Full-spectra hyperfluorescence cesium lead halide perovskite nanocrystals obtained by efficient halogen anion exchange using zinc halogenide salts
Abstract
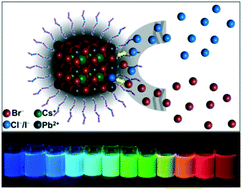
Herein, we demonstrate a facile and less costly approach to deliberately modulate the composition of CsPbX3 (X = Cl, Br, I) NCs. Taking advantage of the highly ionic nature of perovskite structures, complete anion-exchange could be realized on the basis of directly synthesized CsPbBr3 employing zinc halogenide salts as anion source, which embraces the advantages of remarkably short exchange time and room-temperature reaction environment while maintaining the cubic phase for all CsPbX3 NCs, as well as accessing superior perovskite NC qualities with high stability and narrow full width at half maximum peak widths of 18 nm to 38 nm. By controlling the relative concentration of halide anions in a CsPbX3 NC-dispersed solution, their photoluminescence spectra can be finely tuned with ease covering the entire visible spectral region of 410–700 nm.


 Please wait while we load your content...
Please wait while we load your content...