Open Access Article
Open Access ArticleControlling azobenzene photoswitching through combined ortho-fluorination and -amination†
Z.
Ahmed‡
 ,
A.
Siiskonen‡
,
A.
Siiskonen‡
 ,
M.
Virkki
,
M.
Virkki
 and
A.
Priimagi
and
A.
Priimagi
 *
*
Laboratory of Chemistry and Bioengineering, Tampere University of Technology, P.O. Box 541, FI-33101, Tampere, Finland. E-mail: arri.priimagi@tut.fi
First published on 9th October 2017
Abstract
We present a series of visible-light-absorbing azobenzene photoswitches with cis-lifetimes ranging from one second to three days. We combine ortho-fluorination to control the cis-lifetimes, and ortho-amination to boost the visible-light absorption. The synthesis is accomplished by selectively replacing one or more ortho-fluorines with amines in the ortho-fluoroazobenzene precursors.
Photoswitchable azobenzene derivatives have gained increased utility in devising materials whose properties and functions can be remotely controlled with light.1 Their attractiveness is based on their synthetic versatility, high isomerization quantum yields, change in dipole moment upon photoisomerization, and fatigue resistance.2 Equally importantly, their activation wavelengths as well as the photoisomerization dynamics can be significantly altered by simple structural modifications. As a result, azobenzenes have been employed in a variety of applications, including photopharmocology,3 photocontrollable catalysis,4 solar light harvesting,5 and optical-to-mechanical energy conversion.6 Generally, visible-light-switchable azobenzenes are preferred over their UV-switchable counterparts, ideally combined with strong absorptivity to enable switching with low-intensity irradiation. However, the desired switching dynamics may drastically depend on the application. For example, fast bidirectional switching may be required for liquid-crystal photonics,7 whereas in photobiology,3b a bistable system may be more beneficial. Therefore, methods to selectively control the cis-lifetimes of azobenzenes, while maintaining strong visible-light absorption, are of great importance in different fields of research.
The activation wavelength as well as the switching dynamics of azobenzenes can be controlled through ortho-substitution. For example, ortho-fluorination significantly increases the cis-lifetime and separates the n–π* transitions of the trans and cis isomers, enabling quantitative and bidirectional photoswitching.8 The ortho-fluorinated azobenzenes have therefore found use in photoactuators,9 metal–organic frameworks,10 and crystal engineering.11 However, due to their low molar absorption coefficients in the visible region, relatively intense irradiation is required for fast photoswitching, which may be a significant drawback especially in biological systems.
ortho-Amination, on the other hand, significantly increases the molar absorptivity in the visible range, yet at the same time decreases the cis-lifetime.12ortho-Methoxylation increases both the cis-lifetime and the molar absorption coefficient, but the latter only moderately.13 Although ortho-methoxylated azobenzenes have been employed in photobiology,14 they are quite rapidly reduced with glutathione, which may limit their use in biological applications. ortho-Aminated azobenzenes, in turn, are not reduced with glutathione, but as a drawback, the described synthetic route suffers from low reaction yields (2–24%) and does not allow for the synthesis of asymmetric azobenzenes with different substituents at the aromatic rings.12 Although different types of ortho-substituents yield very distinct spectral and photochemical properties, they have not been systematically combined into the same photoswitch. Through rationally designed combination of different ortho-substituents, a versatile control over the photoswitching properties can be achieved.
Herein, we report the design, synthesis and characterization of azobenzene photoswitches that combine ortho-fluorination to increase the cis-lifetimes, and ortho-amination to increase the visible-light absorption. Since the azo group activates vicinal halogens towards nucleophilic aromatic substitution in ortho-halogenated azobenzenes,15 and fluoride is an excellent leaving group, we reasoned that the ortho-amino groups could be conveniently introduced by replacing ortho-fluorines with amines in the easily synthesized ortho-fluoroazobenzenes8 (Scheme 1). Density functional theory (DFT) calculations on the mechanism of the amination reaction showed that in azobenzenes with two ortho-fluorines, the energy barrier for the first amination is several kilocalories per mole lower than for the second amination (ESI†). Hence, by controlling the reaction conditions, it should be possible to selectively replace only one of the fluorines.
