Open Access Article
Open Access ArticleThe impact of hydrogen bonding on 100% photo-switching in solid-state nitro–nitrito linkage isomers†
Lauren E.
Hatcher
 * and
Paul R.
Raithby
* and
Paul R.
Raithby

Department of Chemistry, University of Bath, Bath, BA2 7AY, UK. E-mail: l.e.hatcher@bath.ac.uk
First published on 11th September 2017
Abstract
Two crystal systems: [Pd(Et4dien)(NO2)]OTf [1] and [Pt(Et4dien)(NO2)]OTf [2] (Et4dien = N,N,N′,N′-tetraethyldiethylene-triamine, OTf = trifluoromethanesulfonate) are investigated by steady-state photocrystallographic methods. Both structures contain intermolecular hydrogen bonds to the ground state nitro-(η1-NO2) isomer, which are previously shown to limit the achievable level of nitro → nitrito photo-conversion. Irradiation at 100 K induces a mixture of endo-ONO and exo-ONO isomers in 1 and 2, with overall incomplete photo-activation. In contrast, irradiation at higher temperatures leads to much higher conversion, with 100% excitation in 1 at 150 K. The results show that the detrimental effects of hydrogen bonding on the photo-reaction are overcome at higher temperature, adding a new dimension of control to the isomerisation process.
Introduction
Solid-state photo-switchable systems, which can be reversibly shuttled between two distinct structural forms upon irradiation, are important in the search for new functional materials that can exploit the earth's most abundant clean energy source: the sun. The majority of commercially available photo-switches, for example, those exploited for photo-voltaic applications, are hampered by problems such as low photo-conversion and efficiency levels. As such, there is a need to develop methods to address these issues and ultimately to design new systems capable of high levels of photo-conversion in the solid-state.For many decades it has been known that organometallic systems containing ambidentate ligands exist as one of several, structurally distinct linkage isomers.1 Photo-switching between linkage isomers can be achieved in both solution and the solid-state, with irradiation of the solid leading to the production of a metastable state (MS) isomer below some critical temperature.2In situ photocrystallographic studies have to-date revealed the structures of various MS linkage isomers, including nitrosyl,3,4 nitrite,5–8 sulfur dioxide,9–11 and di-nitrogen12 systems.
Early photocrystallographic studies involving metastable linkage isomers were limited by low levels of photo-activation in the single-crystal.11,13 However, in 2009 the first system to undergo 100% photo-switching between a ground state (GS) nitro-(η1-NO2) and a MS endo-nitrito-(η1-ONO) isomer was reported.7 Since then, we have reported a number of metal–nitrite systems capable of achieving very high levels of photo-induced linkage isomer conversion, designed on the basis of a simple crystal engineering principle. These systems include large, sterically-demanding ancillary fragments in the crystalline array, which are all photo-inert. These static, bulky components can then dominate the crystal packing and define a suitable “reaction cavity” around the potentially isomerisable nitrite group.14,15 Photo-switching can then proceed to a high level within this cavity, without imparting undue strain to the rest of the crystalline array that may otherwise inhibit high conversion.
The successful implementation of this simple crystal engineering principle highlights the important influence that the surrounding crystalline environment has on the photo-switching process. Each molecule in a crystal is involved in a complicated array of intermolecular interactions, which have the potential to both assist and hinder linkage isomerism. In particular, strong-to-moderate intermolecular hydrogen bonds, involving the nitro-(η1-NO2) group in the ground state (GS), are shown to have a detrimental effect on the maximum level of photo-conversion that can be achieved. An example is [Ni(MeDPT)(NO2)2], which contains two, crystallographically distinct nitro-(η1-NO2) environments in its GS structure.16 One NO2 group participates in no significant hydrogen bonding and is found to reach 89% photo-activation in the excited state (ES). By contrast, a conversion level of just 32% is achieved at the second NO2 position, which is bound by moderately strong hydrogen bonds through both of its oxygen atoms.16 This result suggests that, when designing new linkage isomer switches, fragments containing hydrogen bond donor groups should be avoided. This would be an unfortunate design limitation, given that hydrogen bonds are traditionally one of crystal engineering's most important and useful tools.17 Avoiding their presence completely makes the rational design of new photo-switchable crystal systems an even more formidable task.
Here, we report two new metal–nitrite complexes that contain moderately strong intermolecular hydrogen bonds to a nitro-(η1-NO2) ligand. We show that the detrimental effect of these interactions on the nitro → nitrito conversion process can be mitigated by careful control of the experiment temperature, providing a positive new outlook on the compatibility of hydrogen bonds and linkage isomer switching in the solid-state.
