Open Access Article
Open Access ArticleBioorthogonal double-fluorogenic siliconrhodamine probes for intracellular super-resolution microscopy†
E.
Kozma
a,
G.
Estrada Girona
 b,
G.
Paci
b,
G.
Paci
 b,
E. A.
Lemke
b and
P.
Kele
b,
E. A.
Lemke
b and
P.
Kele
 *a
*a
a“Lendület” Chemical Biology Research Group, Institute of Organic Chemistry, Research Centre for Natural Sciences, Hungarian Academy of Sciences, Magyar tudósok krt. 2, 1117 Budapest, Hungary. E-mail: kele.peter@ttk.mta.hu
bStructural and Computational Biology Unit, Cell Biology and Biophysics Unit, European Molecular Biology Laboratory, Meyerhofstrasse 1, Heidelberg, D-69117, Germany
First published on 11th May 2017
Abstract
A series of double-fluorogenic siliconrhodamine probes were synthesized. These tetrazine-functionalized, membrane-permeable labels allowed site-specific bioorthogonal tagging of genetically manipulated intracellular proteins and subsequent imaging using super-resolution microscopy.
Recent years have brought substantial progress in the field of super-resolution microscopy (SRM) enabling the exploration of biomolecular processes in the sub-diffraction range.1 Although the emerged SRM techniques have revealed fine details of live organisms, there is still room for further improvements. Nowadays, the unavailability of dyes suitable for site-specific tagging of intracellular biomolecules is considered as the biggest limitation of SRM techniques.2 Ideal small-sized organic fluorophores can be synthetically tailored in order to meet the criteria necessary for intracellular SRM imaging of live cells. Such criteria are as follows: (a) both the labels themselves and the chemistry applied for tagging should be biocompatible, (b) their photophysical characteristics should allow minimal background and autofluorescence in order to result in a high signal-to-noise (S/N) ratio, (c) their fluorescence should be bright and not or minimally impaired by photobleaching and (d) they should be able to transit through membranes. As for the chemistry applied for installation of such probes, bioorthogonal reactions are extremely useful as they are fully biocompatible.3 Among these transformations, inverse electron demand Diels–Alder (IEDDA) reactions between tetrazines and strained unsaturated ring systems enable the fastest reactions.4 In order to minimize phototoxicity and autofluorescence, far-red/near-infrared (NIR) emitting fluorophores (650–900 nm) are especially well suited, while background fluorescence of unreacted probes bound non-specifically to hydrophobic surfaces can be reduced efficiently by the use of fluorogenic species.5,6
Our continuing efforts to develop fluorescent probes that combine the above listed features have resulted in several bioorthogonally applicable fluorogenic probes with various excitation/emission characteristics.7 Lately, our attention turned to tetrazine quenched fluorescent frameworks as tetrazines can act both as bioorthogonal handles and quencher moieties.7d Tetrazines can exert their quenching effect either by Förster-resonance energy transfer (FRET) or through-bond energy transfer (TBET) mechanisms.8,9 FRET-based fluorogenic probes are limited to tetrazine–fluorophore pairs where the absorption band of the tetrazine (typically between 520 and 540 nm) overlaps with the emission band of the fluorophore. TBET-based systems, on the other hand, are not constrained by the emission band of the fluorescent unit and, in theory, any kind of fluorophore–tetrazine pairs are suitable upon proper design. Moreover, TBET-based quenching is much faster and more efficient, thus enhancement of the fluorescence signal can reach up to 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold upon the IEDDA reaction with dienophiles.9a The tetrazine-based TBET-quenching approach has already been applied to various fluorophores, yet, it has not been performed on SRM compatible dyes.9 However, techniques like SRM would greatly benefit from the high signal-to-noise ratio offered by the TBET-mechanism.
000-fold upon the IEDDA reaction with dienophiles.9a The tetrazine-based TBET-quenching approach has already been applied to various fluorophores, yet, it has not been performed on SRM compatible dyes.9 However, techniques like SRM would greatly benefit from the high signal-to-noise ratio offered by the TBET-mechanism.