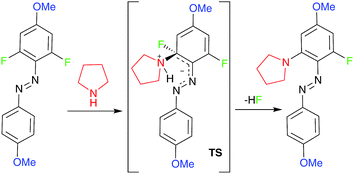 | ||
| Scheme 1 Selective amination of the ortho-fluorinated azobenzene precursor, showing the transition state (TS) and the mono-aminated product. | ||
As precursors, we used three ortho-fluorinated azobenzenes, 1, 2 and 3, containing only one fluorine, one fluorine on each ring, and two fluorines on one ring, respectively. The asymmetric azobenzene precursors 1 and 3 were obtained via a one-pot diazonium coupling reaction of the corresponding phenols with 4-methoxyphenyldiazonium tetrafluoroborate, followed by methylation, whereas the symmetrical ortho-fluorinated precursor 2 was synthesized by oxidation of the corresponding aniline as shown in Scheme 2.8a,16 The precursors were designed to carry methoxy groups at the para positions, to provide a possibility towards further functionalization with, e.g., biocompatible units or polymerizable groups. Pyrrolidine was chosen as the amine (unless otherwise stated) since it red-shifts the absorption maxima and enhances the molar absorption coefficient in the visible region.12
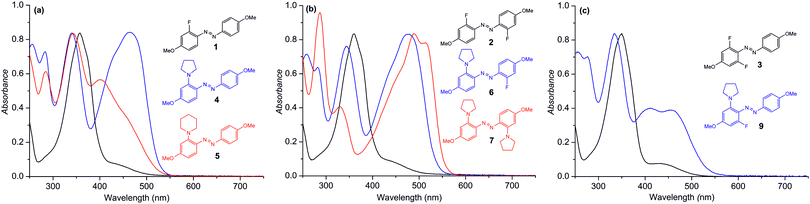
Treatment of the monofluorinated precursor 1 with pyrrolidine at room temperature afforded the ortho-aminated azobenzene 4 in excellent yield (90%). As expected, replacing the fluorine with pyrrolidine decreases the cis-lifetime dramatically (41.8 h → 15.2 s; Fig. S22 and S27, ESI†), at the same time significantly enhancing visible-light absorption (Fig. 1a, Table 1). In addition, 4 can be switched back and forth between the trans- and cis-forms using 435 nm and 595 nm light over several cycles without any sign of fatigue (Fig. S28, ESI†). The fluorine could also be replaced with piperidine under gentle heating (55 °C), to afford 5 in excellent yield (96%). Also in this case the absorption in the visible range was significantly increased, while the cis-lifetime was much less affected (41.8 h → 9.1 h; Fig. S30, ESI†). Therefore, by using the same fluorinated precursor and two different amines, a great variability in the cis-lifetimes (15.2 s for 4vs. 9.1 h for 5) was obtained, while maintaining relatively strong absorption in the visible region. We envision that the reaction can be extended to various amines to control other properties, such as water solubility.
