable patch was present, other uncovered patches of land (‘garden soils’) were used. All samples were stored in Kraft sample bags. Householders were asked to provide information on any modifications made to their residential soils such as the presence of any imported soil or application of compost or manure. Composite indoor dust samples were collected by emptying the contents of the household vacuum cleaner. Information on whether or not there were pets at the property was obtained.2.3. Reagents and standards
Deionised water with resistivity of 18.2 MΩ (Millipore, UK) was used throughout. Nitric (HNO3), hydrochloric (HCl), hydrofluoric (HF) and perchloric (HClO4) acids and hydrogen peroxide (H2O2) were of Romil-SpA™ super purity grade (Romil, UK). Arsenic calibration solutions for dust analysis were from a 1000 mg L−1 PrimAg® grade solution (Romil, UK). Arsenic QC standards (25 μg L−1) were prepared from a multi-element solution with As at 20 mg L−1 (Ultra Scientific, USA). Tellurium (Te) and germanium (Ge) ICP-MS internal standards were prepared from a PlasmaCAL 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1 solution (SCP Science, Canada) and a Fluka Analytical 1000 mg L−1 solution (Sigma-Aldrich, USA), respectively. Reagents used during the bioaccessibility protocol were identical to those reported elsewhere.41
000 mg L−1 solution (SCP Science, Canada) and a Fluka Analytical 1000 mg L−1 solution (Sigma-Aldrich, USA), respectively. Reagents used during the bioaccessibility protocol were identical to those reported elsewhere.41
2.4. Sample preparation and dissolution of soil and dust
Soil samples were oven-dried at 40 °C before being disaggregated and sieved to <2 mm. From this <2 mm fraction, samples for total elemental analysis were ground in an agate ball mill. Pressed sample pellets were prepared using 10 g of sample and 2.5 g of binder wax (PANalytical, UK) for analysis by X-ray fluorescence spectrometry. Vegetable patch soils were further sieved (<250 μm) for bioaccessibility testing. Dust samples were sieved (<250 μm) and weighed (0.25 g) into PFA vials (Savillex, USA) for mixed acid digestion as described for soil dissolution elsewhere.42 It is acknowledged that there is a discrepancy between the size fractions in which total As in soil (<2 mm) and dust (<250 μm) were determined. This was due to operational circumstances (the outsourcing of soil analysis) and was judged to have negligible impact on the key findings of the investigation.Bioaccessible As concentrations in soils were determined using the Bioaccessibility Research Group of Europe (BARGE) Unified Bioaccessibility Method (UBM)43 following previously published methodology.41 In brief, duplicate amounts of 0.6 g of <250 μm sieved soil were added to Nalgene™ oak ridge polycarbonate centrifuge tubes (Fisher Scientific, UK) followed by the addition of simulated digestive fluids designed to replicate the mouth, stomach and small intestine. Either duplicate underwent one of two extractions as follows: (i) stomach phase: saliva and gastric fluid added; adjusted to pH 1.2 ± 0.5; rotated end-over-end at 37 °C for 1 h in a water bath; centrifuged for 15 min at 4500g; (ii) stomach and intestinal phase: stomach phase as above; duodenal and bile fluids added; adjusted to pH 6.3 ± 0.5; rotated end-over-end at 37 °C for a further 4 h; centrifuged as above. Supernatants (10 mL) were collected and preserved with 0.2 mL concentrated HNO3 prior to analysis by ICP-MS. Bioaccessibility was determined by the UBM method in both the stomach and intestinal compartments of the simulated gastrointestinal tract. The highest concentration from the two compartments was selected as the bioaccessible concentration in this study, following common practice.44 The bioaccessible fraction (BAF) of As was calculated as a percentage of the total As concentration and is henceforth employed as an in vitro estimate of the relatively bioavailable fraction in the CLEA model.