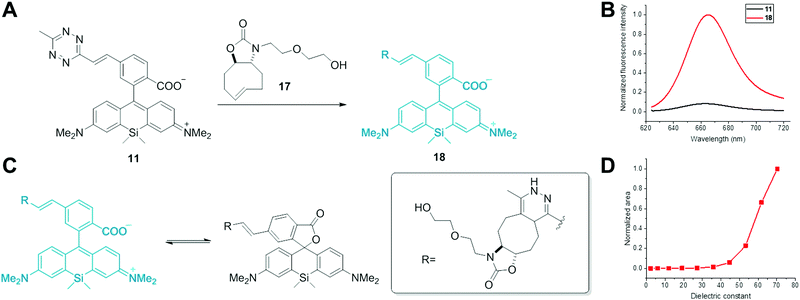
Siliconrhodamine (SiR) is a widely used membrane-permeable NIR dye for SRM applications.10 Besides the high photostability and brightness, the unique environment-dependent fluorescence of carboxy-SiRs due to a polarity dependent lactone-formation offers an appealing opportunity to distinguish between specific and non-specific labeling (polarity-based fluorogenicity).10 When bound to polar protein surfaces, carboxyl-SiRs exist in a fluorescent zwitterionic form, while upon non-specific adhesion to hydrophobic surfaces, they close to a non-fluorescent spirolactone (Fig. 1). SiR derivatives were applied in voltage sensing, in vivo tumor imaging, photodynamic therapy and multicolour imaging studies as well.11 Despite their wide applications, there are only a few examples of SiR-based probes for site-specific labeling of proteins.10a,12,13 Furthermore, no TBET-based carboxyl-SiR fluorogenic probes have been reported yet.
Herein we report the first set of bioorthogonally applicable, NIR-emitting, membrane permeable, double fluorogenic probes suitable for SRM imaging with exceptionally high turn-on rates compared to previously reported tetrazine-based NIR dyes.9d We demonstrate the power of the developed SiR-dyes in site-specific labeling of intracellular skeletal proteins and subsequent SRM imaging applications.
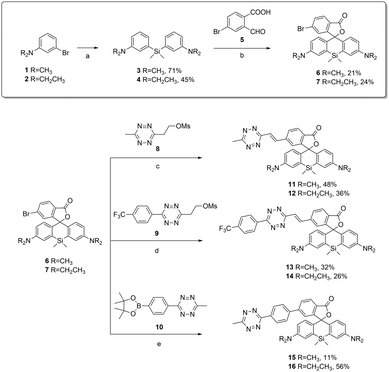
TBET-based fluorogenic probes are composed of a donor fluorophore and an acceptor tetrazine unit that are linked together with a conjugated but electronically decoupled (twisted) linker.14 The tetrazine moiety can be installed onto the phenyl group of SiR directly or via alkene, alkyl and phenyl linkers. Former examples showed that incorporation of the tetrazine through direct conjugation or via alkyl-linkers into fluorophores is less efficient.9c,d,f Therefore we decided to apply alkene and phenyl linkers to connect the tetrazine to carboxy-SiR. Devaraj and co-workers and also our group have recently reported Heck- and Suzuki-type cross-coupling reactions to connect methyl-substituted tetrazines to fluorophores.7d,9b To this end we have synthesized Br-substituted intermediate 6. The general synthetic route for SiR derivatives proceeds through a Si–xantone intermediate which is reacted with aryl lithium reagents via nucleophilic addition.10a Through this route, however, introduction of halogen-substituted aromatic rings is problematic. Therefore we proceeded via an aldehyde condensation reaction developed by Wang et al. (Scheme 1) and acquired Br-SiR derivative 6.15a
We positioned the Br-substituent at the 6-position as previous reports showed superior fluorogenic properties of 6-substituted rhodamine derivatives compared to 5-substitution.9d,f Pd-Catalyzed cross-coupling reactions of 6 with methyl vinyltetrazine precursor (8) and methyltetrazine phenylboronic acid pinacol ester (10) resulted in alkene-(11) and phenyl-linked SiR-tetrazine (15), respectively, in moderate to good yields (11–48%). To improve the reaction rate of alkene-linked SiR-tetrazine in IEDDA, we designed a derivative with a trifluoromethyl-phenyl substituent. Electron-withdrawing groups are known to markedly increase the reactivities of tetrazines in IEDDA reactions.4a To this end, we synthesized trifluoromethyl-tetrazine (9) in two steps via Zn(OTf)2 catalyzed Pinner-synthesis and subsequent O-mesylation.15b Heck reaction of tetrazine 9 with compound 6 proceeded with moderate yields (32%). It is known that different N-alkylation patterns on the rhodamine core can markedly alter the spectral characteristics and brightness of probes.16 Rotation about the C–N bond in –NMe2 substituted rhodamines leads to faster decay of the excited state and hence to lowered quantum yield. Thus we synthesized –NEt2 substituted derivatives (12, 14, 16) starting from Br-SiR derivative 7, which have restricted C–N bond rotation compared to –NMe2 derivatives.