Experimental
Synthetic procedures
K2[Pd(NO2)4] and K2[Pt(NO2)4] were synthesized following a literature procedure.18 Et4dien and NaOTf were used as-purchased from Sigma Aldrich and all preparations were carried out in air.Found: C = 30.36%; H = 5.75%; N = 10.98%.
Found: C = 25.95%; H = 5.26%; N = 9.14%.
Single-crystal X-ray crystallography
Single-crystal X-ray diffraction data were recorded on a Rigaku-Oxford Diffraction Gemini A Ultra diffractometer, equipped with an Atlas CCD detector and Oxford Cryosystems CryojetXL system for temperature control. Data collection, indexing and integration were performed using the Rigaku-Oxford Diffraction software CrysAlisPro. Structures were solved by dual-space methods in SHELXT,19 and refined by full matrix least-squares on F2 in SHELXL.20 Hydrogen atoms were positioned geometrically and refined using a riding model. The hydrogen atom isotropic displacement parameters were fixed to Uiso(H) = 1.5× (for CH3) or Uiso(H) = 1.2× (for CH2 or NH) the Ueq of the parent atom.In situ photocrystallography
For photocrystallographic studies, the X-ray set-up described above was modified to incorporate a purpose-built LED ring array,21 positioning five LEDs (400 nm: Bivar UV5TZ-400-15, λp = 400 ± 2.5 nm, 3.4 V, 15 mA, 40 mW) at a distance of approximately 1 cm from the crystal in a uniform arc. This set-up ensures even irradiation on all sides of the crystal, and enables the collection of complete single-crystal X-ray data while the array remains in place.Results and discussion
Complex 1: ground-state single-crystal X-ray structure
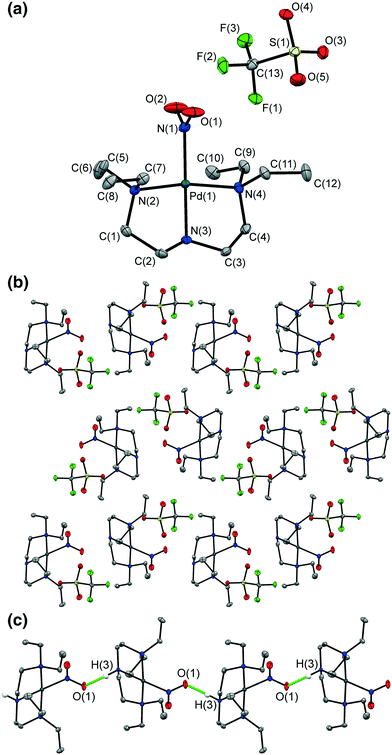
A crystal of 1 was flash-frozen at 100 K and the GS single-crystal X-ray structure was determined in the dark. 1 crystallizes in the monoclinic space group P21/n with one Pd(II) cation and one OTf anion in the asymmetric unit. The Pd(II)-centre adopts square-planar geometry, with one tridentate Et4dien ligand and one monodentate nitrite group occupying the coordination sphere.The ambidentate nitrite ligand adopts solely the nitro-(η1-NO2) geometry in the flash-cooled GS. Fig. 1a shows the full atomic connectivity in the asymmetric unit, and Table 1 gives selected single-crystal X-ray data for the GS structure of 1.
| GS (100 K) | MS (100 K) | GS (150 K) | MS (150 K) | |
|---|---|---|---|---|
| Photo-conversion | 0% | 56% (29% endo, 27% exo) | 0% | 100% (100% endo) |
| Temperature | 100 K | 100 K | 150 K | 150 K |
| Wavelength | 0.71073 Å | 0.71073 Å | 0.71073 Å | 0.71073 Å |
| Empirical formula | C13H29F3N4O5Pd1S1 | C13H29F3N4O5Pd1S1 | C13H29F3N4O5Pd1S1 | C13H29F3N4O5Pd1S1 |
| Formula weight | 516.86 | 516.86 | 516.86 | 516.86 |
| Crystal size | 0.45 × 0.24 × 0.20 | 0.45 × 0.24 × 0.20 | 0.45 × 0.24 × 0.20 | 0.45 × 0.24 × 0.20 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/n | P21/n | P21/n | P21/n |
| Unit cell parameters (constrained) | a = 9.2030(3) Å | a = 9.2897(4) Å | a = 9.2318(4) Å | a = 9.2793(5) Å |
| b = 13.1728(5) Å | b = 13.0472(5) Å | b = 13.1472(6) Å | b = 12.9527(7) Å | |
| c = 16.9033(6) Å | c = 16.6730(8) Å | c = 17.0207(9) Å | c = 16.6542(10) Å | |