2.5. Elemental analyses of soil and dust
Soil total As concentrations were measured by an Axios Advanced wavelength-dispersive XRFS instrument (PANalytical, Nottingham, UK). Dust and bioaccessibility digests were diluted ×40 and ×100, respectively with 1% v/v HNO3 + 0.5% v/v HCl and total As concentrations were determined by inductively coupled plasma mass spectrometry (ICP-MS) (Agilent 7500cx series) using previously reported operating conditions.41 A three-point calibration with concentrations at 1, 10 and 100 μg L−1 and helium collision cell mode was used for total As determination. A multi-element internal standard was introduced via a T-piece and Ge was used to correct for As signal drift. Doubly-charged 150Nd++ and 150Sm++ interferences on As were corrected using single element standards at 100 μg L−1 and the application of a correction factor as described previously.412.6. Quality assurance/quality control
Field duplicate soil samples were collected at seven households from different auger points at the same location of the initial sample. For each dissolution batch of 50 dust samples (2 batches in total), 4 certified reference materials (CRMs), 5 digestion duplicates and 5 reagent blanks were digested. The CRMs used were National Institute of Standards and Technology (NIST) 2584 Indoor Dust (2 × 0.25 g per batch) and NIST 2711a Montana II Soil (2 × 0.25 g per batch). Pearson correlation between soil field duplicate total As concentrations was 0.996 (n = 7) with a mean difference of 8% (geometric mean (GM): 5%; range: 1–21%). The Pearson correlation between dust digestion duplicate total As concentrations was 0.98 (n = 10) with a mean difference of 13% (GM: 5%; range: 1–69%). The mean recovery for NIST 2584 digested with vacuum bag samples was 93 ± 3% (n = 4) and 101 ± 4% (n = 4) for NIST 2711a.2.7. Bioaccessibility adjusted household assessment criteria
Individual household bioaccessibility adjusted assessment criteria (ACBIO) values were derived by changing the soil relative bioavailable fraction parameter in the CLEA software (version 1.071)6 from 1.0 to the bioaccessible fraction measured for individual vegetable patch soils in vitro (n = 68). The CLEA land use and chemical parameters applied were the same as those used for the As C4SL (with produce), of which full details can be found in the C4SL report.8 Only soil relative bioavailable fraction was altered to derive ACBIO values. Total As concentrations measured in this subset of households were then compared with the ACBIO value for that household and recorded as an exceedance if the ACBIO value was lower than the As concentration.2.8. Spatial and historical mining variables
Mapping and spatial analysis was performed using ArcMap version 10.1 (ESRI, USA). Metalliferous mineralisation classification (1 km squares shapefile) was produced by BGS using a dataset originally compiled by Ove Arup.45 This was used during the development of As NBCs for English soils (Fig. 1) to define the mineralised domain. All other areas in Cornwall are classified as principal domain locations as they fall outside of this mineralised classification. A spatial dataset of historical As mining sites was compiled using (i) a BGS publication46 containing details, gathered by George Dines, of former mining sites across south-west England and (ii) the BGS BRITPITS database,47 containing entries of active and inactive mineral workings across the UK. Those listed in Dines' publication as having produced As were located in BRITPITS. Ninety three percent of the sites listed by Dines were obtainable from BRITPITS and a further 5% were located via Google Maps. Where multiple points were present in BRITPITS for the same name, all points were extracted if they were in the expected location. Household proximities to Cu, Sn and As mines from BRITPITS and then to the refined As-specific records were calculated using the ArcMap ‘near’ tool.2.9. Statistical analysis
Data analyses were performed in the R programming environment version 3.2.2 48 (base package unless otherwise specified). Concentration data in soil and dust were found to be positively skewed leading to the calculation of geometric means (GMs) in addition to arithmetic means. For the same reason, data were natural log(ln)-transformed prior to statistical analyses. Unequal variances (determined by F tests) and unequal group sizes led to the use of Welch's test to compare concentration data (ln-transformed) between different groups (e.g. NBC domains). Pearson correlation coefficients (rp) were calculated (including p-values and 95% confidence intervals) to test relationships between, for example, soil total As concentrations and those in dust (ln-transformed). The significance between strength in correlations was determined using Williams' test in the ‘psych’ package.49 Linear regression was used to investigate the relationship between soil As concentration and mine proximity (ln-transformed variables). To assess the spatial correlation of residuals, variogram analysis was performed using the ‘sp’ and ‘gstat’ packages.50,51 Generalised linear modelling was used to assess the relationship between soil As concentrations and proximity to mines with adjustment for spatial correlation using the ‘nlme’ package.523. Results and discussion
3.1. Soil total and bioaccessible As concentrations
Summary statistics for soil As are shown in Table 1.| Sample type or NBC domain | n | Min | Max | Arithmetic mean | Geometric mean |
|---|---|---|---|---|---|
| a Modified indicates that the householder reported vegetable patch soils had been modified. | |||||
| Total As (mg kg −1 ) | |||||
| All | 127 | 12 | 992 | 89 | 57 |
| All vegetable patch | 68 | 12 | 992 | 94 | 58 |
| All garden | 59 | 16 | 474 | 82 | 55 |
| All principal | 56 | 16 | 436 | 55 | 40 |
| Principal vegetable patch | 32 | 16 | 436 | 65 | 45 |
| Principal garden | 24 | 17 | 106 | 41 | 34 |
| Principal modifieda | 13 | 22 | 436 | 96 | 59 |
| Principal unmodifieda | 19 | 16 | 146 | 44 | 38 |
| All mineralised | 71 | 12 | 992 | 115 | 74 |
| Mineralised vegetable patch | 36 | 12 | 992 | 120 | 73 |
| Mineralised garden | 35 | 16 | 474 | 111 | 75 |
| Mineralised modifieda | 13 | 40 | 992 | 171 | 107 |
| Mineralised unmodifieda | 23 | 12 | 395 | 91 | 59 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Bioaccessible As (mg kg −1 ) | |||||
| All vegetable patch | 68 | 2 | 87 | 15 | 10 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Bioaccessible fraction (BAF) (%) | |||||
| All vegetable patch | 68 | 3 | 57 | 19 | 17 |
One hundred and twenty nine households were visited for this study. Residential soil was collected from 127 (98%) households, 68 of which (54%) were using the soil for home-grown produce (vegetable patch soils) and the remaining 59 (46%) were garden soils. Soils (vegetable patch and garden) from 56 (44%) households were located within the principal NBC domain and 71 (56%) from within the mineralised NBC domain. Resident reports shows that 26 (38%) of the vegetable patches had been modified in some way (e.g. addition of imported soil, compost or manure). Welch's tests found no significant difference between As concentrations in vegetable patch and garden soils within NBC domains (principal: p = 0.12; mineralised: p = 0.88). Higher total As concentrations were measured in modified vegetable patch soils compared to unmodified soils but the difference was not significant (principal: p = 0.12; mineralised: p = 0.06). These analyses were constrained by small group sizes and no conclusions can be drawn. The distinction between modified and unmodified soils, while not a focal point of the paper, is presented for reference only. Soils from the mineralised domain were found to contain significantly higher total As concentrations than those from the principal domain (GM: 74 versus 40 As mg per kg). No significant difference was found in the BAF of vegetable patch soils between different NBC domains (p = 0.33) or soil modifications (p = 0.07).