Next, we characterized the spectral properties of SiR-tetrazines 11–16. Measurements were conducted in water containing 0.01% SDS to prevent aggregation of the dyes.10a Excitation maxima were found to be at around 645 nm and 655 nm, and fluorescence emission was detected at around 665 nm and 670 nm for the dimethylamino- and diethylamino-derivatives, respectively (Table 1 and Fig. S1, see the ESI†). We measured the fluorogenic properties of the probes in an IEDDA reaction with OxTCO (17) (Fig. 1 and Fig. S2, ESI,†Table 1).7d To our delight, all derivatives showed much larger fluorescence enhancements (FE) than previously reported fluorogenic tetrazine NIR dyes (cf. 5.6×).9d We propose that this is attributed to the double fluorogenic nature of our SiR dyes. Among dimethylamine derivatives, phenyl-linked 15 showed the highest turn-on ratio (31×). As expected, ethyl substitution on the N-atom improved turn-on rates of the dyes, and up to 49-fold increase was observed. Although energy transfer occurs from the excited state, interestingly, the extinction coefficients were measured to be lower than ε of the parent fluorophores (Table 1).
| Dye | λ exc(max) [nm] | λ em(max) [nm] | ε max , [M−1 cm−1] | ϕ F , | ΔϕFd | FEe |
|---|---|---|---|---|---|---|
| a Values refer to tetrazine-dyes while values in parentheses are that of the respective conjugates with 17. b Measured at absorption maxima of the species. c Cresyl violet in MeOH was used as the quantum yield reference (ϕF = 0.53). d Quantum yield ratios of conjugates with 17 and tetrazine-dyes in 0.1% SDS in water. e Fluorescence intensity enhancements upon the reaction with 17 in 0.1% SDS in water at emission maxima of the products. | ||||||
| 11 | 643 (645) | 665 (667) | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 (19 700 (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) 200) |
0.016 (0.34) | 21 | 22 |
| 12 | 653 (652) | 671 (672) | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 (19 600 (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800) 800) |
0.015 (0.80) | 50 | 49 |
| 13 | 647 (648) | 662 (665) | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 (22 200 (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) 500) |
0.028 (0.26) | 9 | 6 |
| 14 | 655 (654) | 673 (672) | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (25 000 (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600) 600) |
0.037 (0.27) | 7 | 8 |
| 15 | 645 (644) | 660 (660) | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (21 000 (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600) 600) |
0.012 (0.34) | 28 | 31 |
| 16 | 651 (652) | 671 (670) | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (23 000 (23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900) 900) |
0.015 (0.53) | 35 | 40 |
The second order rate constants were also determined and were in good agreement with k2 values of previously reported methyl-tetrazine derivatives with similarly reactive TCO* (for values see the ESI†).17 As expected, trifluoromethylphenyl derivatives, 13 and 14 reacted much faster than methyl derivatized congeners with OxTCO (17) (Fig. S3, ESI†). Due to the lower electron density of the tetrazines in 13 and 14 IEDDA reactions went to completion within minutes compared to methyl substituted probes where the reaction required ca. 1.5 h.