| β = 97.388(4)° | β = 98.853(4)° | β = 96.736(5)° | β = 99.379(4)° | |
| Volume | 2032.4(1) Å3 | 1996.8(2) Å3 | 2051.6(2) Å3 | 1974.9(2) Å3 |
| Z | 4 | 4 | 4 | 4 |
| Density (calculated) | 1.689 Mg m−3 | 1.721 Mg m−3 | 1.673 Mg m−3 | 1.738 Mg m−3 |
| Absorption coefficient μ | 1.074 mm−1 | 1.093 mm−1 | 1.064 mm−1 | 1.105 mm−1 |
| F(000) | 1056 | 1056 | 1056 | 1056 |
| R (int) | 0.0360 | 0.0285 | 0.0301 | 0.0306 |
| Completeness (to θ = 25.00°) | 0.997 | 0.995 | 0.997 | 0.995 |
| R 1 (observed data: I > 2σ(I)) | 0.0284 | 0.0313 | 0.0309 | 0.0323 |
| wR2 (all data) | 0.0603 | 0.0697 | 0.0649 | 0.0668 |
| Reflections (independent) | 9352(4145) | 8848(4068) | 9030(4196) | 8451(4028) |
Fig. 1b shows the GS packing arrangement. 1-D chains of Pd-cations extend parallel to the b-axis, formed by discrete D11(2) hydrogen bonds linking the 2° amine donor group N(3)–H(3) on one molecule to the nitro O(1) acceptor on the next, symmetry-related cation (Fig. 1c). This hydrogen bond motif is significant, as it directly involves the potentially photo-active nitro-(η1-NO2) groups. The hydrogen bonds must be disrupted for photo-isomerisation to proceed and, given the literature precedent,16 could be likely to hinder high photo-conversion in 1. The OTf anions sit in discrete pockets, with each OTf participating in short contacts with 6 nearest neighbour cations. These contacts are primarily weak C–H⋯O or C–H⋯F interactions between OTf and neighbouring Et4dien ligands, and are unlikely to strongly influence any potential photo-isomerisation.
Complex 1: steady-state photocrystallography
There is a unit cell volume decrease of ΔV = −35.6(1) Å3 (or −1.75% of the GS cell) on irradiation of 1 at 100 K, which is statistically significant.24 This is in contrast to the majority of photo-switchable nitrite linkage isomers reported, which more typically show an expansion of the crystal on activation.6–8 The unit cell reduction is anisotropic: while the b and c axes contract by −0.1256(5) Å and −0.230(1) Å respectively, the a-axis lengthens by 0.0867(5) Å. Given that the 1-D chains of metal cations extend parallel to the a-axis, it might be argued that the expansion in this direction is a direct result of the isomerisation. However, the overall decrease in unit cell volume would suggest that the photo-induced nitrito isomers occupy less overall volume than the GS nitro-(η1-NO2) form. This observation can be quantitatively confirmed by comparing the Hirshfeld surface volume for the cation molecules in each of the GS and MS structures (see section 1 in the ESI†).25
The hydrogen bond network in the GS of 1 must be broken for photo-excitation to occur and, following activation, neither of the photo-induced nitrito isomers form significant hydrogen bonds in the MS. This provides a possible explanation for the incomplete photo-conversion achieved at 100 K. It appears that irradiation is insufficient to break all of the hydrogen bonds required for complete photo-excitation, which again indicates that strong intermolecular interactions are detrimental for photo-induced linkage isomer conversion in the single-crystal.