Soil As concentrations are comparable with previously reported As concentrations in residential soils in south west England overall, but lower than three reports of samples taken from mining areas. Those measured in 1154 topsoil samples collected for the G-BASE40 south west England (Devon and Cornwall) campaign ranged from 5 to 1949 mg kg−1 (mean: 50 mg kg−1).53 Culbard and Johnson (1984) reported a range of 119–1130 mg kg−1 in garden soils collected from the former Cornwall mining area of Camborne and Hayle.28 Farago and Kavanagh (1999) reported concentrations of 120–1695 mg kg−1 from gardens in the former mining area of Gunnislake and 345–52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 mg kg−1 at the Devon Great Consols mine.31 Xu and Thornton (1985) reported a range of 144–892 mg kg−1 in soils used for home-grown vegetable production in the former Cornwall mining areas of Hayle, Camborne and Godolphin.29
600 mg kg−1 at the Devon Great Consols mine.31 Xu and Thornton (1985) reported a range of 144–892 mg kg−1 in soils used for home-grown vegetable production in the former Cornwall mining areas of Hayle, Camborne and Godolphin.29
The BAF of vegetable patch soils in the present study (range: 3–57%; mean: 19%; 25th percentile: 13%; 75th percentile: 23%) are comparable with previously reported As bioaccessibility in soils from across the UK (2 to 68%)44 and south west England 10–20%;7 16% (single measurement);54 10 to 34% (mean: 19%).55 Whilst BAF estimates have been found to be higher in mining areas (mean: 15%) compared to other mineralised soils with no previous history of mining activity (mean: 9%), the overall BAF of As in soils is still far below 100%.56 These findings are important because it has been reported that the generic and conservative assumption of 100% relative bioavailability in human health risk assessment can lead to unnecessary remediation of potentially contaminated land and potential blight for homeowners who live within such areas.57,58
3.2. Dust total As concentrations
Dust samples were collected from 99 (77%) households. Summary statistics for total As concentrations measured in these dust samples are presented in Table 2.| NBC domain | n | Min | Max | Arithmetic mean | Geometric mean |
|---|---|---|---|---|---|
| Total As (mg kg−1) | |||||
| All | 99 | 3 | 1078 | 84 | 41 |
| Principal | 40 | 5 | 903 | 54 | 28 |
| Mineralised | 59 | 3 | 1078 | 104 | 54 |
Previous studies have measured total As concentrations in household dust samples in south west England and the findings presented in this paper are within a similar range. Farago and Kavanagh (1999) reported As concentrations of 24 to 3740 and 33 to 1160 mg kg−1 in two separate mining locations.31 Culbard and Johnson (1984) reported a lower concentration range of 1 to 330 mg kg−1 in a former mining village,28 as did Rieuwerts et al. (2006) (43–486 mg kg−1).7 These concentrations can be considered elevated compared to a non-mining area in Cornwall (e.g. 1.7–29 mg kg−1).7 A Canadian survey of household vacuum cleaner dust samples (n = 1025) reported lower concentrations (range: 0.1–153 mg kg−1; GM: 7.7; 95th percentile: 40.6)59 than this study, indicating that many households in Cornwall have elevated As in dust relative to a nationally representative (Canadian) urban background. It was recognised that a Canadian background may not be directly applicable to a UK scenario and, in the absence of background data from the UK, the comparison should be interpreted cautiously.
Dust from households in the mineralised domain contained significantly (p = 0.001) higher As concentrations than those in the principal domain. A weak, but significant (p < 0.01), Pearson correlation was observed between residential soil and dust As concentrations (rp = 0.26; 95% CI = 0.07, 0.44). The median ratio of dust/soil total As concentration was 0.62, broadly comparable to the soil-to-dust transport factor of 0.5 used in the CLEA model.6 Rieuwerts et al. (2006) did not report a significant correlation between soil and dust As concentrations7 whereas Keegan and Hong60 reported a weak (0.40), albeit significant correlation. Of the 97 households where both soil and dust was collected, 48 reported having pets in the house and 29 reported having no pets. For the remaining 20 households, pets were reportedly kept outside or results were ambiguous. A significant (p = 0.01), weak correlation (rp = 0.35; 95% CI = 0.08, 0.58) was found between soil and dust As concentrations in households with pets. There was no significant (p = 0.55) correlation between soil and dust As concentrations in households without pets (rp = 0.12; 95% CI = −0.26, 0.46). Chemical elements and other toxicants have the potential to be tracked indoors by pets and on the surfaces of footwear.8,61 The stronger correlation for houses with pets is consistent with this mechanism, though numbers are small and the p-value for the difference between correlations of dust As versus soil As concentrations for households with and without pets was only 0.31, using Williams' test. Factors such as the number of householders and their occupation as well as environmental considerations such as climatic conditions have previously been associated with indoor As and other element concentrations,62 but were not investigated in the present study.