We also investigated the polarity dependent absorbance of the Diels–Alder product of alkene-SiR (18). In agreement with previous findings, in solvents with lower dielectric constants, absorption maximum characteristic of the spirolactone form at 290 nm was dominant. In polar solvents, however, the zwitterionic form with a characteristic absorption at 645 nm dominated the spectrum (Fig. S4, ESI†). As a consequence, in solvents with dielectric constants <50 conjugate 18 was non-fluorescent while fluorescence gradually reinstated with increasing polarity (Fig. 1).
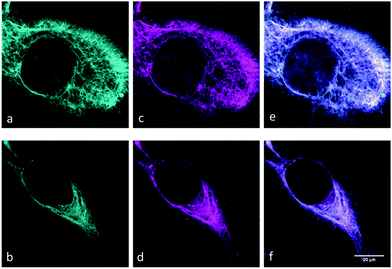
With the SiR-dyes in hand, showing exceptional fluorogenicity in the NIR region, we were eager to evaluate their performance in the cellular environment. We sought to utilize the genetic code expansion (GCE) technology for site-specific labeling with the newly developed double-fluorogenic probes. We and others have recently shown that non-canonical amino acids (ncAA) with strained alkene and alkyne moieties can be genetically encoded in mammalian cells using the pyrrolysine tRNAPyl/PylRS pair from the Methanosarcina species.18 We used the previously reported skeletal protein vimentin116TAG-mOrange to test the intracellular labeling ability of the dyes.13,18e Using the orthogonal tRNAPyl/PylRSAF pair we genetically encoded commercially available cyclooctynylated-lysine (Lys(ε-N-BCNendo), Fig. S5, ESI†) into vimentin116TAG-mOrange and performed live cell labeling experiments with dyes 11–16 in mammalian cells. Clear live cell labeling was observed with dye 11 and 12 (Fig. 2), and even with concentrations as low as 1.5 μM within 10 min at 37 °C (for additional images at different concentrations and labeling times see Fig. S6 and S7 (ESI†), and for a comparison of different post-labeling wash times see Fig. S8, ESI†). The labeling was specific without strong background fluorescence (as quantified by channel colocalisation in Fig. S9, ESI†). Less efficient labeling was observed with phenyl-linked dyes 15 and 16, which is probably due to their slower kinetics (Fig. S10, ESI†). Although CF3-dyes 13 and 14 showed the fastest labeling properties in vitro, they did not give any specific labeling in vivo (results not shown). We attribute this to the low polarity of these dyes, which probably impairs their ability to cross the cell-membrane or to label the protein specifically.
To date, there have only been a few reports on bioorthogonally applicable, fluorogenic tetrazine-mediated intracellular labeling inside living cells and subsequent SRM.13,18e,19 Among them, to the best of our knowledge, there have been no reports of site-specific labeling with TBET-based fluorogens. We set out to test the suitability of dyes 11 and 12 in SRM of vimentin. While GSDIM (ground state depletion microscopy followed by individual molecule return)21 SRM with TIRF illumination was possible with vimentinBCNendo-mOrange labeled with dye 11, we did not get any specific SRM signal with diethyl-derivative 12 (results not shown). SRM imaging with SiR-11 (Fig. 3E and F) clearly gives enhanced resolution compared to the reference channels (Fig. 3A–D and Fig. S11, ESI†).
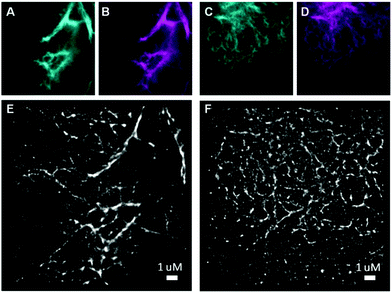 | ||
| Fig. 3 SRM imaging of vimentinBCNendo-mOrange labeled with dye 11. Panels (A and C) (cyan) and (B and D) (magenta) show the mOrange and SiR reference channels, respectively for the corresponding SRM images in panels (E and F) from dye 11 labeling (3 μM for 30 minutes at 37 °C). SRM images (E and F) have resolutions of 35 nm and 28 nm respectively as determined by the Fourier ring correlation criterion (FRC).20 | ||
In conclusion, we have developed double-fluorogenic siliconrhodamine-tetrazine probes with improved kinetics and enhanced fluorescence turn-on ratios in the NIR region upon inverse electron demand Diels–Alder reactions. We have demonstrated the effect of the linker and N-alkylation pattern of the SiR-tetrazine core on the labeling efficiency using confocal microscopy. We successfully applied one derivative in site-specific super-resolution imaging of a cytoskeletal protein, vimentin. Such probes with distinct spectral characteristics would allow multicolor super-resolution imaging of various intracellular structures.