| Compd | λ max (nm) | ε (M−1 cm−1) | τ (s h−1) | cis-% at PSSc |
|---|---|---|---|---|
| a Lifetimes measured at 50, 60, and 70 °C, and extrapolated to 25 °C using the Arrhenius equation. b The long measurement time resulted in slight solvent evaporation during the experiment, which increased the error margin. c Estimated fractions of the cis-isomers at the photostationary state. | ||||
| 1 | 357/432 | 29110/3608 | 41.8 ± 1.3 ha | 93 |
| 2 | 359/432 | 25810/3185 | 60.2 ± 0.5 ha | 93 |
| 3 | 350/432 | 24652/2286 | 430 ± 150 hab | 94 |
| 4 | 340/462 | 12693/12749 | 15.2 ± 0.1 s | 73 |
| 5 | 341/402 | 14924/10075 | 9.1 ± 0.3 h | 75 |
| 6 | 344/479 | 13777/15042 | 258 ± 30 s | 71 |
| 7 | 488/514 | 3701/3456 | 1.21 ± 0.01 s | 62 |
| 9 | 335/411/454 | 16739/8006/7792 | 72 ± 8 ha | 80 |
Next, the selectivity of the amination was tested by reacting the symmetrical difluoro precursor 2 with pyrrolidine. By carrying out the reaction at room temperature, the mono-aminated product 6 was obtained in high yield (81%), along with a minor amount of the bis-aminated product 7 (15%). The two products were easily separated by chromatography. By performing the reaction at 55 °C, the yields were reversed and the bis-aminated product 7 was obtained in 74% yield (along with 6 in 20% yield). Thus, as predicted by the DFT calculations (ESI†), the reactivity difference of the first and second amination is large enough to enable mono-amination in good selectivity. In other words, depending on the reaction temperature, we could replace either one or both of the ortho-fluorines with pyrrolidine, thereby controlling the selectivity of the reaction and obtaining either symmetric (7) or asymmetric (6) amination of 2.
Compounds 6 and 7 showed a substantial difference in their absorption profile and photoswitching behavior. Compound 7 exhibits an absorption maximum at 488 nm, with a pronounced low-energy shoulder at 514 nm, yet with moderate molar absorption coefficient of <4 000 M−1 cm−1. Compound 6, in turn, displays a comparable absorption maximum wavelength (479 nm) accompanied with a significant increase in the molar absorption coefficient (>15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1). In addition to the four-fold enhancement in the absorption coefficient, the cis-lifetime of 6 was increased from 1.2 s to 258 seconds (Table 1, Fig. S32 and S33, ESI†), i.e., by more than a factor of 200. This can be attributed to the presence of the ortho-fluorine, pinpointing the benefit of our design concept of simultaneous ortho-fluorination and ortho-amination.
000 M−1 cm−1). In addition to the four-fold enhancement in the absorption coefficient, the cis-lifetime of 6 was increased from 1.2 s to 258 seconds (Table 1, Fig. S32 and S33, ESI†), i.e., by more than a factor of 200. This can be attributed to the presence of the ortho-fluorine, pinpointing the benefit of our design concept of simultaneous ortho-fluorination and ortho-amination.
Finally, we studied the selective amination of 3, bearing two ortho-fluorines on the same phenyl ring. Performing the reaction in neat pyrrolidine afforded the bis-aminated product 8 which was highly unstable and therefore not studied further. However, conducting the reaction in acetonitrile, with pyrrolidine only as a reagent, the mono-aminated product 9 was obtained in excellent yield (92%). It has very attractive properties, combining strong molar absorptivity in the visible range and an exceptionally long cis-lifetime of 72 h at 25 °C (Fig. 1c, Table 1, Fig. S35, ESI†). This lifetime is substantially longer than for any other ortho-aminated azobenzene reported up to date. The trans–cis and cis–trans photoswitching of 9 can be triggered by 405 nm and 595 nm irradiation, respectively, with no sign of photodegradation over repeated switching cycles (Fig. S36, ESI†). We used 1H NMR spectra, obtained before and after irradiation with the visible light (405 nm), to estimate the cis-fraction of 9, concluding that >80% of the azobenzenes exist in the cis-form in the photostationary state (Fig. S37, ESI†).
Fig. 2 illustrates the pronouncedly different cis-lifetimes achievable through our molecular design, ranging from one second (compound 7; the red curve) up to several days (compound 9; the blue curve). This is a highly attractive feature, enabling the use of our synthetic pathway to rationally design visible-absorbing azobenzenes with optimized isomerization dynamics for a multitude of applications.