3.3. Generic assessment criteria (C4SL) and normal background concentrations
Study soil concentrations compared to the As C4SL (with and without produce) and the As NBC are shown in Table 3.| Sample type or NBC domain | n | Value (As mg per kg) | n exceeding | % exceeding |
|---|---|---|---|---|
| C4SL (with home-grown produce) | ||||
| All vegetable patch | 68 | 37 | 48 | 71 |
| Principal | 32 | 20 | 63 | |
| Mineralised | 36 | 28 | 78 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| C4SL (without home-grown produce) | ||||
| All garden | 59 | 40 | 39 | 66 |
| Principal | 24 | 9 | 38 | |
| Mineralised | 35 | 30 | 86 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Principal NBC | ||||
| All principal | 56 | 32 | 29 | 52 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Mineralised NBC | ||||
| All mineralised | 71 | 290 | 6 | 8 |
A high proportion of households across the study region exceeded the As C4SL, both for soils with home-grown produce (71%) and without (66%). This proportion was higher in the mineralised domain, especially for the group without home grown produce. The NBC for the mineralised domain (290 mg kg−1) is almost 10-times the value of the C4SL, suggesting that an appreciable number of households in England, located within the mineralised domain for As, are also likely to be in exceedance. In this study, only 8% of households in the mineralised domain exceeded the NBC, whereas 52% exceeded the lower NBC in the principal domain. It is possible that the spatial resolution of the NBC domains may have resulted in the misclassification of households in the study area. For example, households categorised as principal domain that reside on localised, unmapped mineralisation.
3.4. Bioaccessibility-adjusted household assessment criteria
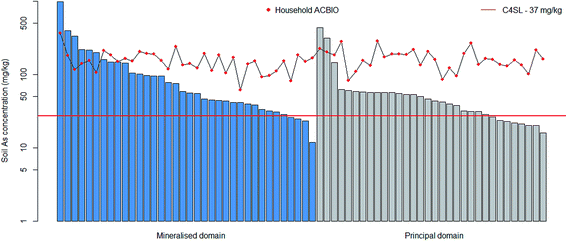
ACBIO values were derived using CLEA by changing the default C4SL relative bioavailable fraction of 1.0 to the in vitro BAF estimates for each household with vegetable patches as determined by UBM. The land-use scenario applied was residential with consumption of home-grown produce, which assumes As exposure of a 0–6 year old female child receptor. This is conservative since children were not resident in most households (16% of households with occupants <16 years old) and 46% did not grow their own produce. It was not justifiable to use anything but the generic female child receptor because detailed quantitative risk assessment for each property is outside the scope of this paper.Eight (13%) of the 68 households for which a bioaccessibility adjusted assessment criteria (ACBIO value) was derived exceeded their respective values. Six (9%) households in the mineralised NBC domain exceeded their ACBIO values, whilst only two (3%) exceeded in the principal NBC domain. Household exceedances of total As concentrations with respect to both the C4SL and the household specific ACBIO values are shown in Fig. 2 for the 68 households with a vegetable patch present.
The findings presented in Fig. 2 illustrate that the derivation of ACBIO for this study resulted in a large reduction in the number of household exceedances relative to comparing to the C4SL without incorporating bioaccessibility data. However, a small number of residential soil As concentrations (n = 8), particularly in the mineralised domain, still exceeded their respective ACBIO values. Rieuwerts et al. (2006) used the CLEA model to derive average daily exposures (μg per kg per day) using bioaccessibility data and reported that 0.3 μg per kg per day was exceeded by 75% of households.7 However, this proportion applied exclusively to a former mining area. Whilst it is acknowledged that the household-specific ACBIO value estimates made in the present study were still conservative, they are consistent with previous reports25 that infants and small children may be particularly vulnerable to As exposure in particularly elevated spatial locations such as mineralisation and in proximity to former mining sites. The geochemical controls on bioaccessibility were not investigated in this study, but likely dictate how the number of assessment criteria exceedances varies by region.