The crystal held in its photostationary state was next subject to variable temperature parametric studies, to ascertain the behaviour of the photo-active isomers with temperature. The crystal was warmed in 10 K steps and an X-ray dataset obtained at each interval to reveal the nitrite isomer ratio. The results of these studies are given in Table 2. Between 100 and 120 K, while the GS isomer occupancy remains constant, within error, the exo-ONO occupancy is found to decrease, with a concomitant increase in endo-ONO. This suggests that the exo-ONO isomer, while sufficiently metastable to be observed at very low temperature, decays directly into the endo-ONO geometry with only a small thermal energy barrier. As such, the relative nitrite isomer stability in 1 at 100 K is exo-nitrito-(η1-ONO) < endo-nitrito-(η1-ONO) < nitro-(η1-NO2).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nitrito isomer occupancy ratios, refined from single-crystal X-ray data in steady-state photocrystallography studies with 1 at 100 K
nitrito isomer occupancy ratios, refined from single-crystal X-ray data in steady-state photocrystallography studies with 1 at 100 K
| Temp/K | Irradiation time/h | Nitrite occupancy level | ||
|---|---|---|---|---|
| Nitro | endo-Nitrito | exo-Nitrito | ||
| 100 | 0 | 1.00 | 0.00 | 0.00 |
| 100 | 1 | 0.44 | 0.28 | 0.27 |
| 100 | 2 | 0.44 | 0.28 | 0.27 |
| 110 | 2 | 0.43 | 0.40 | 0.17 |
| 120 | 2 | 0.43 | 0.57 | 0.00 |
| 130 | 2 | 0.43 | 0.57 | 0.00 |
| 140 | 2 | 0.43 | 0.57 | 0.00 |
| 150 | 2 | 0.44 | 0.56 | 0.00 |
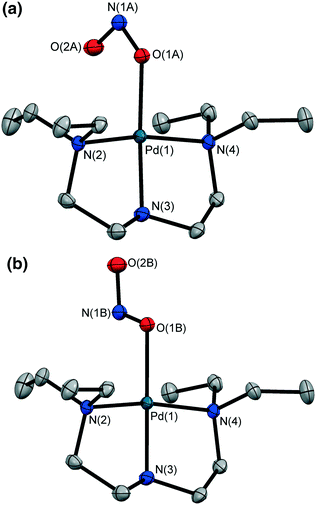
The crystal was held at 150 K and irradiated with 400 nm LED light. After 1 h the LEDs were switched off and the MS structure determined at 150 K. In contrast to results at 100 K, irradiation at 150 K induces 100% conversion to the endo-ONO form (Fig. 2a), with no evidence of an exo-ONO isomer. The unit cell volume again decreases, with ΔV = −76.7(2) Å3 (or −3.74% of the GS unit cell). The GS hydrogen bond network is now entirely disrupted to enable complete photo-conversion, and the MS endo-ONO structure shows no significant hydrogen bonding to neighbouring molecules.
Finally, variable temperature parametric studies conducted at 150–300 K determine the temperature range over which the endo-ONO isomer is metastable (Table 3). The crystal regains GS nitro geometry by 190 K, indicating the MS limit region in 1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nitrito isomer occupancy ratios refined from single-crystal X-ray data in steady-state photocrystallography studies with 1 at 150 K
nitrito isomer occupancy ratios refined from single-crystal X-ray data in steady-state photocrystallography studies with 1 at 150 K
| Temp/K | Irradiation time/h | Nitrite occupancy level | ||
|---|---|---|---|---|
| Nitro | endo-Nitrito | exo-Nitrito | ||
| 150 | 0 | 1.00 | 0.00 | 0.00 |
| 150 | 1 | 0.00 | 1.00 | 0.00 |
| 170 | 1 | 0.05 | 0.95 | 0.00 |
| 180 | 1 | 0.37 | 0.63 | 0.00 |
| 190 | 1 | 1.00 | 0.00 | 0.00 |
| 210 | 1 | 1.00 | 0.00 | 0.00 |
| 250 | 1 | 1.00 | 0.00 | 0.00 |
| 300 | 1 | 1.00 | 0.00 | 0.00 |
Complex 2: ground-state single-crystal X-ray structure
A crystal of 2 was flash-frozen at 100 K and a GS X-ray structure determined in the dark. 2 is both isomorphous and isostructural with 1 and, excepting the exchange of Pd(II) for Pt(II), its GS structure is otherwise well described by Fig. 1 (see also Fig. S2†). Table 4 shows selected X-ray data for the 100 K GS of 2.| GS | MS | GS | MS | |
|---|---|---|---|---|
| Photo-conversion | 0% | 68% (56% endo, 12% exo) | 0% | 93% (93% endo) |
| Temperature | 100 K | 100 K | 200 K | 200 K |
| Wavelength | 0.71073 Å | 0.71073 Å | 0.71073 Å | 0.71073 Å |
| Empirical formula | C13H29F3N4O5Pt1S1 | C13H29F3N4O5Pt1S1 | C13H29F3N4O5Pt1S1 | C13H29F3N4O5Pt1S1 |
| Formula weight | 605.55 | 605.55 | 605.55 | 605.55 |
| Crystal size | 0.50 × 0.26 × 0.07 | 0.50 × 0.26 × 0.07 | 0.49 × 0.21 × 0.09 | 0.49 × 0.21 × 0.09 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/n | P21/n | P21/n | P21/n |
