Cu(II) and Zn(II) based phthalocyanines as hole selective layers for perovskite solar cells†
Laura
Caliò
a,
Jorge
Follana-Berná
 c,
Samrana
Kazim
a,
Morten
Madsen
d,
Horst-Günter
Rubahn
d,
Ángela
Sastre-Santos
c,
Samrana
Kazim
a,
Morten
Madsen
d,
Horst-Günter
Rubahn
d,
Ángela
Sastre-Santos
 *c and
Shahzada
Ahmad
*c and
Shahzada
Ahmad
 *ab
*ab
aAbengoa Research, Abengoa, C/ Energía Solar no. 1, Campus Palmas Altas-41014, Sevilla, Spain. E-mail: shahzada.ahmad@bcmaterials.net; Tel: +34 946128811
bBasque Center for Materials, Applications and Nanostructures (BCMaterials), Parque Tecnológico de Bizkaia, Building 500, Derio, Spain
cÁrea de Química Orgánica, Instituto de Bioingeniería, Universidad Miguel Hernández, Avda. Universidad s/n, Elche 03202, Spain
dSDU NanoSYD, Mads Clausen Institute, University of Southern Denmark, Alsion 2, 6400 Sønderborg, Denmark
First published on 15th September 2017
Abstract
Recently, significant progress has been achieved in the fabrication of highly efficient perovskite solar cells, while major challenges, such as the commercial viability of exotic materials and their instability, remain an obstacle. Hole transporting materials (HTMs) represent a tricky choice for the fabrication of efficient solar cells and cost ineffective Spiro-OMeTAD continues to be so far the most obvious candidate. Organometallic complexes, such as phthalocyanine metal complexes, appeared as a promising class of p-type material, since they are less expensive and more stable. Herein, we report the synthesis of a novel Cu(II)-based phthalocyanine [(tOctPhO)8CuPc 1] with 4-tert-octylphenoxy-substituted functional groups that possesses a very good solubility in a wide range of organic solvents, and thus can be applied using solution processing in a wide range of electro-optical devices. In the present work (tOctPhO)8CuPc 1 and its analogous Zn(II) phthalocyanine [represented as (tOctPhO)8ZnPc 2] were tested in mixed perovskites (FAPbBr3)0.85(MAPbI3)0.15 for the fabrication of perovskite solar cells. These phthalocyanine based HTMs exhibited competitive power conversion efficiencies and demonstrated superior stability when compared to classical HTMs.
Introduction
In recent years, organic–inorganic lead halide perovskite solar cells (PSCs) have received huge interest in photovoltaic research due to their high efficiency, low cost, and easy processability.1 Notable progress has been achieved since their first introduction in 2009,2 and their power conversion efficiencies (PCEs) have increased from just 3.8% to >22% in 2016.3 However, some important issues such as use of low-cost materials and long-term stability still remain unsolved, which represent major obstacles for their commercial use. Recent studies have been reported on perovskite compositional engineering or architecture optimization to improve the long-term stability of PSC devices.4,5 A mixed halide or mixed cation perovskite was reported to enhance the long-term stability;6 however, perovskite is not the only component of the device architecture that needs optimization. Though perovskite behaves as an ambipolar material and it can conduct holes and electrons by itself, the use of a hole transporting material (HTM) is paramount in order to fabricate efficient devices.7 The key role of HTMs is to efficiently extract holes and rapidly inject them toward the metal contact, in order to avoid charge recombination. An ideal p-type material used as a HTM for efficient and stable PSCs should fulfil the following requirements: easy synthetic and purification process to allow them to be cost-effective; high charge mobility, to avoid recombination within the perovskite interface; proper energetic level matching with active materials, i.e. HOMO–LUMO levels; along with high thermal stability and moisture insensitivity. To date, 2,2′,7,7′-tetrakis-(N,N-di-p-methoxyphenylamino)-9,9′-spirobifluorene (Spiro-OMeTAD) was found to be the most efficient and so far the most explored small organic HTM for PSCs,8 even though it doesn't fulfil all the requirements for an ideal HTM, mostly due to its complex synthetic route and multi-step purification process that increase its cost and decrease its potential large-scale application in commercial photovoltaics. Moreover, it suffers from a relatively poor charge mobility (1 × 10−5 to 1 × 10−4 cm2 V−1 s−1) which compels the usage of additives such as lithium bis(trifluoromethanesulfonyl)imide (LiTFSI), 4-tert-butyl pyridine (t-BP) and tris(2-(1H-pyrazol-1-yl)-4-tert-butylpyridine) cobalt(III) tri[bis(trifluoromethane)sulfonimide] (FK209)9 in order to enhance both the conductivity and the charge mobility of the HTM. The use of such additives induces instability in the devices since they accelerate degradation processes due to their deliquescent and hygroscopic properties.10,11 Recently sincere efforts have been made to design novel HTMs in order to replace the expensive Spiro-OMeTAD, by design of new polymeric, organometallic or small organic molecules with improved opto-electrical properties.12 Small organic molecules, i.e. simplified Spiro-type,8,13 carbazole,14 azulene15 and truxene-based16 HTMs, have been recently reported and successfully integrated into PSCs, yielded good performance, they can be easily synthesized to achieve low-cost materials. Metal organic complexes based on phthalocyanines17–23 have been extensively employed in opto-electrical devices as charge transporting materials, since they can be easily synthesized and purified to cap their cost. Moreover their 18-π electron macrocyclic conjugated structure endows them with excellent semiconductor properties, such as high charge mobility and excellent thermal and chemical stability. These properties of Cu(II), Ni(II) and Zn(II)-based phthalocyanines (Pc) have recently attracted considerable attention and have been applied in PSCs, exhibiting an efficiency of 17%.20–23 However, most of the Cu(II) and Zn(II)-based phthalocyanines developed have poor solubility in common organic solvents, and thermal evaporation procedures are required to deposit them as a thin layer to be used as HTMs; this can be tricky and can destroy the underlying perovskite layer. Recently, tetra n-butyl CuPc, as a mixture of regioisomers, has been developed in order to increase the solubility and allow solution processability.24 Here, we report the synthesis of a novel and easy to produce Cu(II) phthalocyanine, substituted by eight 4-tert-octylphenoxy groups that can efficiently reduce aggregation and possess an excellent solubility in all common organic solvents.Herein, the single isomer Cu(II) phthalocyanine [(tOctPhO)8CuPc 1] and its Zn(II) homologue [(tOctPhO)8ZnPc 2]25 have been used as HTMs in [(FAPbBr3)0.85(MAPbI3)0.15] perovskite solar cells. We have optimized the thickness of the HTM layers, by employing three different concentrations, to tune their behaviour as p-type materials and photovoltaic properties.
Results and discussion
Synthesis and characterization of (tOctPhO)8CuPc 1 and (tOctPhO)8ZnPc 2
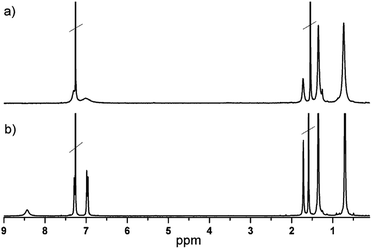
Phthalocyanines were prepared by cyclotetramerization of 4,5-bis[p-(tert-octyl)phenoxy]-phthalonitrile using a metal acetate salt as the template in the presence of DBN as the base and DMAE as the solvent (Scheme 1). The tert-octylphenoxy group was chosen due to its strong electron-donor character and furthermore it increases the solubility and prevents the π–π stacking due to its high volume. The new compounds were characterized by UV-vis, 1H-NMR spectroscopy and HR-MALDI-TOF mass spectrometry.The 1H NMR spectra of (tOctPhO)8CuPc 1 and (tOctPhO)8ZnPc 2 in CDCl3 are shown in Fig. 2. The spectrum of (tOctPhO)8ZnPc 2 (b) shows two aromatic signals for the protons of tert-octylphenoxy groups at 6.97 and 7.28 ppm and one broad signal for the nonperipheral protons of the phthalocyanine core at 8.43 ppm. This broad signal is attributed to a small aggregation phenomenon in CDCl3 as solvent as compared with the sharp singlet observed at 8.96 ppm when THF-d8 was used (Fig. S2†). Nevertheless, due to the paramagnetic character of copper, there is no signal for the phthalocyanine core and the signals of tert-octylphenoxy groups in (tOctPhO)8CuPc 1 (a) are broader than those of (tOctPhO)8ZnPc 2.
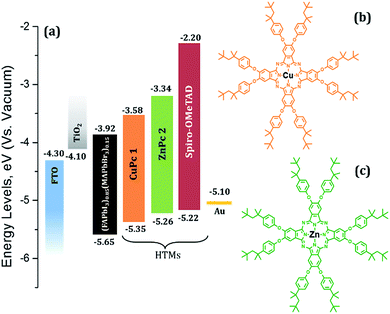 | ||
| Fig. 1 (a) Energy level diagram of the materials used in this study and (b) chemical structure of (tOctPhO)8CuPc 1 and (c) (tOctPhO)8ZnPc 2. | ||
The UV-vis spectra of the compounds in CHCl3 (Fig. 3) show Soret bands around 350 nm and Q bands around 680 nm, characteristic of non-aggregated phthalocyanines.