An additional line of useful research would be to assess the importance of home-grown vegetable consumption in the adult population within the study area. This would be approached by surveying residents to quantify their intake and compare this to the generic parameters in CLEA. The generic assumption used to derive C4SLs is that the fraction contribution of home-grown produce to vegetable intake is between 2 and 9% depending on product type. It is noted in the C4SL guidance document8 that this probably underestimates the contribution for many population sub-groups. This is likely the case in Cornwall, where a high prevalence of home-grown vegetable production was observed during fieldwork. Studies conducted elsewhere have found this to be a substantial pathway of exposure. One study63 investigated gardening and home-grown vegetable consumption at properties in the vicinity of a former mining and smelting site in Arizona. They reported that garden soils and home-grown vegetables accounted for 16 and 7% of daily As intake, respectively. In a different approach to that used in this paper, the authors used correlations between As concentrations in soils and vegetables to derive maximum allowable concentrations in soils to limit excess cancer risk to 10−6 (i.e. one in a million). These estimates ranged from 1.56 mg kg−1 to 12.4 mg kg−1 As in soil depending on the different vegetable families grown in them. All of the soils collected in the present study exceeded the estimates previously presented,63 barring one soil in the case of the upper estimate. A detailed assessment of home-grown vegetables consumption was not conducted in the present study, but the findings mentioned above make this a topic worthy of further investigation.
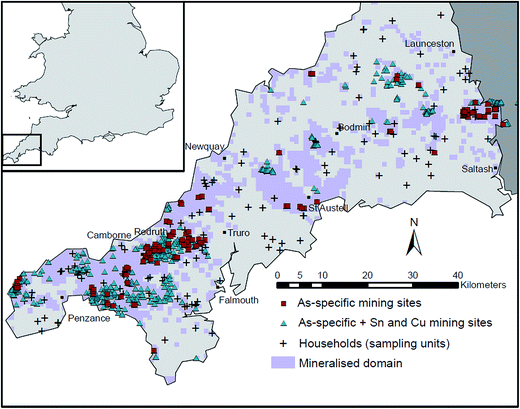
3.5. Spatial influences on residential As concentrations
A dataset containing the names and locations of 103 As-producing mines in Cornwall was generated from BRITPITS and Dines' publication.46 These mines, in addition to other Cu and Sn mines and mineralisation in relation to households are plotted in Fig. 3. The names of individual mines are shown in Fig. S1.† Geometric mean household distances to all mining sites were 4.4 and 1.2 km for the principal and mineralised domains, respectively and 7.1 and 3.2 km for As-specific mines. Due to the inter-domain differences and the significantly higher soil As concentrations in the mineralised domain, regression analysis was performed on separate domains.Several linear regression models were initially tested for log-residential As concentrations as a function of log-mining proximity (log transformed because mining proximities were negatively skewed). These models showed that distance to all mines was not a significant predictor (r2 = 0.03; p = 0.15) of total soil As in the mineralised domain, however, it was significantly, but weakly, (r2 = 0.11; p = 0.01) inversely associated with soil As in the principal domain. Distance to As-specific mines was significantly (r2 = 0.28; p < 0.001) inversely related with total soil As in the mineralised domain but not (r2 < 0.01; p = 0.65) in the principal domain. Distance to mine (all or As-specific) was not a significant predictor of dust As concentration in either domain.
The inverse relationship between soil As concentration and As-specific mine proximity (both ln transformed) in the mineralised domain was chosen for further investigation. Model 1 is the simplest – with no spatial correlation adjustment (simply, ln(soil As) as a function of ln(distance to nearest mine)).
Due to the spatial nature of the data under investigation, points occupying nearby locations in space with similar mining proximities and soil As concentrations had the potential to violate model independence from confounding spatial factors. To validate the relationship between soil As concentration and mine proximity, the Model-1 residuals were investigated for spatial correlation using a variogram. Residuals exhibited a spatial correlation up to approximately 8 km and, therefore a spatial correlation structure was added to a generalised least squares model (Model-2) of soil As concentration against mine proximity (the same structure as Model 1). Several correlation structures64 were tested and, using Akaike's Information Criterion (AIC), a spherical structure yielded the best fit and was significantly better than the GLS without the addition of the correlation structure (AIC: 159 versus 168; ANOVA p = 0.002). The Model-2 residuals exhibited no spatial correlation following normalisation with the spherical structure and soil As concentration remained significantly inversely correlated with mine proximity. The regression coefficient in Model 1 was −0.54 per ln-unit and −0.47 in Model 2, both significant (p < 0.001). A description of the methods employed in this paper for spatial correlation normalisation has been published in detail.64
The widespread As contamination resulting from the extensive mining operations in Cornwall's past have been widely reported12,13,25,27 as well as high concentrations at specific, heavily contaminated locations.65,66 The utility of the As-specific refined mining dataset generated in this study highlights the importance of individual mining site characteristics in how residential As, and other elemental contamination is distributed in the study region.
It is noted that the observed correlation between soil As and mining proximity does not necessarily reflect emissions from mining operations. For example, the locations of As mining were likely dictated by local As ore grades – dependant on local geochemistry. Therefore, it is possible that the correlation between soil As and mining proximity is an indirect relationship between residential soil and parent material As concentrations. Further investigation would require a representative spread of households across a range of lithology groups. Another limitation of the data used in the present paper is acknowledged, in that site-specific variables were not available to include in analyses. Sites require investigation to quantify the levels of contamination present at a given mining site26 and the spread of contamination around former workings.65 Nevertheless, this paper presents a correlation between residential soil As concentrations and proximity to historical As mining sites in the study region using datasets not previously exploited for this purpose.
