Highlights from the Faraday Discussion meeting “Atmospheric chemistry in the Anthropocene”, York, 2017
Lucy J.
Carpenter
 *a,
Steve R.
Arnold
*a,
Steve R.
Arnold
 b,
Colette L.
Heald
b,
Colette L.
Heald
 c,
A. R.
Ravishankara
c,
A. R.
Ravishankara
 d and
Jonathan
Williams
e
d and
Jonathan
Williams
e
aWolfson Atmospheric Chemistry Laboratories, University of York, UK. E-mail: lucy.carpenter@york.ac.uk
bSchool of Earth and Environment, University of Leeds, UK
cEarth, Atmospheric and Planetary Sciences, MIT, USA
dDepartments of Chemistry and Atmospheric Science, Colorado State University, USA
eMax Planck Institute for Chemistry, Germany
First published on 9th November 2017
1. Introduction
Human civilization evolved in the Holocene, an era of reasonably invariant climate where the influence of humans on the Earth was minimal and natural emissions (and natural changes) were the dominant factors. The beginning of industrialization about 150 years ago brought a rapid increase in human influence. Human-induced emissions into the atmosphere started to influence the Earth's atmosphere, in many cases acting as “catalysts” for various processes such that small emissions had disproportionately larger impacts. Examples include the emissions of nitrogen oxides that catalyzed tropospheric ozone formation, and the emissions of chlorofluorocarbons (CFCs) that catalytically destroyed stratospheric ozone. It was clear that humans could influence the Earth's atmospheric composition on a global scale. Now, human activities are not only catalyzing changes, but are at the verge of dominating over natural causes of change. Thus, we are clearly in a new epoch – the Anthropocene. The consequences of this dominance in the atmosphere – observed and predicted – result in changes in the oceans, terrestrial regions, and biosphere, and have raised important societal issues such as climate change, air quality, acid precipitation, ozone layer depletion, and food security.In response to these influences, in May 2017, the University of York hosted the ‘Atmospheric Chemistry in the Anthropocene’ Faraday Discussions meeting, which brought together more than 100 scientists from around the world to discuss how our understanding of atmospheric chemistry and our ability to measure and predict it is evolving in this new epoch. Such an understanding is essential if humans want to take action to avoid or reverse human influence to exit the Anthropocene back to an epoch where human influence is again smaller than that of the natural system.
The impacts of evolving pollutant emissions in the Anthropocene on human health and welfare depends upon how they are processed and transported in the atmosphere. Understanding and quantifying these processes underpins policies aimed at protecting public health from harmful atmospheric pollutants, mitigating the effects of chemically active climate forcers, and reducing the harmful effects of air pollution on ecosystems. Fundamental new understanding from laboratory and field experiments can provide information on the sources, distribution, and processing of pollutants, as well as the atmospheric response to emission changes. Modelling is used to assess the efficacy of proposed pollutant emission controls, evaluate the impacts of the controls on various socio-economic changes, and to understand the response of atmospheric pollution to complex scenarios of e.g. climate and emissions, or to new fundamental understanding. New advances in knowledge across each of these frontiers were presented at the meeting.
The Discussion was introduced with a lecture by Barbara Finlayson-Pitts who gave an expert overview of the tropospheric chemistry associated with the Anthropocene, focusing on areas of high uncertainty including heterogeneous chemistry, reactions at liquid interfaces, organic oxidations, and biogenic–anthropogenic interactions. All of these processes are impacted by activities associated with a burgeoning population and the pursuit of a better quality of life (which means more energy usage and more exploitation of natural resources), greater use of fossil fuels, and agricultural activities.