| Unit cell parameters (constrained) | a = 9.2331(3) Å | a = 9.3067(4) Å | a = 9.2636(3) Å | a = 9.3268(2) Å |
| b = 13.1391(4) Å | b = 13.0046(5) Å | b = 13.1409(4) Å | b = 12.9755(3) Å | |
| c = 16.9695(5) Å | c = 16.7285(8) Å | c = 17.1074(6) Å | c = 16.7508(4) Å | |
| β = 97.100(3)° | β = 99.001(4)° | β = 96.567(3)° | β = 99.170(2)° | |
| Volume | 2042.9(1) Å3 | 1999.7(2) | 2068.9(1) Å3 | 2001.3(1) |
| Z | 4 | 4 | 4 | 4 |
| Density (calculated) | 1.969 Mg m−3 | 2.011 Mg m−3 | 1.944 Mg m−3 | 2.010 Mg m−3 |
| Absorption coefficient μ | 7.028 mm−1 | 7.180 mm−1 | 6.940 mm−1 | 7.174 mm−1 |
| F(000) | 1184 | 1184 | 1184 | 1184 |
| R (int) | 0.0267 | 0.0355 | 0.0399 | 0.0388 |
| Completeness (to θ = 25.00°) | 0.998 | 0.997 | 0.997 | 0.998 |
| R 1 (observed data: I > 2σ(I)) | 0.0279 | 0.0358 | 0.0213 | 0.0187 |
| wR2 (all data) | 0.0666 | 0.0854 | 0.0487 | 0.0429 |
| Reflections (independent) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 322(4173) 322(4173) |
9264(4085) | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 464(4226) 464(4226) |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 907(4086) 907(4086) |
Complex 2: steady-state photocrystallography
After 1 h irradiation, an X-ray dataset revealed that 22% of the crystal was converted to a MS endo-ONO isomer, 7% to exo-ONO, with the remaining 71% in GS NO2 geometry (Fig. S3†). Further irradiation increased the ES isomer occupancy levels, with a photostationary state of 32% NO2, 56% endo-ONO and 12% exo-ONO isomers achieved after a total of 3 h illumination. Analysis of the Hirshfeld surface volume for the cation in the GS and MS structures of 2 confirm that, as for 1, the photo-isomers occupy less volume than the GS NO2 form (Fig. S4 and Table S2†).
Variable temperature parametric studies next followed the variation in the nitro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nitrito occupancies on warming. Table 5 confirms that exo-ONO is observed between 100 and 130 K, however, as for the 100 K MS of 1, this isomer gradually decays into the endo-ONO geometry across this temperature range.
nitrito occupancies on warming. Table 5 confirms that exo-ONO is observed between 100 and 130 K, however, as for the 100 K MS of 1, this isomer gradually decays into the endo-ONO geometry across this temperature range.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nitrito isomer occupancy ratios, refined from single-crystal X-ray data in steady-state photocrystallography studies with 2 at 100 K
nitrito isomer occupancy ratios, refined from single-crystal X-ray data in steady-state photocrystallography studies with 2 at 100 K
| Temp/K | Irradiation time/h | Nitrite occupancy level | ||
|---|---|---|---|---|
| Nitro | endo-Nitrito | exo-Nitrito | ||
| 100 | 0 | 1.00 | 0.00 | 0.00 |
| 100 | 1 | 0.71 | 0.22 | 0.07 |
| 100 | 2 | 0.40 | 0.50 | 0.10 |
| 100 | 3 | 0.32 | 0.56 | 0.12 |
| 110 | 3 | 0.32 | 0.57 | 0.10 |
| 120 | 3 | 0.32 | 0.62 | 0.05 |
| 130 | 3 | 0.33 | 0.63 | 0.04 |
| 140 | 3 | 0.34 | 0.66 | 0.00 |
| 150 | 3 | 0.33 | 0.67 | 0.00 |
The crystal was held at 200 K and irradiated in situ with 400 nm LED light. The results of these studies are given in Table 6. A photostationary state of 93% endo-ONO and 7% GS nitro NO2 isomers was achieved after 3 h irradiation, which is – to the author's knowledge – the highest-reported photo-conversion in a Pt(II) linkage isomer to-date. Similarly to 1, no evidence of the exo-ONO form was found at this higher temperature. Variable temperature parametric studies show that the MS limit for the photo-induced endo-ONO isomer in 2 lies in the region of 230–240 K, with the crystal returning to its GS NO2 isomer by 250 K.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nitrito isomer occupancy ratios, refined from single-crystal X-ray data in steady-state photocrystallography studies with 2 at 150 K
nitrito isomer occupancy ratios, refined from single-crystal X-ray data in steady-state photocrystallography studies with 2 at 150 K
| Temp/K | Irradiation time/h | Nitrite occupancy level | ||
|---|---|---|---|---|
| Nitro | endo-Nitrito | exo-Nitrito | ||
| 200 | 0 | 1.00 | 0.00 | 0.00 |
