RE-p-halobenzoic acid–terpyridine complexes, Part II: structural diversity, supramolecular assembly, and luminescence properties in a series of p-bromobenzoic acid rare-earth hybrid materials†‡
Abstract
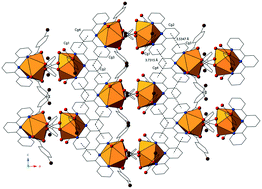
Twenty-four new rare-earth p-bromobenzoic acid complexes, [RE(L1)3(L2)(H2O)]2 (RE = La3+ (1), Ce3+ (2), Pr3+ (3), Nd3+ (4)), [RE(L1)3(L2)(H2O)]2 (RE = La3+ (1′), Ce3+ (2′), Pr3+ (3′), Nd3+ (4′)), [RE(L1)3(L2)]2·H2O (RE = Sm3+ (5), Eu3+ (6), Gd3+ (7), Tb3+ (8)), RE(L1)3(L2)(H2O) (RE = Gd3+ (7′), Tb3+ (8′), Dy3+ (9), Ho3+ (10), Er3+ (11), Tm3+ (12), Yb3+ (13), Lu3+ (14), Y3+ (15)), and RE2(L1)4(L2)2(Ox) (RE = Tm3+ (12′), Yb3+ (13′) Lu3+ (14′)); L1: p-bromobenzoic acid; L2: 2,2′:6′,2′′-terpyridine; Ox: oxalic acid, have been hydrothermally synthesized and structurally characterized by single crystal and powder X-ray diffraction. The series includes binuclear molecular complexes [(La3+–Nd3+), (La′3+–Nd′3+), (Sm3+–Tb3+)], which transition to pseudo-dimeric units (Gd3+–Y3+) as a result of the lanthanide contraction. The range of structures is completed by distinct oxalate-bridged binuclear complexes (Tm3+–Lu3+), formed via in situ synthesis, that appear only with the smallest lanthanides and only from higher temperature syntheses. There are subtle differences in coordination environments between the five structure types as a result of the lanthanide contraction, despite all complexes in this study containing binuclear units. All twenty-four complexes feature halogen- and π-based supramolecular interactions which assemble the molecular complexes into one, two, and three dimensions. Visible and near-IR solid state luminescence spectra were collected on complexes 3, 4, 5, 6, 8, 8′, 9, 12, and 13 and characteristic terpyridine sensitized luminescence was observed.


 Please wait while we load your content...
Please wait while we load your content...