The Discussion meeting then focused on the issues of new mechanisms important for atmospheric chemistry, interactions between anthropogenic and biogenic emissions, air quality in the past, present and future, and new instrumental tools and platforms for atmospheric chemistry (Fig. 1). Below, we discuss the three main areas of new understanding that emerged from the meeting: new insights into atmospheric processes, new technological advances, and atmospheric chemistry in policy making and public health impacts of pollution.
 | ||
| Fig. 1 A schematic of the main areas for discussion in the Faraday Discussion meeting “Atmospheric chemistry in the Anthropocene”. | ||
2. New insights into atmospheric processes
Over recent decades, a great deal of progress has been made in understanding the key initiators of atmospheric chemical transformations, the conversion of chemicals into products that influence environmental issues, the processes that move the constituents around the atmosphere and alter their properties, and how these constituents are irreversibly (on time scales of interest) removed from the atmosphere. Important recent advances in our understanding of such processes were presented at the meeting.The major initiators of chemical transformation during daytime are understood to be the hydroxyl (OH) radical and ozone (O3), while night-time transformations are known to be dominated by O3 and the nitrate (NO3) radical. From research over the last few years, it is becoming clear that the Criegee intermediate, formed predominantly by ozonolysis of olefins, is an additional important propagator of chemical transformation, especially for the formation and growth of aerosols. There are many Criegee intermediates and examinations of their formation, reactivity, and impact are being studied. During this meeting, it was made clearer in the general discussion that this radical, though it looks like a peroxy radical, does not react like one since it is a zwitterion. The reaction of this intermediate with NO appears to proceed via addition and its reaction with NO2 appears to be different than that of an ordinary organic peroxide radical (DOI: 10.1039/C7FD00007C) (R1).
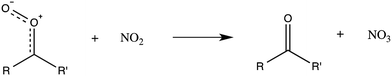 | (R1) |
Methods are being developed to detect a large number of Criegee intermediates via spin trapping techniques (DOI: 10.1039/C7FD00025A). Clearly, the Criegee intermediate is the “hydroxyl radical” of this decade in terms of the attention being paid to it and there is much that needs to be done.
In addition to the Criegee intermediate, the presentations and discussion shed more light on the role of halogens and the NO3 radical (DOI: 10.1039/C7FD00026J and DOI: 10.1039/C7FD00001D) in the troposphere. In this regard, the transformation of the old “smog chamber” into a modern platform for studying the details of kinetics and mechanisms, especially for the formation and growth of aerosols, is noteworthy. These studies are allowing production of realistic particles, enabling studies of their properties, and helping to understand their health impacts.
The evolution of secondary aerosols, which are important for their health and climate impacts, is being examined in highly polluted areas. The surprising results of such studies, where new small particles are found (implying formation of new particles) in the presence of high levels of existing large particles, have called into question our understanding of aerosol formation and growth (DOI: 10.1039/C6FD00257A). It could be that nanometer sized particles are being directly emitted; however, that possibility is not clear. Suggestions are that molecular cluster scavenging is being overestimated or that the rates of growth of existing particles is underestimated (Fig. 2).
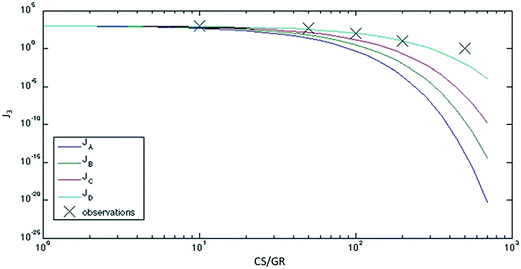 | ||
| Fig. 2 The formation rate of 3 nm particles (J3) as a function of the survival parameter P = CS (condensation sink)/GR (particle growth rate). The stars present observations from Hyytiälä, SPC and Shanghai. Curve JA assumes a fixed formation rate of 1.5 nm particles of 1000 cm3 s−1. Curves JB to JD show calculations with increased growth rates of clusters and decreased scavenging of these clusters. The theoretical value of J3 starts to deviate from the observed as P exceeds 30–40, and the theoretical predictions completely disagree with the observations under highly-polluted conditions. Reproduced from DOI: 10.1039/C6FD00257A with permission from the Royal Society of Chemistry. | ||
Interesting work on the composition of aerosol particles has been carried out and was presented. In particular, it was suggested that ammonium salts of organic acids are present in areas where there are insufficient sulfate and nitrate anions, attributed to ammonia being neutralized by organic acids (DOI: 10.1039/C7FD00027H). This finding may have important consequences, for example on the composition of preindustrial aerosols. Similarly, the role of PAHs in the formation of biogenic aerosols was suggested (DOI: 10.1039/C7FD00032D) and is worthy of further pursuit.