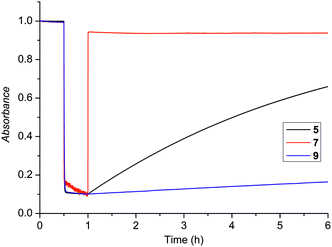 | ||
| Fig. 2 Normalized cis–trans thermal back relaxation for compounds 5, 7, 9. The irradiation was switched on at 30 min and ceased at 60 min. | ||
In summary, we describe a novel approach for controlling the cis-lifetimes and absorption coefficients of azobenzenes by combining ortho-fluoro- and ortho-amino-functionalities in the same photoswitch. This combination stabilizes the cis-isomer and at the same time increases the absorption in the visible region. The attained control over the cis-lifetimes and broad coverage of absorption spectrum makes the studied compounds promising candidates for a variety of applications such as sunlight harvesting and photobiology. The synthetic route allows for selective replacement of one or more fluorines with an amine in the fluorinated azobenzenes in good to excellent yields. The presence of methoxy groups in the para positions leaves a possibility to attach the photoswitches with different polymers/systems, according to the field of application. We envision that the design concept of including different types of ortho-substituents to control the spectral and photochemical properties of azobenzenes can be expanded to other functionalities besides fluorines and amines, to obtain photoswitchable compounds with even a wider range of properties.
We thank the financial support of the European Research Council (Grant Agreement No. 679646). Computing resources provided by the CSC – IT Center for Science Ltd are gratefully acknowledged.
Conflicts of interest
There are no conflicts to declare.Notes and references
- M. M. Russew and S. Hecht, Adv. Mater., 2010, 22, 3348–3360 CrossRef CAS PubMed.
- (a) H. M. Bandara and S. C. Burdette, Chem. Soc. Rev., 2012, 41, 1809–1825 RSC; (b) A. A. Beharry and G. A. Woolley, Chem. Soc. Rev., 2011, 40, 4422–4437 RSC; (c) R. Klajn, Pure Appl. Chem., 2010, 82, 2247–2279 CrossRef.
- (a) J. Broichhagen, J. A. Frank and D. Trauner, Acc. Chem. Res., 2015, 48, 1947–1960 CrossRef CAS PubMed; (b) M. M. Lerch, M. J. Hansen, G. M. van Dam, W. Szymanski and B. L. Feringa, Angew. Chem., Int. Ed., 2016, 55, 10978–10999 CrossRef CAS PubMed.
- (a) R. S. Stoll and S. Hecht, Angew. Chem., Int. Ed., 2010, 49, 5054–5075 CrossRef CAS PubMed; (b) R. Gostle, A. Senf and S. Hecht, Chem. Soc. Rev., 2014, 43, 1982–1996 RSC.
- (a) A. K. Saydjari, P. Weis and S. Wu, Adv. Energy Mater., 2017, 7, 1601622 CrossRef; (b) A. M. Kolpak and J. C. Grossman, Nano Lett., 2011, 11, 3156–3162 CrossRef CAS PubMed; (c) A. Lennartson, A. Roffey and K. Moth-Poulsen, Tetrahedron Lett., 2015, 56, 1457–1465 CrossRef CAS.
- (a) O. S. Bushuyev, M. Aizawa, A. Shishido and C. J. Barrett, Macromol. Rapid Commun., 2017, 1700253 CrossRef PubMed; (b) T. Ube and T. Ikeda, Angew. Chem., Int. Ed., 2014, 53, 10290–10299 CrossRef CAS PubMed; (c) T. J. White, Photomechanical Materials, Composites, and Systems: Wireless Transduction of Light into Work, John Wiley & Sons, 2017 Search PubMed.
- U. A. Hrozhyk, S. V. Serak, N. V. Tabiryan, L. Hoke, D. M. Steeves and B. R. Kimball, Opt. Express, 2010, 18, 8697–8704 CAS; L. De Sio, N. Tabiryan, T. J. Bunning, B. R. Kimball and C. Umeton, Prog. Opt., 2013, 58, 1–64 Search PubMed.