4. Conclusions
This study is the largest of its kind to be conducted at residential properties in Cornwall to date and has confirmed widespread elevated concentrations of As in soil and dust in the region. A high proportion of households exceeded the C4SL for As in residential soils. The human ingestion soil As bioaccessibility data measured for this study using the UBM enabled the derivation of ACBIO that accounted for the low bioaccessibility of As in the soils collected in the study region. The number of household exceedances of ACBIO were substantially reduced in comparison to the C4SL. A small number (n = 8) of households, particularly in mineralised areas, remained in exceedance of ACBIO. Further investigation is warranted to assess the exposure of the local population, particularly small children and home-grown vegetable consumers, to As in residential soil and dust. Using an As-specific historical mining dataset, residential soil As concentration were found to be inversely correlated with proximity to historical As workings, but more work is needed to qualify this as a causal relationship.Terminology
In this text, “relative bioavailability” refers to the parameter in the contaminated land assessment model and “bioaccessible fraction” is that which is determined in vitro using the unified bioaccessibility method. In this context, bioaccessibility is employed as a proxy of bioavailability.Disclaimer
This paper does not reflect the organisational opinions or recommendations of Public Health England (PHE). The methods used in this paper are for research purposes and are not endorsed by PHE for the purpose of contaminated land risk assessment.Abbreviations
| ACBIO | Bioaccessibility adjusted assessment criteria |
| AIC | Akaike's information criterion |
| BAF | Bioaccessible fraction |
| BW | Bodyweight |
| C4SL | Category 4 screening level |
| CLEA | Contaminated land exposure assessment (model) |
| GM | Geometric mean |
| NBC | Normal background concentration |
| UBM | Unified bioaccessibility method |
Acknowledgements
The authors gratefully acknowledge the contributions of Mark Cave for statistical and scientific review of the manuscript. Andrew Dunne and Andrew Marriott are thanked for their participation in field work and Louise Ander for help with constructing the field database. Joshua Coe is thanked for contributions to laboratory analysis. Helen Crabbe, Karen Exley, Amy Rimell and Mike Studden are thanked for their contributions to the wider project. Funding for this research was provided by the Natural Environment Research Council (NERC) via a University of Manchester/British Geological Survey (BGS) University Funding Initiative (BUFI) PhD studentship (Contract No. GA/125/017, BUFI Ref: S204.2) and the Centre for Environmental Geochemistry, BGS. The participation of the 215 volunteers in the wider study is gratefully acknowledged.References
- IARC, IARC Monogr Eval Carcinog Risks Hum., 2012, vol. 100C, pp. 41–85 Search PubMed.
- WHO, World Health Organisation, Geneva, Environmental health criteria 224: Arsenic and arsenic compounds, World Helath Organisation, Geneva, 2001 Search PubMed.
- D. Chakraborti, M. M. Rahman, B. Das, M. Murrill, S. Dey, S. Chandra Mukherjee, R. K. Dhar, B. K. Biswas, U. K. Chowdhury and S. Roy, Water Res., 2010, 44, 5789–5802 CrossRef CAS PubMed.
- D. Chakraborti, B. Das, M. M. Rahman, U. K. Chowdhury, B. Biswas, A. Goswami, B. Nayak, A. Pal, M. K. Sengupta and S. Ahamed, Mol. Nutr. Food Res., 2009, 53, 542–551 CAS.
- I. Thornton, Sources and pathways of arsenic in the geochemical environment: Health implications, Geological Society, London, Special Publications, 1996, vol. 113, pp. 153–161 Search PubMed.
- J. Jeffries and I. Martin, Updated technical background to the CLEA model, Environment Agency, 2009, available: https://www.gov.uk/government/publications/updated-technical-background-to-the-clea-model Search PubMed.
- J. S. Rieuwerts, P. Searle and R. Buck, Sci. Total Environ., 2006, 371, 89–98 CrossRef CAS PubMed.
- CL: AIRE, SP1010 – Development of Category 4 Screening Levels for assessment of land affected by contamination final project report (revision 2). Contaminated Land: Applications in Real Environments (CL: AIRE), 2014, available: http://randd.defra.gov.uk/Default.aspx?Module=More%26Location=None%26ProjectID=18341 Search PubMed.
- WHO, Arsenic in drinking-water - background document for development of WHO guidelines for drinking-water quality, World Helath Organisation, 2011, http://who/sde/wsh/03.04/75/rev/1 Search PubMed.
- E. L. Ander, C. C. Johnson, M. R. Cave, B. Palumbo-Roe, C. P. Nathanail and R. M. Lark, Sci. Total Environ., 2013, 454, 604–618 CrossRef PubMed.
- B. G. Rawlins, R. Webster and T. R. Lister, Earth Surf. Processes Landforms, 2003, 28, 1389–1409 CrossRef CAS.
- I. Thornton, Appl. Geochem., 1996, 11, 355–361 CrossRef CAS.
- P. Abrahams and I. Thornton, Trans. Inst. Min. Metall., Sect. B, 1987, 96, 1–8 Search PubMed.