Exquisite details of gas–liquid and gas–solid interactions are being teased out using very sophisticated measurements, often on isolated particles. Presentations at the meeting showed the measurements of the thermodynamic and optical properties of single particles. As noted above, the interaction of PAHs with SOA appears to be very strong, which may facilitate enhanced transport of PAHs over long distances (DOI: 10.1039/C7FD00032D). One paper suggested that soot and SOA can act as ice nuclei and hence influence precipitation (DOI: 10.1039/C7FD00010C). Advances are being made in elucidating the optical properties (real and imaginary refractive indices) and Rayleigh cross-sections of aerosol particles (DOI: 10.1039/C7FD00008A). A key challenge will be translating findings from single particle and detailed laboratory studies into generalized or simplified processes or constraints for complex atmospheric models.
Atmospheric composition is altered not only by the chemical transformations that take place in the gas phase but also those in the condensed phase present in the atmosphere. Interesting developments in this area were presented in this meeting. They include examinations of the fraction of isoprene that actually leads to secondary aerosols (DOI: 10.1039/C7FD00014F), the mechanisms of liquid phase transformations, and the processes at the air–liquid interfaces. The latter aspect was exemplified by a study on the role of glycolic acid organic sulfur chemicals at the interface. Another very intriguing possibility that was highlighted was the production of volatile organic compounds via light absorption at the interface (DOI: 10.1039/C7FD00022G). These surface photochemical transformations are likely to be quite important in some special cases and merit further scrutiny.
Overall, the meeting highlighted the most important advances over the past few years in understanding atmospheric chemical processes.
3. New technological advances
New technological advances in mass spectrometry and optical techniques continue to improve limits of detection for many species, which now extend to sub-ppt levels with excellent specificity. These high sensitivity, fast-response detectors have been paired with suitable platforms for atmospheric measurements. Now, scientists are able to measure reactive free radicals in the atmosphere, which are in the sub-ppt ranges, while our abilities were limited to measuring ppm-ranges a few decades back. This continual improvement in detection capabilities is opening up new avenues for research, quantification of processes, and a better understanding of atmospheric chemistry.This ability to detect very low concentrations at high frequency has enabled many new advances, for example the ability to measure chemical fluxes and deposition, and fast chemical transformations. Examples of the utilization of this high-sensitivity instrumentation were evident in many talks and discussions, for example in the use of proton-transfer reaction–mass spectrometry (PTR-MS) for airborne measurements of volatile organic compound (VOC) fluxes (DOI: 10.1039/C7FD00002B). In this study, high frequency 3-D wind velocities and fast (5 Hz) VOC measurements were used to calculate fluxes using the technique of eddy covariance, analysed using a continuous wavelet transform (CWT). Using a footprint model to calculate the origin of the emissions, the measurements indicated that isoprene and monoterpene fluxes over South East England are significantly underestimated by current inventories. In the case of optical techniques, significant advances have been enabled by cavity-enhanced spectroscopy, a direct absorption technique which can mimic the capabilities of long path DOAS or remote sensing instruments with highly sensitive and accurate measurement, in a lightweight and robust package (DOI: 10.1039/C7FD00001D). These advances in new technologies have also enabled major new abilities in laboratory studies. For example, coupling of these CIMS and spectroscopic techniques has revitalized the use of “smog chambers” into facilities that can simulate the atmosphere and enable the elucidation of mechanisms and quantification of rates of reactions (DOI: 10.1039/C7FD00014F).
Organic compounds in the atmosphere cover a vast range of structures, molecular composition and properties in gas and particle phases. A new generation of field-deployable mass spectrometers offers unprecedented detail and specificity in quantifying organic composition, however it is important to identify where gaps remain in our ability to measure this chemical complexity. The current capabilities of a suite of in situ mass spectrometers have been systematically analysed (DOI: 10.1039/C7FD00021A). Together, these in situ mass spectrometers access nearly the entire range of chemical space, however they are limited in their ability to provide structural information. The use of an innovative sampling technique – spin trapping coupled with mass spectrometry – to detect reactive Criegee intermediates was demonstrated (DOI: 10.1039/C7FD00025A). This new method can be employed for a wide range of Criegee intermediates, which have previously eluded direct experimental measurement, and allows simultaneous measurements in laboratory studies.