| 200 | 1 | 0.11 | 0.89 | 0.00 |
| 200 | 2 | 0.08 | 0.92 | 0.00 |
| 200 | 3 | 0.07 | 0.93 | 0.00 |
| 210 | 3 | 0.08 | 0.92 | 0.00 |
| 220 | 3 | 0.09 | 0.91 | 0.00 |
| 230 | 3 | 0.18 | 0.82 | 0.00 |
| 240 | 3 | 0.66 | 0.34 | 0.00 |
| 250 | 3 | 1.00 | 0.00 | 0.00 |
| 300 | 3 | 1.00 | 0.00 | 0.00 |
Comparison of photo-isomerisation behaviour at low vs. higher temperature in 1 and 2
The dramatic contrast in the photo-response of 1 and 2 at low vs. higher temperatures indicates that one or more factors influencing isomerisation are strongly temperature-dependent.The first contrast is the fact that the exo-ONO isomer is only present in the MS structures obtained at 100 K. Previous reports of photo-induced exo-ONO isomers suggest that this species is an intermediate existing in the pathway between GS NO2 and MS endo-ONO forms.5 The present results support this theory, as exo-ONO decays directly into endo-ONO on warming with no change in GS NO2 occupancy. Despite this, the fact that the exo-ONO occupancy is as high as 27% for 1 and 12% for 2, even after the light is removed, does suggest that the exo-ONO isomer is an independent ES with its own stability range. The exo-ONO isomer has decayed by 120 K in 1 and by 140 K in 2, which indicates that its ES lifetime is too short to be observed by steady-state photocrystallographic methods above these temperatures.26 This would explain why there is no evidence of the exo-ONO form at 150 or 200 K.
It is also interesting to compare the overall photo-activation level in 1 and 2 at each temperature. Despite the presence of multiple MS isomers at 100 K, excitation remains incomplete: for 1 the combined exo- and endo-ONO ratios give an overall conversion of 56%, in 2 the sum is 68%. In contrast, much higher photo-conversion is achieved in 1 and 2 at higher temperature, with 100% excitation in 1 after just 1 h at 150 K.
There are several structural factors that may explain these observations. Firstly, we reflect that all of the structures contain hydrogen bonding to the GS NO2 group. In the GS structures of 1 and 2, the NO2⋯amine interaction is linear and a comparison of the hydrogen bond distances is given in Tables 7 and 8. In line with thermal lattice expansion, the N(3)–H(3)⋯O(1) hydrogen bonds are significantly longer at higher temperature, consistent with these interactions becoming weaker on warming. If, as expected, hydrogen bonds to the GS NO2 ligand inhibit photo-isomerism,16 the weaker interaction at high temperature is expected to facilitate higher conversion. Secondly, it has been previously shown that “reaction cavity” analysis – where a reaction cavity is defined as the volume encapsulating the photo-active group – provides insight into local structural changes and helps to rationalize how or why a photo-reaction proceeds.14,15Tables 9 and 10 (and Fig. S5 and S6†) show reaction cavity analyses for the GS and MS structures of 1 and 2. For both complexes, the GS reaction cavity volumes are larger at higher temperatures. This indicates that there is more available space, and so fewer steric restrictions, in the vicinity of the NO2 ligand at higher temperatures, which may help the reaction to proceed to higher conversion. In addition, the overall change in reaction cavity volume on photo-activation (ΔVc) can be used to provide a measure of how much the local structure must change in order to accommodate linkage isomer photo-switching. For a single-crystal-to-single-crystal transformation, we expect that the reaction proceeding with the least amount of movement or structural rearrangement will impart the least strain to the surrounding crystalline array, and will therefore be most favourable.28 As such, we expect that the reaction requiring the smallest change in the vicinity of the photo-switching nitrite ligand (i.e. smallest ΔVc) will lead to highest photo-conversion. For 1, ΔVc is ∼3.4× smaller at 150 K than 100 K, while for 2 ΔVc is ∼1.8× smaller at 200 K than 100 K. This provides a rationale for why higher conversion levels can be accommodated at higher temperatures in both of the complexes.