- E. Dartey, K. Sarpong, G. Darko and M. Acheampong-Marfo, J. Environ. Chem. Technol., 2013, 5, 113–118 CAS.
- K. A. Asante, T. Agusa, A. Subramanian, O. D. Ansa-Asare, C. A. Biney and S. Tanabe, Chemosphere, 2007, 66, 1513–1522 CrossRef CAS PubMed.
- J. B. Wickre, C. L. Folt, S. Sturup and M. R. Karagas, Arch. Environ. Health, 2004, 59, 400–409 CrossRef CAS PubMed.
- D. C. Pearce, K. Dowling and M. R. Sim, J. Exposure Sci. Environ. Epidemiol., 2012, 22, 248–257 CrossRef CAS PubMed.
- A. L. Hinwood, M. R. Sim, D. Jolley, N. de Klerk, E. B. Bastone, J. Gerostamoulos and O. H. Drummer, Environ. Geochem. Health, 2004, 26, 27–36 CrossRef CAS PubMed.
- H. C. Flynn, V. Mc Mahon, G. C. Diaz, C. S. Demergasso, P. Corbisier, A. A. Meharg and G. I. Paton, Sci. Total Environ., 2002, 286, 51–59 CrossRef CAS PubMed.
- C. Fillol, F. Dor, L. Labat, P. Boltz, J. Le Bouard, K. Mantey, C. Mannschott, E. Puskarczyk, F. Viller and I. Momas, Sci. Total Environ., 2010, 408, 1190 CrossRef CAS PubMed.
- C. Candeias, R. Melo, P. F. Ávila, E. F. da Silva, A. R. Salgueiro and J. P. Teixeira, Appl. Geochem., 2014, 44, 12–26 CrossRef CAS.
- A. L. Hinwood, M. R. Sim, D. Jolley, N. de Klerk, E. B. Bastone, J. Gerostamoulos and O. H. Drummer, Environ. Health Perspect., 2003, 111, 187 CrossRef CAS PubMed.
- M. Button, G. R. Jenkin, C. F. Harrington and M. J. Watts, J. Environ. Monit., 2009, 11, 610–617 RSC.
- S. Murcott, Arsenic contamination in the world: An international sourcebook 2012, Water Intelligence Online, 2012, vol. 11, p. 9781780400396 Search PubMed.
- P. Mitchell and D. Barr, Environ. Geochem. Health, 1995, 17, 57–82 CrossRef CAS PubMed.
- B. Klinck, B. Palumbo-Roe, M. Cave and J. Wragg, Arsenic dispersal and bioaccessibility in mine contaminated soils: A case study from an abandoned arsenic mine in Devon, UK. (RR/04/003). British Geological Survey, Nottingham, UK, 2005, available: http://nora.nerc.ac.uk/3681/ Search PubMed.
- E. I. Hamilton, Sci. Total Environ., 2000, 249, 171–221 CrossRef CAS PubMed.
- E. Culbard and L. Johnson, Trace Substances in Environmental Health, XVIII, University of Missouri, Columbia, 1984, pp. 311–319 Search PubMed.
- J. Xu and I. Thornton, Environ. Geochem. Health, 1985, 7, 131–133 CrossRef CAS PubMed.
- G. Norton, C. Deacon, A. Mestrot, J. Feldmann, P. Jenkins, C. Baskaran and A. A. Meharg, Environ. Sci. Technol., 2013, 47, 6164–6172 CAS.
- M. Farago, P. Kavanagh and H. Ármannsson, 1999, High arsenic-containing soils in SW England and human exposure assessment, in Proceedings of the Geochemistry of the earth's surface Proceedings of the 5th International Symposium, AA Balkema, Reykjavik, Iceland, 15–20 August 1999, pp. 181–184 Search PubMed.
- E. Ander, M. Watts, P. Smedley, E. Hamilton, R. Close, H. Crabbe, T. Fletcher, A. Rimell, M. Studden and G. Leonardi, Environ. Geochem. Health, 2016, 1–20 Search PubMed.
- L. R. Johnson and J. G. Farmer, Environ. Geochem. Health, 1989, 11, 39–44 CrossRef CAS PubMed.
- P. Kavanagh, M. E. Farago, I. Thornton, P. Elliott, W. Goessler and K. J. Irgolic, Occup. Environ. Med., 1997, 54, 840 CrossRef CAS PubMed.
- P. Kavanagh, M. Farago, I. Thornton, W. Goessler, D. Kuehnelt, C. Schlagenhaufen and K. Irgolic, Analyst, 1998, 123, 27–29 RSC.
- D. Middleton, M. Watts, E. Hamilton, E. Ander, R. Close and K. Exley, et al. , Sci. Rep., 2016, 25656 CrossRef CAS PubMed.
- D. R. S. Middleton, M. J. Watts, E. M. Hamilton, T. Fletcher, G. Leonardi, R. Close, K. Exley, H. Crabbe and D. Polya, Environ. Sci.: Processes Impacts, 2016, 18, 562–574 CAS.
- D. F. Peach and D. W. Lane, Environ. Geochem. Health, 1998, 20, 231–237 CrossRef CAS.