Alongside these advances in highly sophisticated instrumentation, the pursuit of small, inexpensive sensors for atmospheric monitoring and large-scale measurements is progressing well. There are interesting technological solutions including the use of clusters of sensors, machine learning, and sensor networks, that have been employed to compensate for the variability, drift, and other sources of inaccuracies (DOI: 10.1039/C7FD00020K). These technological advances could deliver sensor assemblies with good qualitative measures of pollution trends and could enable more widespread pollution monitoring, especially in places where expensive but accurate sensors cannot be deployed or there are resource limitations. Quantitative measurements are now possible with frequent multi-parameter calibration and clusters of inexpensive, very small sensors (Fig. 3) and this advance would enable measurements from developing countries with the fastest rates of emission changes.
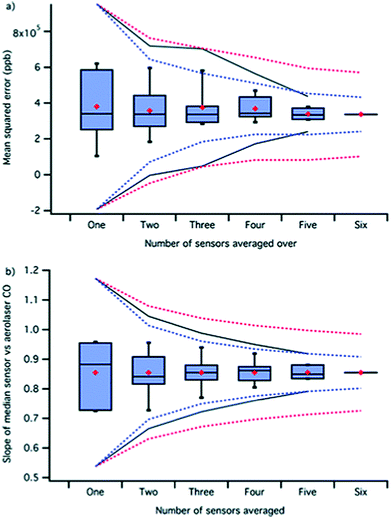 | ||
| Fig. 3 Mean squared error (a) and slope (b) for the correlation of CO reference fluorescence instrument data with metal oxide sensor (MOS) data calculated as the average of different combinations of sensor signals. The dashed lines show the calculated ±3 standard deviations on the mean using a 1/square root of N decrease (red) and 1/N decrease (blue) from the individual sensor versus the reference instrument statistic. Reproduced from DOI: 10.1039/C7FD00020K with permission from the Royal Society of Chemistry. | ||
The abilities of photochemical or “smog” chambers to use sensitive instruments and to measure reactive free radicals (see above) has greatly advanced our understanding of atmospheric chemistry. These chambers have also been able to study secondary organic aerosol formation and the properties of aerosols at unprecedented rates and detail. Recent advances on the microscopic scale now allow the physiological properties of single particles to be investigated under controlled conditions, so that the behavior of key parameters (e.g. hydroscopicity, refractive index and surface tension) can be characterized for use in the next generation of aerosol models (DOI: 10.1039/C7FD00008A).
The exquisite chemical detail now provided in both gas and particle phase measurements and the spatial coverage provided by aircraft or low cost sensor networks will fuel the transformation of generic, simplified models into more comprehensive, chemically and physically accurate simulations. The benefits will extend through explicit models (e.g. MCM), regional pollution and climate modelling (e.g. WRF), to global models that in their newest forms can be used to determine the social cost of pollution providing policy-makers with tractable information (DOI: 10.1039/C7FD00009J).
4. Atmospheric chemistry in policy making and public health impacts of pollution
Atmospheric chemistry underpins policies aimed at protecting public health from harmful atmospheric pollutants, mitigating the effects of chemically active climate forcers, and reducing the harmful effects of air pollution on ecosystems.New information on the complex response of pollution, and its effects, to emission changes was presented. The overall social cost of methane (CH4), as a result of harmful effects on public health, agriculture, forests and infrastructure (Fig. 4), is estimated to be 75 times larger than that of carbon dioxide (CO2) (DOI: 10.1039/C7FD00009J). Increases in CH4 over the past decade may have offset much of the societal benefit of a slowdown in CO2 growth rate over the same period. Policies aimed at controlling CH4 emissions have the potential to benefit both the climate and air quality at relatively low cost, contributing to efforts to meet a range of Sustainable Development Goals across climate, energy, heath and ecosystems.