| Temp/K | Graph set | Symmetry operation | D⋯A distance/Å |
|---|---|---|---|
| 100 | D11(2) | ½ − x, −½ + y, ½ − z | 2.801(3) |
| 150 | D11(2) | ½ − x, −½ + y, ½ − z | 2.815(3) |
| Temp/K | Graph set | Symmetry operation | D⋯A distance/Å |
|---|---|---|---|
| 100 | D11(2) | ½ − x, −½ + y, ½ − z | 2.798(6) |
| 200 | D11(2) | ½ − x, −½ + y, ½ − z | 2.810(4) |
| Temp/K | V c per unit cell/Å3 | V c per molecule/Å3 | ΔVc (MS–GS)/% | |
|---|---|---|---|---|
| GS | 100 | 127.66 | 31.92 | |
| MS | 100 | 139.04 | 34.76 | 8.91 |
| GS | 150 | 133.05 | 33.26 | |
| MS | 150 | 136.51 | 34.13 | 2.60 |
| Temp/K | V c per unit cell/Å3 | V c per molecule/Å3 | ΔVc (MS–GS)/% | |
|---|---|---|---|---|
| GS | 100 | 132.30 | 33.08 | |
| MS | 100 | 138.02 | 34.51 | 4.32 |
| GS | 200 | 138.23 | 34.56 | |
| MS | 200 | 141.53 | 35.38 | 2.37 |
Comparison of photo-isomerisation behaviour in 1 and 2
Under steady-state photocrystallographic conditions, the isomerisation behaviour in the isostructural crystals of 1 and 2 is generally very similar, indicating that the primary factor controlling linkage isomer photo-switching in these materials is the surrounding crystal structure. However, there are two key differences in the photo-responses of 1 and 2 that should be considered. The first is that the photo-reaction rate is much slower for the Pt-cation in 2 than for the Pd system 1. There is precedent for slower reaction kinetics in photo-active linkage isomer crystals with heavier transition metals (TMs),29 and this has generally been linked to the expectation of lower kinetic lability for ligands attached to heavier TMs.30 Secondly, though high photo-activation is achieved in both 1 and 2 at 150 and 200 K respectively, despite structural similarities 100% conversion is only reached in the Pd-system 1. Alongside similar arguments for the relative kinetic lability of the nitrite ligand in each complex, this slight discrepancy in excitation level could also be attributed to their absorption profiles (Fig. S7†). There is a blue shift in the absorption spectrum of 2 compared with 1, suggesting that lower excitation wavelengths could improve conversion. However, test studies at wavelengths between 365 and 395 nm could not induce 100% photo-excitation in 2, and instead led to crystal degradation.Conclusions
The results of this, and previous,16 photocrystallographic studies confirm that intermolecular hydrogen bonding involving the photo-active group will influence the progress of nitro → nitrito conversion in the solid-state. However, in contrast to previous work, this study has illustrated that the presence of such intermolecular interactions does not necessarily limit the crystal system to only a low photo-activation level. Careful control of the experiment temperature not only influences the ES lifetime,5,31 but can also be used to control the maximum level of excitation in hydrogen bonded systems. There has been a prior assumption that, for each crystal system, there is a fixed overall level of achievable MS occupation, bound either by the steric properties of the crystal or the electronic properties of the active molecule.4,8,32,33 This study challenges that supposition, and the observation of temperature-regulated photo-activation adds a new dimension of control for single-crystal-to-single-crystal linkage isomer transformations, which may be exploited in future device technologies. Importantly, in terms of crystal engineering, the inclusion of hydrogen bond donor groups need not be avoided, a factor that will assist in the targeted design of new linkage isomer systems aimed towards specific photo-switching applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to that the UK Engineering and Physical Sciences Research Council (EPSRC) for financial support. This work is supported by EPSRC Programme Grant EP/K004956/1.Notes and references
- A. Werner, Ber. Dtsch. Chem. Ges., 1907, 40, 765–788 CrossRef CAS.
- M. D. Carducci, M. R. Pressprich and P. Coppens, J. Am. Chem. Soc., 1997, 119, 2669–2678 CrossRef CAS.
- P. Coppens, I. Novozhilova and A. Kovalevsky, Chem. Rev., 2002, 102, 861–884 CrossRef CAS PubMed.
- B. Cormary, S. Ladeira, K. Jacob, P. G. Lacroix, T. Woike, D. Schaniel and I. Malfant, Inorg. Chem., 2012, 51, 7492–7501 CrossRef CAS PubMed.
- L. E. Hatcher, J. Christensen, M. L. Hamilton, J. Trincao, D. R. Allan, M. R. Warren, I. P. Clarke, M. Towrie, D. S. Fuertes, C. C. Wilson, C. H. Woodall and P. R. Raithby, Chem. – Eur. J., 2014, 20, 3128–3134 CrossRef CAS PubMed.
- L. E. Hatcher, M. R. Warren, D. R. Allan, S. K. Brayshaw, A. L. Johnson, S. Fuertes, S. Schiffers, A. J. Stevenson, S. J. Teat, C. H. Woodall and P. R. Raithby, Angew. Chem., Int. Ed., 2011, 50, 8371–8374 CrossRef CAS PubMed.
- M. Warren, S. Brayshaw, A. Johnson, S. Schiffers, P. Raithby, T. Easun, M. George, J. Warren and S. Teat, Angew. Chem., 2009, 121, 5821–5824 CrossRef.