- J. Barnes, A. Ledbrooke, B. Parsons, L. Salter and A. Q. Unit, Monitoring of ambient air particulate arsenic concentrations at nine sites in Cornwall, 2006 Search PubMed.
- C. Johnson and N. Breward, G-BASE: Geochemical Baseline Survey of the Environment. British Geological Survey, Nottingham, UK, 2004, (CR/04/016N) (Unpublished), available: http://nora.nerc.ac.uk/509527/ Search PubMed.
- E. M. Hamilton, T. S. Barlow, C. J. Gowing and M. J. Watts, Microchem. J., 2015, 123, 131–138 CrossRef CAS.
- M. J. Watts, M. Button, T. S. Brewer, G. R. Jenkin and C. F. Harrington, J. Environ. Monit., 2008, 10, 753–759 RSC.
- S. Denys, J. Caboche, K. Tack, G. Rychen, J. Wragg, M. Cave, C. Jondreville and C. Feidt, Environ. Sci. Technol., 2012, 46, 6252–6260 CrossRef CAS PubMed.
- J. Appleton, M. Cave and J. Wragg, Sci. Total Environ., 2012, 435, 21–29 CrossRef PubMed.
- O. Arup, Mining instability in Britain, Department of environment contract, Hardcopy maps digitised and adapted by the British Geological Survey, 1990.
- H. G. Dines, The metalliferous mining region of south-west England, HM Stationery Office, 1956 Search PubMed.
- D. Cameron, 2013. User guide for the BRITPITS GIS dataset. British Geological Survey, Nottingham. available, accessed: 20/02/16, http://nora.nerc.ac.uk/503964/.
- R Core Team, A language and environment for statistical computing, R foundation for statistical computing, Vienna, Austria, 2013, www.R-project.Org Search PubMed.
- W. Revelle, Psych: Procedures for psychological, psychometric, and personality research, Northwestern University, Evanston, Illinois, 2014, http://cran.R-project.Org/package=psych Search PubMed.
- E. J. Pebesma and R. S. Bivand, Multivariable geostatistics in S: The gstat package, Computers & Geosciences, 2004, vol. 30, pp. 683–691 Search PubMed.
- E. J. Pebesma and R. S. Bivand, Classes and methods for spatial data in R. R news 5(2), 2005, http://cran.R-project.Org/doc/rnews/ Search PubMed.
- J. Pinheiro, D. Bates, S. DebRoy, D. Sarkar and R. Core Team, Nlme: Linear and nonlinear mixed effects models, R package version 3.1-126, 2016, http://cran.R-project.Org/package=nlme Search PubMed.
- BGS, G-BASE regional geochemistry map for arsenic (As) in soils in south-west England, 2016, British Geological Survey, Nottinghamshire, UK, 2016, available: http://www.bgs.ac.uk/gbase/gbasesw.html Search PubMed.
- M. Cave, J. Wragg, B. Palumbo, B. Klinck and K. Mcleod, Physico-chemical controls on the bioaccessibility of arsenic in UK soils, in Proceedings of the EGS General Assembly Conference Abstracts, 2002, vol. 27, p. 3908 Search PubMed.
- M. Button, M. J. Watts, M. R. Cave, C. F. Harrington and G. T. Jenkin, Environ. Geochem. Health, 2009, 31, 273–282 CrossRef CAS PubMed.
- B. Palumbo-Roe and B. Klinck, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2007, 42, 1251–1261 CrossRef CAS PubMed.
- C. P. Nathanail and R. Smith, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2007, 42, 1193–1202 CrossRef CAS PubMed.
- C. Nathanail, Professional practice note: Reviewing human health risk assessment reports invoking contaminant oral bioavailability measurements or estimates, Chartered Institute for Environment and Health, 2009 Search PubMed.
- P. E. Rasmussen, C. Levesque, M. Chénier, H. D. Gardner, H. Jones-Otazo and S. Petrovic, Sci. Total Environ., 2013, 443, 520–529 CrossRef CAS PubMed.
- T. Keegan and B. Hong, J. Exposure Sci. Environ. Epidemiol., 2002, 12, 179–185 CrossRef CAS PubMed.
- P. J. Lioy, N. C. Freeman and J. R. Millette, Environ. Health Perspect., 2002, 110, 969 CrossRef CAS PubMed.
- I. Meyer, J. Heinrich and U. Lippold, Environ. Res., 1999, 81, 32–44 CrossRef CAS PubMed.
- M. D. Ramirez-Andreotta, M. L. Brusseau, P. Beamer and R. M. Maier, Sci. Total Environ., 2013, 454, 373–382 CrossRef PubMed.
- A. F. Militino, J. R. Stat. Soc., 2010, 173, 938–939 CrossRef.
- G. S. Camm, H. J. Glass, D. W. Bryce and A. R. Butcher, J. Geochem. Explor., 2004, 82, 1–15 CrossRef CAS.
- A. Dybowska, M. Farago, E. Valsami-Jones and I. Thornton, Chem. Speciation Bioavailability, 2006, 17, 147–160 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6em00690f |
| This journal is © The Royal Society of Chemistry 2017 |