The present work was supported by the Hungarian Scientific Research Fund (OTKA, NN-116265) and the “Lendület” Program of the Hungarian Academy of Sciences (LP2013-55/2013). E. K. is grateful for the support of The New National Excellence Program of The Ministry of Human Capacities (Hungary). EAL acknowledges the SPP1623 and SFB1129 for funding.
Notes and references
- (a) M. Heilemann, J. Biotechnol., 2010, 149, 243 CrossRef CAS PubMed; (b) B. Huang, H. Babcock and X. Zhuang, Cell, 2010, 143, 1047 CrossRef CAS PubMed.
- (a) M. Fernández-Suárez and A. Y. Ting, Nat. Rev. Mol. Cell Biol., 2008, 9, 929 CrossRef PubMed; (b) T. J. Chozinski, L. A. Gagnon and J. C. Vaughan, FEBS Lett., 2014, 588, 3603 CrossRef CAS PubMed.
- (a) C. P. Ramil and Q. Lin, Chem. Commun., 2013, 49, 11007 RSC; (b) J. A. Prescher and C. R. Bertozzi, Nat. Chem. Biol., 2005, 1, 13 CrossRef CAS PubMed; (c) E. M. Sletten and C. R. Bertozzi, Angew. Chem., Int. Ed., 2009, 48, 6974 CrossRef CAS PubMed.
- (a) A.-C. Knall and C. Slugovc, Chem. Soc. Rev., 2013, 42, 5131 RSC; (b) E. Kozma, O. Demeter and P. Kele, ChemBioChem, 2017, 18, 486 CrossRef CAS PubMed.
- (a) G. Cserép, A. Herner and P. Kele, Methods Appl. Fluoresc., 2015, 3, 042001 CrossRef; (b) L. D. Lavis and R. T. Raines, ACS Chem. Biol., 2014, 9, 855 CrossRef CAS PubMed.
- A. Nadler and C. Schultz, Angew. Chem., Int. Ed., 2013, 52, 2408 CrossRef CAS PubMed.
- (a) A. Herner, I. Nikić, M. Kállay, E. A. Lemke and P. Kele, Org. Biomol. Chem., 2013, 11, 3297 RSC; (b) A. Herner, G. Estrada Girona, I. Nikić, M. Kállay, E. A. Lemke and P. Kele, Bioconjugate Chem., 2014, 25, 1370 CrossRef CAS PubMed; (c) O. Demeter, E. A. Fodor, M. Kállay, G. Mező, K. Németh, P. T. Szabó and P. Kele, Chem. – Eur. J., 2016, 22, 6382 CrossRef CAS PubMed; (d) G. Knorr, E. Kozma, A. Herner, E. A. Lemke and P. Kele, Chem. – Eur. J., 2016, 22, 8972 CrossRef CAS PubMed.
- (a) N. K. Devaraj, S. Hilderbrand, R. Upadhyay, R. Mazitschek and R. Weissleder, Angew. Chem., Int. Ed., 2010, 49, 2869 CrossRef CAS PubMed; (b) J. Yang, J. Šečkutė, C. M. Cole and N. K. Devaraj, Angew. Chem., Int. Ed., 2012, 51, 7476 CrossRef CAS PubMed.