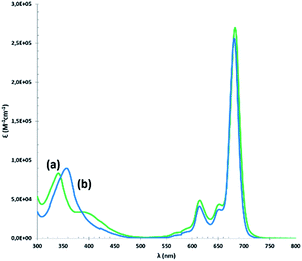 | ||
| Fig. 3 UV-vis absorption spectra of (a) (tOctPhO)8CuPc 1 (green) and (b) (tOctPhO)8ZnPc 2 (blue) measured in CHCl3. | ||
Electrochemical characterization was performed using cyclic voltammetry in dry THF as solvent containing 0.1 M TBAPF6 as the supporting electrolyte (Fig. 4). (tOctPhO)8CuPc 1 shows one oxidation potential at 0.55 V and one reduction potential at −1.22 V (vs. Fc/Fc+). (tOctPhO)8ZnPc 2 shows one oxidation potential at 0.39 V and one reduction potential at −1.48 V (vs. Fc/Fc+).
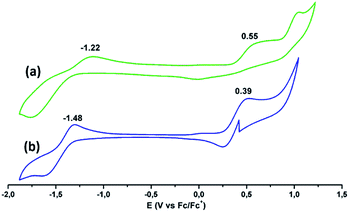 | ||
| Fig. 4 Cyclic voltammograms of (a) (tOctPhO)8CuPc 1 (green) and (b) (tOctPhO)8ZnPc 2 (blue) in deaerated THF solutions containing TBAPF6 (0.1 M) obtained at 298 K. | ||
Photovoltaic performance of PSCs with (tOctPhO)8CuPc 1 and (tOctPhO)8ZnPc 2 as HTMs
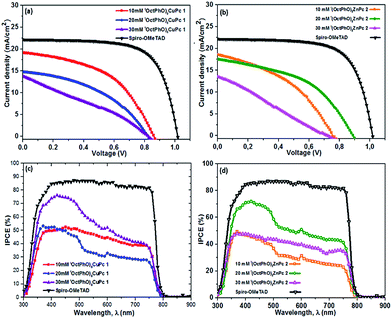
Fig. 1a presents the band alignment and the relative energy levels of all the materials used in this work. The novel Cu(II) and Zn(II) phthalocyanine-based HTMs (chemical structure is depicted in Fig. 1b and c, respectively) possess a HOMO of −5.35 eV and −5.26 eV respectively, and have a favourable energy level match with (FAPbI3)0.85(MAPbBr3)0.15. PSCs were fabricated using these phthalocyanines as HTMs, in a conventional mesoporous architecture such as FTO/c-TiO2/meso-TiO2/(FAPbI3)0.85(MAPbBr3)0.15/HTM/Au. For both (tOctPhO)8CuPc 1 and (tOctPhO)8ZnPc 2, the thickness of HTM layers was tuned by using three different concentrations, 10–30 mM. Fig. 5a and b depict the current–voltage (J–V) characteristics of (tOctPhO)8CuPc 1 and (tOctPhO)8ZnPc 2-based PSCs obtained for each concentration, respectively. The photovoltaic parameters, including short-circuit current density (Jsc), open-circuit voltage (Voc), fill factor (FF), and PCE, are summarized in Table 1, while the statistical values including the average for each configuration are presented in Table S1.† We have noted that the thinner layers of (tOctPhO)8CuPc 1 and (tOctPhO)8ZnPc 2 worked more effectively in a mixed perovskite based environment. By minimizing the concentrations, thinner layers of the HTMs can be deposited and they showed improved performance. This is mainly due to a decrease in series resistance and more effective charge extraction. On decreasing the concentration of CuPc solution, the series resistance decreases from 211.22 to 136.57 and 71.55 Ω cm2 for 30, 20 and 10 mM concentrations, respectively. Similarly, for ZnPc it decreases from 399.45 to 107.23 and 128.82 Ω cm2 as the concentration of the solution decreases. As shown in Table 1, for the (tOctPhO)8CuPc 1-based devices the best photovoltaic parameters were obtained at 10 mM concentration, resulting in an improved Jsc (19.01 mA cm−2) and FF of 50.56%. In the case of (tOctPhO)8ZnPc 2, the 20 mM-based devices gave the best efficiency, resulting in an improved Voc (890 mV) and FF of 46.52%. Though encouraging photovoltaic parameters were obtained, by optimizing the concentration (thickness) of HTMs, the reported PCE values are relatively lower than those in the recent report of phthalocyanine-based molecules as HTMs in PSCs.20 In particular, the relatively low FF for both Cu and Zn-based phthalocyanines could be addressed due to the poor hole transport ability of these materials, supported by the presence of high series resistance. This can be achieved partially by reducing the concentration of HTM. This poor charge transport ability can be ascribed to an unfavourable molecular packing in the film formation, originating due to the steric hindrance of the bulky substituents on the phthalocyanine moiety. The presence of eight orthogonal tert-octylphenoxy groups not only possibly hinders the π-stacking interaction, reducing the possibility of aggregation, but also hampers the intermolecular charge-transfer process. Subsequently, the presence of bulky tert-octylphenoxy substituents has a significant effect on the solubility, especially in perovskite-friendly solvents like chlorobenzene and toluene. In spite of the presence of such hindered substituents, these newly developed phthalocyanine-based HTMs were produced by a simple synthetic route, without the need for any further purification steps, and are solution processable. The open circuit voltage remains relatively constant, even on reducing the thickness of the hole transporting layer. Since the mismatch in the energetic levels of these new molecules with the perovskites is relatively low, the relatively low Voc obtained is likely due to poor electron blocking properties, which produce an undesired recombination of excitons at the HTM–perovskite interface. The introduction of a buffer layer between the developed HTMs and the perovskite can prevent such kinds of losses.24 In order to further elucidate the effects of HTMs on the device performance, the incident photon-to-electron conversion efficiency (IPCE) of each type of perovskite solar cell was measured (Fig. 5c and d). The IPCE results were in good agreement with the electrical characteristics of each type of device. The (tOctPhO)8CuPc 1-based and (tOctPhO)8ZnPc 2-based devices with the resultant optimum concentration of 10 mM and 20 mM, respectively, exhibited enhanced IPCE over the whole visible wavelength range, from 300 to 800 nm.| HTM | V oc (mV) | J sc (mA cm−2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| (tOctPhO)8CuPc 1 10 mM | 870 | 19.01 | 50.56 | 8.33 |
| (tOctPhO)8CuPc 1 20 mM | 730 | 16.85 | 35.00 | 4.29 |
| (tOctPhO)8CuPc 1 30 mM | 820 | 13.78 | 29.63 | 3.35 |
| (tOctPhO)8ZnPc 2 10 mM | 760 | 18.76 | 40.56 | 5.75 |
| (tOctPhO)8ZnPc 2 20 mM | 890 | 17.52 | 46.52 | 7.25 |
| (tOctPhO)8ZnPc 2 30 mM | 720 | 13.49 | 25.29 | 2.45 |
| Spiro-OMeTAD | 1040 | 21.52 | 68.87 | 15.20 |
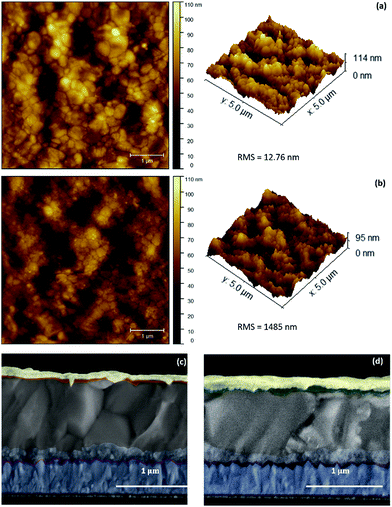
To elucidate the microstructure of (tOctPhO)8CuPc 1 (a) and (tOctPhO)8ZnPc 2 (b) layers, it was deposited atop of perovskites and scanning probe microscopy was employed to image the surface. Both these HTMs, showed smooth and uniform coverage as HTLs (Fig. 6a and b). (tOctPhO)8CuPc 1 (a) was found to be relatively smoother than (tOctPhO)8ZnPc 2 (b). The root mean square (RMS) roughness was 12.76 nm and 14.85 nm respectively, when measured on a 5 × 5 μm2 for these HTLs. This hypothesis was also supported by the top-view morphology recorded by using an interference microscope (Fig. S3†). Microscopy experiments revealed a uniform layer, with fewer pinholes and less surface irregularity. The cross-sectional image of a full device is presented in Fig. 6c and d and it shows well interconnected and defined layers. Here perovskites are the thickest layers, while the HTM layer is buried (due to a very low thickness) between the perovskites and gold layer, which is acting as the cathode (top layer). A very thin compact layer and mesoporous TiO2 are also represented in the anatomy of the device, and these are in accordance with the structure adopted for the fabrication of the device, i.e. FTO/c-TiO2/meso-TiO2/(FAPbI3)0.85(MAPbBr3)0.15/HTM/Au.
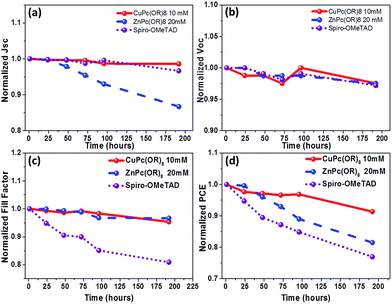
The stability of PSCs and its charge selective contacts are also an important issue that needs to be addressed. Metal phthalocyanines exhibit high thermal and chemical stability and this can help to improve the preservation of the underlying perovskite layer, and will allow enhancing the stability of the PSCs. The air stability of unencapsulated devices with the configurations of Cu(II) and Zn(II) Pc-based PSCs was examined for over 200 hours and for comparative purposes Spiro-OMeTAD based standard devices were also given the same treatment. The unsealed PSCs were kept in the ambient environment at 25 °C and under a relative humidity of 50–60%. The corresponding performance changes versus time are summarized in Fig. 7. Fig. 7d illustrates the normalized PCEs of PSCs with (tOctPhO)8CuPc 1 as the HTM only losing less than 10% of the initial value after a 7 day stability test, and (tOctPhO)8ZnPc 2 based devices have shown a drop of 15% of their initial value, while the reference devices with Spiro-OMeTAD, show a loss of over 20% of their initial efficiency value. From Fig. 7a–c, it can be deduced that the influential loss for (tOctPhO)8ZnPc 2 based devices corresponds to a drop of the Jsc of 15%, while for Spiro-OMeTAD the degradation corresponds to a significant decrease in the FF, around 20%, whereas all the other parameters remained constant. These results point towards the necessity of inorganic based materials such as Cu(II) and Zn(II) Pc derivatives as promising alternatives in order to fabricate efficient devices with an improved stability.
Experimental
Materials
All the chemicals were purchased from Sigma Aldrich, whereas 2,2′,7,7′-tetrakis(N,N-di-p-methoxyphenylamine)-9,9-spirobifluorene (Spiro-OMeTAD) was acquired from Merck KGaA, methylammonium iodide (MAI), formamidinium iodide (FAI), and methylammonium bromide (MABr) from Dyesol, and PbI2 and PbBr2 were purchased from Tokyo Chemical Industry (TCI); all the chemicals were employed without any treatment or purification.All reactions were carried out under an argon atmosphere. 1-Hexanol was dried with K2CO3, followed by filtration and distillation before use. The solvent for spectroscopic studies was of spectroscopic grade and used as received. UV-vis absorbance was measured with a Helios Gamma spectrophotometer. High-resolution mass spectra were obtained using a Bruker Microflex LRF20 matrix assisted laser desorption/ionization time of flight (MALDI-TOF) instrument using dithranol as the matrix. 4,5-Bis[p-(tert-octyl)phenoxy]phthalonitrile and (tOctPhO)8ZnPc 2 were synthesized according to the processes described in the literature.25
Synthesis of (tOctPhO)8CuPc 1
300 mg (0.56 mmol) of 4,5-bis[p-(tert-octyl)phenoxy]phthalonitrile, 38 mg (0.28 mmol) of CuCl2 and two drops of DBN were dissolved in 1 mL of DMAE and heated to reflux under an argon atmosphere overnight. After being cooled to room temperature, the crude was washed with MeOH. Purification by chromatography using toluene as the solvent afforded (tOctPhO)8CuPc 1 (86 mg, 28%) as a green powder. 1H NMR (CDCl3): δ = 0.73 (s, 72H; tert-butyl), 1.35 (s, 48H; –CH3), 1.72 (s, 16H; –CH2–), 7.02 (s, 16H; phenol), 7.30 (s, 16H; phenol). UV-vis: λmax (CHCl3)/nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 341 (4.92), 615 (4.69), 683 (5.43). HRMS MALDI-TOF (dithranol): m/z: for C144H176CuN8O8 calcd, 2208.2902 [M+]; found 2208.2930.
ε): 341 (4.92), 615 (4.69), 683 (5.43). HRMS MALDI-TOF (dithranol): m/z: for C144H176CuN8O8 calcd, 2208.2902 [M+]; found 2208.2930.
Electrochemical measurements
Cyclic voltammetry was performed in a conventional three-electrode cell using a μ-AUTOLAB type III potentiostat/galvanostat at 298 K over benzonitrile and deaerated sample solutions containing 0.10 M tetrabutylammonium hexafluorophosphate (TBAPF6) as the supporting electrolyte at a scan rate of 100 mV s−1. A platinum working electrode, an Ag/AgNO3 reference electrode, and a platinum wire counter electrode were employed. Ferrocene/ferrocenium was used as an internal standard for all measurements.Device fabrication
Initially, fluorine doped tin oxide (FTO) coated glasses (TEC15), used as substrates were cleaned by ultrasonication in 2% Hellmanex water solution for 30 minutes followed by rinsing with deionized water; then the same process was repeated with the use of acetone and isopropanol and finally heated up to 500 °C to remove any organic residue. Then, a TiO2 compact layer was deposited on FTO substrates via spray pyrolysis at 450 °C from a precursor solution of titanium diisopropoxide bis(acetylacetonate) in anhydrous ethanol. After the spraying, the substrates were left at 450 °C for 30 minutes and allowed to cool down to room temperature. Afterwards, a 150–200 nm mesoporous TiO2 layer was deposited by spin coating a (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) TiO2 paste (Dyesol 30 NR-D) diluted in ethanol for 20 s at 4000 rpm. The samples were immediately dried at 125 °C and then annealed until 500 °C through a four-step temperature ramp and maintained at 500 °C for 30 min in order to allow the transformation of amorphous TiO2 into anatase. Upon cooling to room temperature, the substrates were transferred to an argon-filled glove-box in order to deposit the upper perovskite layer. The precursor solution for preparing the mixed perovskite was prepared by dissolving FAI (1 M), PbI2 (1.2 M), MABr (0.2 M), and PbBr2 (0.2 M) in anhydrous DMF
7) TiO2 paste (Dyesol 30 NR-D) diluted in ethanol for 20 s at 4000 rpm. The samples were immediately dried at 125 °C and then annealed until 500 °C through a four-step temperature ramp and maintained at 500 °C for 30 min in order to allow the transformation of amorphous TiO2 into anatase. Upon cooling to room temperature, the substrates were transferred to an argon-filled glove-box in order to deposit the upper perovskite layer. The precursor solution for preparing the mixed perovskite was prepared by dissolving FAI (1 M), PbI2 (1.2 M), MABr (0.2 M), and PbBr2 (0.2 M) in anhydrous DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO 4
DMSO 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v). The perovskite solution was spin coated in a two-step sequence at 1000 and 6000 rpm for 10 and 20 s, respectively. During the second step, 100 μL of chlorobenzene was used as an antisolvent and poured on the substrates for 10 s prior to the end of the spinning. The substrates were then annealed at 100 °C for 1 h in a glovebox. After the perovskite annealing and cooling, the hole transport layer, consisting of Spiro-OMeTAD (70 mM), or (tOctPhO)8CuPc 1 and (tOctPhO)8ZnPc 2 (in three different concentrations: 10, 20 and 30 mM), was prepared by dissolving the corresponding amount of materials in chlorobenzene. Spiro-OMeTAD was doped by adding bis-(trifluoromethylsulfonyl)imide lithium salt (Li-TFSI, from Aldrich), tris(2-(1H-pyrazol-1-yl)-4-tert-butylpyridine) cobalt(III)-tris(bis-(trifluoro-methylsulfonyl)imide) (FK209), and 4-tert-butylpyridine (t-BP, from Aldrich) in the molar ratio of 0.5, 0.01, and 3.3 for Li-TFSI, FK209, and t-BP, respectively. (tOctPhO)8CuPc 1 and (tOctPhO)8ZnPc 2 were doped by adding LiTFSI and t-BP as additives in the molar ratio of 0.5 and 3.3 respectively. 35 μL of HTM solutions were spin-coated onto the perovskite layer at 4000 rpm for 20 s, in a dry air filled glove-box. Finally, a 70–80 nm gold layer as the cathode was thermally evaporated under low vacuum (10−6 torr) to complete the device fabrication. All solutions were prepared inside an argon-filled glovebox under controlled moisture and oxygen conditions (O2 < 10 ppm, H2O < 2 ppm).
1 (v/v). The perovskite solution was spin coated in a two-step sequence at 1000 and 6000 rpm for 10 and 20 s, respectively. During the second step, 100 μL of chlorobenzene was used as an antisolvent and poured on the substrates for 10 s prior to the end of the spinning. The substrates were then annealed at 100 °C for 1 h in a glovebox. After the perovskite annealing and cooling, the hole transport layer, consisting of Spiro-OMeTAD (70 mM), or (tOctPhO)8CuPc 1 and (tOctPhO)8ZnPc 2 (in three different concentrations: 10, 20 and 30 mM), was prepared by dissolving the corresponding amount of materials in chlorobenzene. Spiro-OMeTAD was doped by adding bis-(trifluoromethylsulfonyl)imide lithium salt (Li-TFSI, from Aldrich), tris(2-(1H-pyrazol-1-yl)-4-tert-butylpyridine) cobalt(III)-tris(bis-(trifluoro-methylsulfonyl)imide) (FK209), and 4-tert-butylpyridine (t-BP, from Aldrich) in the molar ratio of 0.5, 0.01, and 3.3 for Li-TFSI, FK209, and t-BP, respectively. (tOctPhO)8CuPc 1 and (tOctPhO)8ZnPc 2 were doped by adding LiTFSI and t-BP as additives in the molar ratio of 0.5 and 3.3 respectively. 35 μL of HTM solutions were spin-coated onto the perovskite layer at 4000 rpm for 20 s, in a dry air filled glove-box. Finally, a 70–80 nm gold layer as the cathode was thermally evaporated under low vacuum (10−6 torr) to complete the device fabrication. All solutions were prepared inside an argon-filled glovebox under controlled moisture and oxygen conditions (O2 < 10 ppm, H2O < 2 ppm).
Device characterization
Current density–voltage (J–V) curves were recorded with a Keithley 2400 source-measurement-unit under AM 1.5G, 100 mW cm2 illumination from a 450 W AAA solar simulator (ORIEL, 94023 A). It was calibrated using a NREL certified calibrated monocrystalline silicon solar cell. A black metal mask (0.16 cm2) was used over the square solar cell active area (0.5 cm2) to reduce the influence of scattered light. Photovoltaic parameters including Jsc, Voc, fill factor (FF), and power conversion efficiency (PCE) were extracted from the photocurrent–voltage (J–V) curves of the solar cells (active surface area: 0.16 cm2, scan rate: 100 mV s−1, and pre-sweep delay: 10 s). The IPCE measurements were performed using a Newport 150 W xenon lamp coupled with an Oriel Cornerstone 260 motorized 1/4 m monochromator as the light source and a 2936 R power meter to measure the short circuit current. Atomic force microscopy experiments were performed using a Veeco Dimension 3100 and the data were analysed by using Gwyddion software, while the cross-sectional scanning probe electron images were recorded by using a Hitachi S-4800 SEM.Conclusions
To conclude, we have reported the usage of two non-aggregated Cu and Zn based phthalocyanines, which are highly soluble in most organic solvents. These (tOctPhO)8CuPc 1 and (tOctPhO)8ZnPc 2 were then used as a hole-selective contact for perovskite solar cell fabrication. The thickness of hole selective layers was optimized by tuning the concentration and it was found that the device fabricated with the thinnest layer gave the best performance. The Cu based phthalocyanine outperformed the Zn based one and competitive power conversion efficiencies in excess of 8% were achieved, along with superior stability compared to classical small molecule based hole transport materials. Our work demonstrates that these easily prepared phthalocyanines can be used as HTMs for perovskite solar cells, and opens up a new pathway to design by molecular engineering new systems, with improved charge transfer abilities for efficient hole extraction.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project has received funding from the European Union Seventh Framework Programme under grant agreement no. 607232 [THINFACE]. This research was financially supported by the Spanish Ministerio de Economía Industria y Competitividad of Spain (CTQ2014-55798-R).Notes and references
- S. Kazim, M. K. Nazeeruddin, M. Grätzel and S. Ahmad, Angew. Chem., Int. Ed., 2014, 53, 2812–2824 ( Angew. Chem. , 2014 , 126 , 2854–2867 ) CrossRef CAS PubMed.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed.
- D. Bi, W. Tress, M. I. Dar, P. Gao, J. Luo, C. Renevier, K. Schenk, A. Abate, F. Giordano, J. C. Baena, J. Decoppet, S. M. Zakeeruddin, M. K. Nazeeruddin, M. Grätzel and A. Hagfeldt, Sci. Adv., 2016, 2, e1501170 Search PubMed.
- Y. Reyna, M. Salado, S. Kazim, A. Perez-Tomas, S. Ahmad and M. Lira-Cantu, Nano Energy, 2016, 30, 570–579 CrossRef CAS.
- M. Deepa, M. Salado, L. Calio and S. Kazim, Phys. Chem. Chem. Phys., 2017, 19, 4069–4077 RSC.
- M. Salado, L. Calio, R. Berger, S. Kazim and S. Ahmad, Phys. Chem. Chem. Phys., 2016, 18, 27148–27157 RSC.
- Z. Yu and L. Sun, Adv. Energy Mater., 2015, 5(12), 1500213 CrossRef.
- D. Bi, B. Xu, P. Gao, L. Sun, M. Grätzel and A. Hagfeldt, Nano Energy, 2016, 23, 138–144 CrossRef CAS.
- J. H. Noh, N. J. Jeon, Y. C. Choi, M. K. Nazeeruddin, M. Grätzel and S. Il Seok, J. Mater. Chem. A, 2013, 1, 11842–11847 CAS.
- A. Abate, T. Leijtens, S. Pathak, J. Teuscher, R. Avolio, M. E. Errico, J. Kirkpatrik, J. M. Ball, P. Docampo, I. McPherson and H. J. Snaith, Phys. Chem. Chem. Phys., 2013, 15, 2572–2579 RSC.
- A. Abate, D. J. Hollman, J. Teuscher, S. Pathak, R. Avolio, G. D'Errico, G. Vitiello, S. Fantacci and H. J. Snaith, J. Am. Chem. Soc., 2013, 135, 13538–13548 CrossRef CAS PubMed.
- L. Calió, S. Kazim, M. Graetzel and S. Ahmad, Angew. Chem., Int. Ed., 2016, 55, 14522–14545 ( Angew. Chem. , 2016 , 128 , 14740–14764 ) CrossRef PubMed.
- M. Saliba, S. Orlandi, T. Matsui, S. Aghazada, M. Cavazzini, J.-P. Correa-Baena, P. Gao, R. Scopelliti, E. Mosconi, K.-H. Dahmen, F. De Angelis, A. Abate, A. Hagfeldt, G. Pozzi, M. Graetzel and M. K. Nazeeruddin, Nat. Energy, 2016, 1, 15017 CrossRef CAS.
- M. Daskeviciene, S. Paek, Z. Wang, T. Malinauskas, G. Jokubauskaite, K. Rakstys, K. T. Cho, A. Magomedov, V. Jankauskas, S. Ahmad, H. J. Snaith, V. Getautis and M. K. Nazeeruddin, Nano Energy, 2017, 32, 551–557 CrossRef CAS.
- H. Nishimura, I. Naoki, A. Shimazaki, A. Wakamiya, A. Saeki, S. Lawrence and Y. Murata, J. Am. Chem. Soc., 2015, 137, 15656–15659 CrossRef CAS PubMed.
- C. Huang, W. Fu, C. Li, Z.-L. Z. Zhang, W. Qiu, M. Shi, P. Heremans, A. K.-Y. Jen and H. Chen, J. Am. Chem. Soc., 2016, 138, 2528–2531 CrossRef CAS PubMed.
- Q. Dao, A. Fujii, R. Tsuji, Y. Takeoka and M. Ozaki, Org. Electron., 2017, 43, 156–161 CrossRef CAS.
- G. Yanga, Y.-L. Wangb, J.-J. Xub, H.-W. Leia, C. Chena, H.-Q. Shanb, X.-Y. Liub, Z.-X. Xub and G.-J. Fanga, Nano Energy, 2016, 31, 322–330 CrossRef.
- Y. Wang, X. Liu, H. Shan, Q. Chen, T. Liu, X. Sun, D. Ma, Z. Zhang, J. Xu and Z. Xu, Dyes Pigm., 2017, 139, 619–626 CrossRef.
- K. T. Cho, O. Trukhina, C. Roldán-carmona, M. Ince, P. Gratia, G. Grancini, P. Gao, T. Marszalek, W. Pisula, P. Y. Reddy, T. Torres and M. K. Nazeeruddin, Adv. Energy Mater., 2017, 7, 1601733 CrossRef.
- P. Gao, K. T. Cho, A. Abate, G. Grancini, P. Y. Reddy, M. Srivasu, M. Adachi, A. Suzuki, K. Tsuchimoto, M. Grätzel and M. K. Nazeeruddin, Phys. Chem. Chem. Phys., 2016, 27083–27089 RSC.
- J. Guo, X. Meng, J. Niu, Y. Yin, M. Han and X. Ma, Synth. Met., 2016, 220, 462–468 CrossRef CAS.
- A. Z. Yu, X. Jiang, J. Lai, Y. Zhang, X. Jiang, Z. Yu, J. Lai, Y. Zhang, M. Hu and N. Lei, ChemSusChem, 2017, 10, 1838–1845 CrossRef PubMed.
- E. Nouri, Y. L. Wang, Q. Chen, J. J. Xu, G. Paterakis, V. Dracopoulos, Z. X. Xu, D. Tasis, M. R. Mohammadi and P. Lianos, Electrochim. Acta, 2017, 233, 36–43 CrossRef CAS.
- F. J. Céspedes-Guirao, K. Ohkubo, S. Fukuzumi, F. Fernández-Lázaro and Á. Sastre-Santos, Chem.–Asian J., 2011, 6, 3110–3121 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: MALDI-TOF, NMR, interference microscope images and device statistics. See DOI: 10.1039/c7se00367f |
| This journal is © The Royal Society of Chemistry 2017 |