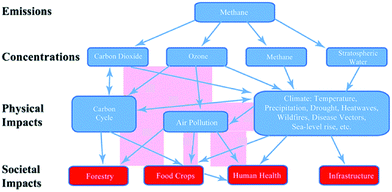 | ||
| Fig. 4 Schematic diagram of the pathways by which methane emissions, and its consequent oxidation in both the troposphere and stratosphere, lead to distinct societal impacts. Reproduced from DOI: 10.1039/C7FD00009J with permission from the Royal Society of Chemistry. | ||
Thermodynamic modelling and field measurements together demonstrate a steep increase in the pH of aerosol particles with an increase in the ammonium/(bi)sulfate ratio (DOI: 10.1039/C7FD00086C). Such increases may have important consequences for chemical processing and the environmental impacts of aerosol particles, in response to emission control policies.
Two-way interactions between atmospheric chemistry and the biosphere were examined during the meeting, relevant for both pollution control and land use change policies. New model estimates suggest that the atmospheric chemistry response to anthropogenic land use change leads to a positive radiative forcing, resulting from a decrease in biogenic VOC emissions and subsequent SOA formation, which outweighs the negative radiative forcing of reduced O3 and CH4 (through OH response) (DOI: 10.1039/C7FD00028F). The large-scale response of global ecosystems to the radiative effects of aerosol particulate pollution were also discussed. Aerosols decrease direct radiation at the surface, but increase diffuse sunlight, with a net cooling and drying of the climate system. It was also shown that aerosols increase net primary productivity (NPP) in the Amazon and suppress NPP in the Boreal region (DOI: 10.1039/C7FD00033B). These studies continue to enhance the horizons of atmospheric chemistry to go beyond the composition of the atmosphere to components that the atmosphere interacts with.
New measurements suggest that interactions of mineral dust with SOA particles lead to the formation of reactive oxygen species (ROS) in aqueous solution (DOI: 10.1039/C7FD00023E). Such interactions may lead to the release of ROS in the human respiratory tract, with subsequent oxidative stress and health effects. Another study showed that the allergenic potential of proteins in bioaerosols may be enhanced in the presence of NO2 and O3 pollution through nitration and oligomerization processes, and postulated that an increase in tropospheric O3 over the Anthropocene may be responsible for promoting widespread protein modifications, contributing to the observed increase of allergies over recent decades (DOI: 10.1039/C7FD00005G).
The public health impact of atmospheric pollution is a major driver for atmospheric chemistry research. New estimates of health impacts from air pollution in Africa were presented at the meeting, based on new emission scenarios and atmospheric modelling (DOI: 10.1039/C7FD00011A). These results suggest that residential energy use contributes most to the ambient particulate matter abundances and associated mortality in this region. Although there are uncertainties in our knowledge of atmospheric distributions of ground level air pollutants, and of their toxicity and detailed interactions with the human body, the consensus emerging from the meeting was that there is an important role for the atmospheric chemistry community in reporting best estimates of air pollution burdens to health. Improved interdisciplinary working between the atmospheric chemistry, emissions and epidemiological communities would allow more robust framing and communication of uncertainties and caveats associated with such estimates, again highlighting the central role of atmospheric chemistry in societal issues.
Despite the substantial estimated global effects of air pollution on public health and ecosystems, “Clean Air” is not identified as one of the Sustainable Development Goals (SDG) of the United Nations. However, our understanding of emissions, processing, and deposition (both out of the atmosphere and into living organisms) of air pollutants are all developing rapidly, building an increasingly robust evidence base for the adoption of such a goal, as proposed in the concluding talk by Jos Lelieveld.
5. Concluding remarks
From the Industrial Revolution to the present day, the human population has climbed rapidly from under a billion to almost 8 billion, and it continues to rise. The burning of fossil fuels – coal, oil, and gas – associated with planet-wide industrialization, along with agricultural and land-use change, have caused dramatic impacts that are likely to be felt far into the future, even when human activities might impact the Earth’s system less. The combined human-driven changes to the Earth's environment have been identified to be on such a large scale that they characterize a new epoch – the Anthropocene. The goal of humanity is to move out of this epoch such that humans are not the dominating influence on Earth. It is clear that this goal can be met only by continued research into atmospheric chemistry and use of that scientific information to make informed choices and policies for the betterment of people and the planet. The studies presented at the meeting were a positive sign of the future when such a goal can be met.| This journal is © The Royal Society of Chemistry 2017 |