- (a) L. G. Meimetis, J. C. T. Carlson, R. J. Giedt, R. H. Kohler and R. Weissleder, Angew. Chem., Int. Ed., 2014, 53, 7531 CrossRef CAS PubMed; (b) H. Wu, J. Yang, J. Šečkute and N. K. Devaraj, Angew. Chem., Int. Ed., 2014, 53, 5805 CrossRef CAS PubMed; (c) A. Wieczorek, T. Buckup and R. Wombacher, Org. Biomol. Chem., 2014, 12, 4177 RSC; (d) A. Wieczorek, P. Werther, J. Euchner and R. Wombacher, Chem. Sci., 2017, 8, 1506 RSC; (e) P. Agarwal, B. J. Beahm, P. Shieh and C. R. Bertozzi, Angew. Chem., Int. Ed., 2015, 54, 11504 CrossRef CAS PubMed; (f) J. C. T. Carlson, et al. , Angew. Chem., Int. Ed., 2013, 52, 6917 CrossRef CAS PubMed.
- (a) G. Lukinavičius, et al. , Nat. Chem., 2013, 5, 132 CrossRef PubMed; (b) S. Uno, et al. , Nat. Chem., 2014, 6, 681 CAS; (c) P. Shieh, M. S. Siegrist, A. J. Cullen and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 5456 CrossRef CAS PubMed.
- Y. Kushida, T. Nagano and K. Hanaoka, Analyst, 2015, 140, 685 RSC.
- T. Peng and H. C. Hang, J. Am. Chem. Soc., 2016, 138, 14423 CrossRef CAS PubMed.
- C. Uttamapinant, et al. , J. Am. Chem. Soc., 2015, 137, 4602 CrossRef CAS PubMed.
- (a) T. G. Kim, et al. , J. Phys. Chem. A, 2006, 110, 20 CrossRef CAS PubMed; (b) M. Steeger, S. Griesbeck, A. Schmiedel, M. Holzapfel, I. Krummenacher, H. Braunschweig and C. Lambert, Phys. Chem. Chem. Phys., 2015, 17, 11848 RSC.
- (a) B. Wang, X. Chai, W. Zhu, T. Wang and Q. Wu, Chem. Commun., 2014, 50, 14374 RSC; (b) J. Yang, M. R. Karver, W. Li, S. Sahu and N. K. Devaraj, Angew. Chem., Int. Ed., 2012, 51, 5222 CrossRef CAS PubMed.
- J. B. Grimm, B. P. English, J. Chen, J. P. Slaughter, Z. Zhang, A. Revyakin, R. Patel, J. J. Macklin, D. Normanno, R. H. Singer, T. Lionnet and L. D. Lavis, Nat. Methods, 2015, 12, 244 CrossRef CAS PubMed.
- J. E. Hoffmann, T. Plass, I. Nikić, I. V. Aramburu, C. Koehler, H. Gillandt, E. A. Lemke and C. Schultz, Chem. – Eur. J., 2015, 21, 12266 CrossRef CAS PubMed.
- (a) T. Plass, et al. , Angew. Chem., Int. Ed., 2012, 51, 4166 CrossRef CAS PubMed; (b) I. Nikić, T. Plass, O. Schraidt, J. Szymański, J. A. Briggs, C. Schultz and E. A. Lemke, Angew. Chem., Int. Ed., 2014, 53, 2245 CrossRef PubMed; (c) E. Kozma, I. Nikić, B. R. Varga, I. V. Aramburu, J. H. Kang, O. T. Fackler, E. A. Lemke and P. Kele, ChemBioChem, 2016, 17, 1518 CrossRef CAS PubMed; (d) K. Lang, L. Davis, J. Torres-Kolbus, C. Chou, A. Deiters and J. W. Chin, Nat. Chem., 2012, 4, 298 CrossRef CAS PubMed; (e) I. Nikić, et al. , Angew. Chem., Int. Ed., 2016, 55, 16172 CrossRef PubMed.
- R. S. Erdmann, et al. , Angew. Chem., Int. Ed., 2014, 53, 10242 CrossRef CAS PubMed.
- N. Banterle, et al. , J. Struct. Biol., 2013, 183, 363 CrossRef CAS PubMed.
- J. Fölling, et al. , Nat. Methods, 2008, 5, 943 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7cc02212c |
| This journal is © The Royal Society of Chemistry 2017 |