- L. E. Hatcher, CrystEngComm, 2016, 18, 4180–4187 RSC.
- J. M. Cole, K. Y. M. Yeung, G. Pace, S. O. Sylvester, D. Mersch and R. H. Friend, CrystEngComm, 2015, 17, 5026–5031 RSC.
- K. F. Bowes, J. M. Cole, S. L. G. Husheer, P. R. Raithby, T. L. Savarese, H. A. Sparkes, S. J. Teat and J. E. Warren, Chem. Commun., 2006, 2448–2450 RSC.
- A. Y. Kovalevsky, K. A. Bagley, J. M. Cole and P. Coppens, Inorg. Chem., 2002, 42, 140–147 CrossRef PubMed.
- D. V. Fomitchev, K. A. Bagley and P. Coppens, J. Am. Chem. Soc., 2000, 122, 532–533 CrossRef CAS.
- P. Coppens, M. D. Carducci, K. Culp and D. Fomitchev, Abstracts of Papers of the American Chemical Society, 1997, vol. 214, p. 318 Search PubMed.
- Y. Ohashi, Crystallogr. Rev., 2013, 19, 2–146 CrossRef CAS.
- Y. Ohashi, Acc. Chem. Res., 1988, 21, 268–274 CrossRef CAS.
- L. E. Hatcher, E. J. Bigos, M. J. Bryant, E. M. MacCready, T. P. Robinson, L. K. Saunders, L. H. Thomas, C. M. Beavers, S. J. Teat, J. Christensen and P. R. Raithby, CrystEngComm, 2014, 16, 8263–8271 RSC.
- G. R. Desiraju, Chem. Commun., 1997, 1475–1482 RSC.
- R. D. Feltham, G. Elbaze, R. Ortega, C. Eck and J. Dubrawski, Inorg. Chem., 1985, 24, 1503–1510 CrossRef CAS.
- G. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3–8 CrossRef PubMed.
- G. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 CrossRef PubMed.
- S. K. Brayshaw, J. W. Knight, P. R. Raithby, T. L. Savarese, S. Schiffers, S. J. Teat, J. E. Warren and M. R. Warren, J. Appl. Crystallogr., 2010, 43, 337–340 CrossRef CAS.
- R. Wen, I. Bernal, S. S. Massoud, R. K. Thalji, D. R. Billodeaux and F. R. Fronczek, Inorg. Chim. Acta, 1999, 295, 91–105 CrossRef CAS.
- D. Das, I. Rahaman Laskar, A. Ghosh, A. Mondal, K.-i. Okamoto and N. Ray Chaudhuri, J. Chem. Soc., Dalton Trans., 1998, 3987–3990 RSC.
- A. J. Blake, W. Clegg and J. M. Cole, Crystal Structure Analysis: Principles and Practice, Oxford University Press, 2009 Search PubMed.
- M. A. Spackman and J. J. McKinnon, CrystEngComm, 2002, 4, 378–392 RSC.
- L. E. Hatcher and P. R. Raithby, Coord. Chem. Rev., 2014, 277–278, 69–79 CrossRef CAS.
- C. F. Macrae, I. J. Bruno, J. A. Chisholm, P. R. Edgington, P. McCabe, E. Pidcock, L. Rodriguez-Monge, R. Taylor, J. v. d. Streek and P. A. Wood, J. Appl. Crystallogr., 2008, 41, 466–470 CrossRef CAS.
- A. Natarajan and B. R. Bhogala, in Supramolecular Photochemistry, John Wiley & Sons, Inc., 2011, pp. 175–228 Search PubMed.
- M. R. Warren, S. K. Brayshaw, L. E. Hatcher, A. L. Johnson, S. Schiffers, A. J. Warren, S. J. Teat, J. E. Warren, C. H. Woodall and P. R. Raithby, Dalton Trans., 2012, 41, 13173–13179 RSC.
- P. Atkins, P. W. Atkins and D. F. Shriver, Shriver & Atkins Inorganic Chemistry, W.H. Freeman, 2006 Search PubMed.
- L. E. Hatcher, J. M. Skelton and P. R. Raithby, Phys. Chem. Chem. Phys., 2017 Search PubMed , under review.
- L. E. Hatcher and P. R. Raithby, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2013, 69, 1448–1456 CrossRef CAS PubMed.
- D. Schaniel and T. Woike, Phys. Chem. Chem. Phys., 2009, 11, 4391–4395 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1564437–1564451 (1) and 1564462–1564480 (2) contain the crystallographic data for this paper. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ce01366c |
| This journal is © The Royal Society of Chemistry 2017 |