Alkyl groups as electron density donors in π-hole bonding†
Abstract
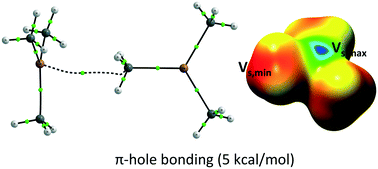
Whereas sp2 and sp-hybridized carbon compounds have been described as electron-rich species able to interact with electron-deficient moieties to form π and σ-hole bonds, to the best of our knowledge there is no report in the literature of sp3 carbons acting as Lewis bases in hole-bonds. By means of a combined structural and computational analysis we have demonstrated the unexpected ability of alkyl groups to act as electron density donors in π-hole bonding. We focused on the interaction between alkyl groups and typical Lewis bases, namely ER3 (E = B, Al, Ga, In, Tl) molecular frameworks. Structural database searches revealed a clear directionality for these short contacts. The attractive nature of the interaction was confirmed by “atoms in molecules” (AIM) and “non-covalent interactions” (NCI) analysis. The interaction strength was evaluated at the MP2/aug-cc-pVTZ level of theory on several models and its nature further investigated by MEP, NBO and EDA analysis. In terms of energy, they are comparable to other π-hole and weak hydrogen bonds, which opens the door to their use in crystal engineering and supramolecular design.


 Please wait while we load your content...
Please wait while we load your content...