- (a) C. Knie, M. Utecht, F. Zhao, H. Kulla, S. Kovalenko, A. M. Brouwer, P. Saalfrank, S. Hecht and D. Bleger, Chem. – Eur. J., 2014, 20, 16492–16501 CrossRef CAS PubMed; (b) D. Bleger, J. Schwarz, A. M. Brouwer and S. Hecht, J. Am. Chem. Soc., 2012, 134, 20597–20600 CrossRef CAS PubMed; (c) D. Bleger and S. Hecht, Angew. Chem., Int. Ed., 2015, 54, 11338–11349 CrossRef CAS PubMed; (d) M. J. Hansen, M. M. Lerch, W. Szymanski and B. L. Feringa, Angew. Chem., Int. Ed., 2016, 55, 13514–13518 CrossRef CAS PubMed.
- (a) S. Iamsaard, E. Anger, S. J. Asshoff, A. Depauw, S. P. Fletcher and N. Katsonis, Angew. Chem., Int. Ed., 2016, 55, 9908–9912 CrossRef CAS PubMed; (b) K. Kumar, C. Knie, D. Bleger, M. A. Peletier, H. Friedrich, S. Hecht, D. J. Broer, M. G. Debije and A. P. Schenning, Nat. Commun., 2016, 7, 11975 CrossRef CAS PubMed.
- (a) K. Mueller, A. Knebel, F. Zhao, D. Bleger, J. Caro and L. Heinke, Chem. – Eur. J., 2017, 23, 5434–5438 CrossRef CAS PubMed; (b) S. Castellanos, A. Goulet-Hanssens, F. Zhao, A. Dikhtiarenko, A. Pustovarenko, S. Hecht, J. Gascon, F. Kapteijn and D. Bleger, Chem. – Eur. J., 2016, 22, 746–752 CrossRef CAS PubMed.
- (a) O. S. Bushuyev, A. Tomberg, T. Friscic and C. J. Barrett, J. Am. Chem. Soc., 2013, 135, 12556–12559 CrossRef CAS PubMed; (b) O. S. Bushuyev, T. C. Corkery, C. J. Barrett and T. Friscic, Chem. Sci., 2014, 5, 3158–3164 RSC; (c) S. K. Rastogi, R. A. Rogers, J. Shi, C. Gao, P. L. Rinaldi and W. J. Brittain, J. Org. Chem., 2015, 80, 11486–11490 CrossRef PubMed; (d) M. Saccone, A. Siiskonen, F. Fernandez-Palacio, A. Priimagi, G. Terraneo, G. Resnati and P. Metrangolo, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2017, 73, 227–233 CAS.
- O. Sadovski, A. A. Beharry, F. Zhang and G. A. Woolley, Angew. Chem., Int. Ed., 2009, 48, 1484–1486 CrossRef CAS PubMed.
- A. A. Beharry, O. Sadovski and G. A. Woolley, J. Am. Chem. Soc., 2011, 133, 19684–19687 CrossRef CAS PubMed.
- S. Samanta, A. A. Beharry, O. Sadovski, T. M. McCormick, A. Babalhavaeji, V. Tropepe and G. A. Woolley, J. Am. Chem. Soc., 2013, 135, 9777–9784 CrossRef CAS PubMed.
- (a) G. M. Badger, J. W. Cook, W. P. Vidal, H. H. Hodgson and E. R. Ward, J. Chem. Soc., 1947, 1109 RSC; (b) J. T. Manka, V. C. Mckenzie and P. Kaszynski, J. Org. Chem., 2004, 69, 1967–1971 CrossRef PubMed; (c) J. Miller, Aromatic Nucleophilic Substitution, Elsevier, New York, 1968 Search PubMed.
- E. Merino, Chem. Soc. Rev., 2011, 40, 3835–3853 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7cc07308a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |