Open Access Article
Open Access ArticleVersatile telluracycle synthesis via the sequential electrophilic telluration of C(sp2)–Zn and C(sp2)–H bonds†
Bin
Wu
,
Melvina
,
Xiangyang
Wu
,
Edwin Kok
Lee Yeow
and
Naohiko
Yoshikai
 *
*
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371, Singapore. E-mail: nyoshikai@ntu.edu.sg
First published on 10th April 2017
Abstract
We report herein a new approach for the synthesis of tellurium-bridged aromatic compounds based on the sequential electrophilic telluration of C(sp2)–Zn and C(sp2)–H bonds with tellurium(IV) chlorides. A combination of transition metal-catalyzed (migratory) arylmetalation of alkynes and sequential telluration allows for the expedient construction of a library of functionalized benzo[b]tellurophenes. Furthermore, a variety of heteroarene-fused benzotellurophenes and other novel tellurium-embedded polycyclic aromatics can be readily synthesized from the corresponding 2-iodoheterobiaryls.
Introduction
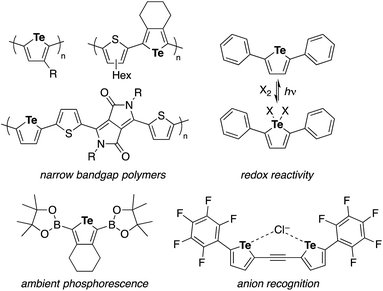
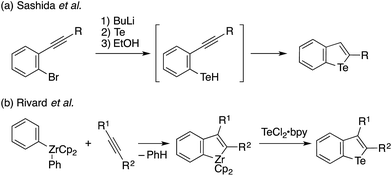
Tellurophene has gained growing interest as a structural element of polymeric materials for organic electronics and other applications with features such as narrow band gaps, low LUMO levels, high charge carrier mobilities, the redox capability of Te, and Te–Te interactions.1,2 Studies of tellurophene-containing small molecules focused on their redox and photochemical reactivity,3 photoluminescence properties,4 and anion recognition ability through chalcogen bonds5 have also emerged (Chart 1). In these studies, the synthesis and functionalization of substituted tellurophenes have been achieved using a variety of methods, such as the cyclization of 1,3-diyne with sodium telluride6 and zirconacyclopentadiene transfer to TeCl2·bpy.2c,7In contrast to the extensive studies on tellurophenes, benzo-fused tellurophenes and other tellurium-bridged aromatic systems, unlike their sulfur and selenium congeners,8 have been much less explored.4b,9 Indeed, there remains a scarcity of synthetic methods for making such tellurium compounds,10 as the C–S and C–Se bond-forming methods can not always be extended to C–Te bond formation.11 Sashida developed a benzo[b]tellurophene synthetic route via the trapping of ortho-alkynylaryllithium with elemental tellurium and subsequent intramolecular cyclization (Scheme 1a).12 Rivard extended the zirconacycle transfer method to achieve benzo[b]tellurophene synthesis via a zirconaindene intermediate (Scheme 1b).4b However, these methods may not be suitable for the rapid preparation of diversely functionalized benzotellurophenes. Likewise, synthetic methods for making other tellurium-bridged (hetero)aromatic systems remain scarce.9a,13
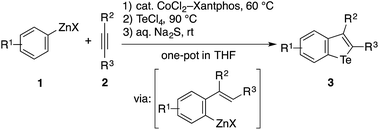
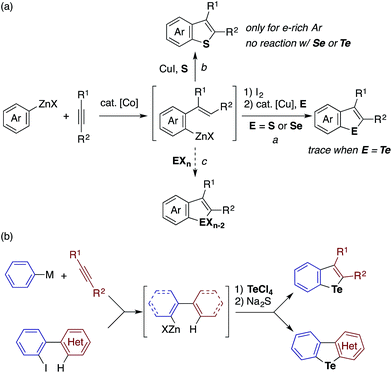
Recently, we developed methods for the synthesis of benzothiophenes and benzoselenophenes based on the cobalt-catalyzed addition of an arylzinc reagent to an alkyne involving 1,4-cobalt migration (migratory arylzincation), which affords an ortho-alkenylarylzinc species as a key intermediate (Scheme 2a).14,15 The iodination of this zinc intermediate is followed by the reaction of the resulting aryl iodide and elemental sulfur or selenium under copper-catalyzed Ullmann-type conditions, allowing for the two-step synthesis of benzothiophene or benzoselenophene (route a). Only when an electron-rich arylzinc reagent is employed can the zinc intermediate be directly converted to the corresponding benzothiophene using stoichiometric CuI and elemental sulfur (route b). Our attempt to synthesise benzotellurophene using elemental tellurium via route a or b was futile.
 | ||
| Scheme 2 Approaches to benzochalcogenophenes via cobalt-catalyzed migratory arylzincation (a) and a summary of the telluracycle synthesis developed in this study (b). | ||
We envisioned that the above limitation in our previous benzochalcogenophene synthesis could be removed using stronger chalcogen electrophiles (EXn), such as TeCl4, SeCl4, and SCl2, to intercept the zinc intermediate (route c). If successful, the electrophilic trapping of the zinc intermediate might be followed by intramolecular dehydrohalogenative cyclization onto the olefinic moiety to furnish benzochalcogenophene.16 Our study along this line allowed us not just to develop a modular one-pot method for benzotellurophene synthesis, but also to establish the sequential electrophilic telluration of C(sp2)–Zn and C(sp2)–H bonds as a general approach to tellurium-bridged aromatic systems (Scheme 2b). Being moderately nucleophilic, the C(sp2)–Zn bond undergoes selective monosubstitution of TeCl4, which is followed by the intramolecular telluration of the proximal alkenyl or (hetero)aryl C(sp2)–H bond to form a telluracycle. A variety of functionalized benzotellurophenes can be synthesized in a one-pot manner starting from arylzinc or aryl Grignard reagents and alkynes by way of cobalt- or nickel-catalyzed (migratory) arylmetalation.14,17 Furthermore, 2-heterobiarylzinc reagents that were generated from the corresponding iodides gave rise to a series of heteroarene-fused benzotellurophenes.
Results and discussion
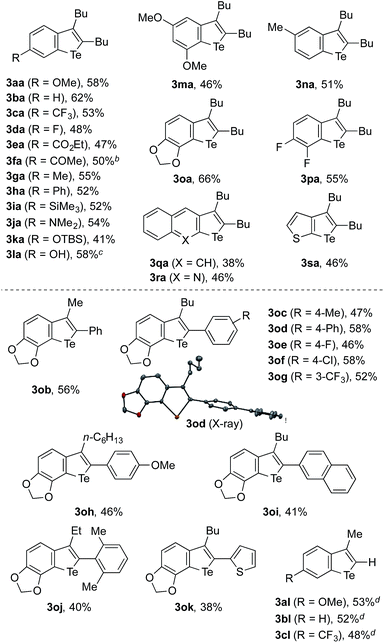
The feasibility of the sequential telluration of C(sp2)–Zn and C(sp2)–H bonds was first demonstrated by the one-pot synthesis of benzotellurophenes from arylzinc reagents and alkynes capitalizing on the cobalt-catalyzed migratory arylzincation (Table 1).13 As a typical example, the reaction of a 4-methoxyphenylzinc reagent (1a) and 5-decyne (2a) in the presence of a CoCl2–Xantphos catalyst was followed by the treatment of the resulting ortho-alkenylarylzinc species with TeCl4 at 90 °C and then with aqueous Na2S at room temperature, affording a benzotellurophene 3aa in 58% yield. Na2S is considered to reduce Te(IV) to Te(II) in the last step.13b The putative Te(IV) intermediate, i.e., the 1,1-dichlorobenzotellurophene derivative, was detected using mass spectrometry (ESI) analysis of the reaction mixture before the addition of Na2S. We obtained the product 3aa even without the addition of Na2S, albeit in a significantly lower yield (<30% GC yield). It should be noted that the use of a Te(II) electrophile, such as TeCl2·bipy instead of TeCl4, resulted in an even poorer yield of 3aa (<10% GC yield). It is also worth noting that all of the attempts using other chalcogen electrophiles such as SeCl4, SeCl2, S2Cl2, SOCl2, or SO2Cl2 in place of TeCl4 failed to produce the corresponding benzochalcogenophene derivative.18| a The reaction was performed using 0.5 mmol of 2a as the limiting agent. See the ESI for the detailed procedure. b The starting arylzinc reagent was protected in the form of p-anisidine imine. c The starting arylzinc reagent was protected with a Boc group, which was removed during the reaction. d 1-Trimethylsilyl-1-propyne was used as the alkyne. |
|---|
|
A wide variety of arylzinc reagents could be employed for the one-pot cyclization with 2a and TeCl4, affording the corresponding benzotellurophenes 3ba–3qa in moderate to good yields. In particular, the method allowed for the installation of various functional groups, both electron-donating and electron-withdrawing, to the 6-position of the benzotellurophene core. The benzotellurophenes 3na–3qa were obtained with exclusive regioselectivity as a result of regioselective 1,4-cobalt migration to the less hindered position (for 3na and 3qa) or the position proximal to the ether oxygen or the fluorine atom (for 3oa and 3pa).14 The reactions of 2-quinolinyl- and 3-thienylzinc reagents with 2a allowed for the preparation of the corresponding fused tellurophenes 3ra and 3sa in moderate yields. Besides these 2,3-dialkylbenzotellurophenes, a series of 2-aryl-3-alkylbenzotellurophenes 3ob–3ok could be synthesized in decent yields from the 3,4-methylenedioxyphenylzinc reagent and the corresponding aryl(alkyl)acetylenes. Furthermore, the use of 1-trimethylsilyl-1-propyne as the alkyne reactant resulted in a loss of the trimethylsilyl group during the cyclization process, thus furnishing 3-methylbenzotellurophenes 3al–3cl. The generally moderate efficiency of the present synthesis of benzotellurophenes and other telluracycles (vide infra) is mainly attributed to the moderate efficiency of the electrophilic trapping of the organozinc intermediate with TeCl4, which results in its protonated derivative as the major byproduct. It is worth noting that the crystal packing of the benzotellurophene 3od displayed a rather short Te–Te distance of 3.67 Å, indicating that there were significant Te–Te interactions.1,19
Because migratory arylzincation is not applicable to a diarylalkyne due to the substantial E/Z isomerization of the alkenylcobalt intermediate and incomplete 1,4-cobalt migration,14 the above protocol did not allow for the synthesis of a 2,3-diarylbenzotellurophene. This gap can be filled by a modified protocol employing a normal arylmetalation reaction instead of the migratory arylzincation (Scheme 3). Thus, a one-pot sequence of the nickel-catalyzed arylmagnesiation of diphenylacetylene (2m),17,20 the transmetalation of the resulting alkenylmagnesium species with ZnCl2·TMEDA (TMEDA = N,N,N′,N′-tetramethylethylenediamine), and treatment with TeCl4 and aqueous Na2S allowed for the preparation of 2,3-diphenylbenzotellurophenes 3am, 3bm, and 3jm in moderate yields. It should be noted that the Mg-to-Zn transmetalation is essential for this protocol. The omission of this step resulted in only a trace amount of the desired benzotellurophene due to multiple substitution reactions on Te(IV) with the alkenylmagnesium species, as indicated from the GCMS analysis of the crude product.
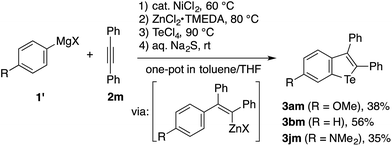 | ||
| Scheme 3 One-pot benzotellurophene synthesis based on the Ni-catalyzed arylmagnesiation of diphenylacetylene. | ||
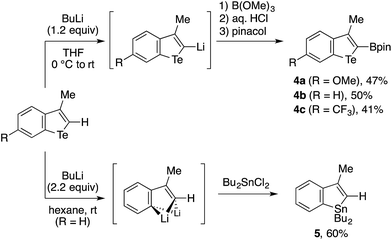
The reaction chemistry of benzotellurophene and n-BuLi21 enabled the further transformations of the 3-methylbenzotellurophenes 3al–3cl (Scheme 4). Thus, these benzotellurophenes were amenable to C2-lithiation using n-BuLi in THF, and the resulting 2-lithiobenzotellurophenes were readily transformed into 2-borylated benzotellurophenes 4a–4c. Not unexpectedly, 4a–4c and the other benzotellurophenes (Table 1 and Scheme 3) were not emissive in organic solvents.3a,4,8d Among 4a–4c, only 4c exhibited weak luminescence at 525 nm in a THF/water mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9), presumably due to aggregation-induced emission.22 This luminescence was found to have a short lifetime in the order of ns (see the ESI†), and the relevance of this to the phosphorescent nature of Rivard’s borylated (benzo)tellurophenes in the solid state4 remains to be explored. A treatment of 3bl with excess n-BuLi in hexane resulted in double tellurium–lithium exchange,21 and subsequent trapping with Bu2SnCl2 furnished benzostannole 5 in 60% yield. The success of this conversion would hold promise for the use of benzotellurophenes as versatile precursors for different benzoheteroles,10 such as benzosilole23 and benzophosphole.24
9), presumably due to aggregation-induced emission.22 This luminescence was found to have a short lifetime in the order of ns (see the ESI†), and the relevance of this to the phosphorescent nature of Rivard’s borylated (benzo)tellurophenes in the solid state4 remains to be explored. A treatment of 3bl with excess n-BuLi in hexane resulted in double tellurium–lithium exchange,21 and subsequent trapping with Bu2SnCl2 furnished benzostannole 5 in 60% yield. The success of this conversion would hold promise for the use of benzotellurophenes as versatile precursors for different benzoheteroles,10 such as benzosilole23 and benzophosphole.24
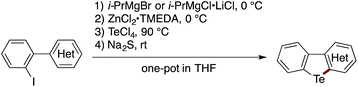
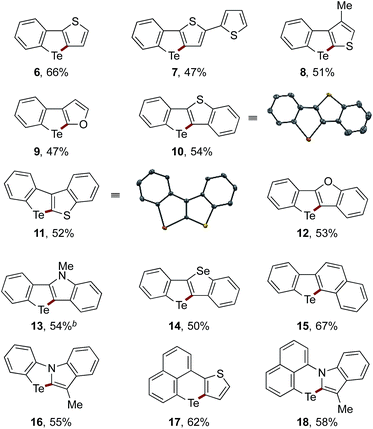
The scope of the sequential C(sp2)–Zn and C(sp2)–H telluration was further extended to the synthesis of tellurium-bridged heterobiaryls starting from 2-iodoheterobiaryls, which can be readily prepared in two steps from 2-bromoaniline and heteroarylboronic acids (Table 2). Iodine–magnesium exchange with i-PrMgBr or i-PrMgCl·LiCl was followed by Mg-to-Zn transmetalation and sequential treatment with TeCl4 and Na2S, thus furnishing benzotellurophenes fused with (benzo)thiophene, (benzo)furan, indole, or benzoselenophene 6–14 in decent yields. Again, the Mg-to-Zn transmetalation proved to be a crucial step, without which the double substitution on Te(IV) with the arylmagnesium species took place predominantly to afford a diaryltellurium derivative. Although the present protocol failed to convert 2-iodobiphenyl to the parent dibenzotellurophene in an appreciable yield, it allowed for the conversion of 2-(2-iodophenyl)naphthalene to benzo[b]naphtho[2,1-d]tellurophene 15via the regioselective telluration of the naphthalene 1-position. Furthermore, tellurium-bridged heteroarenes 16–18, which feature non-tellurophene-type telluracycles, could also be synthesized in reasonable yields.
The planar structures of the benzothiophene-fused derivatives 10 and 11 were confirmed using X-ray crystallographic analysis.19 While 10 adopted a so-called sandwiched herringbone-type packing structure without significant Te–Te interactions (the shortest Te–Te distance being 6.80 Å),2511 assumed a herringbone arrangement with close Te–Te contacts (3.71 Å) between the π-stacks (Fig. 1). This sharp difference suggests the importance of the molecular framework and the peripheral structures in achieving efficient Te–Te interactions in the condensed phase, which may be relevant for the potential application of tellurium-embedded polyaromatic systems in organic electronics.
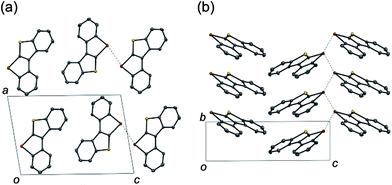 | ||
| Fig. 1 Packing structures of 11 in the (a) ac-plane and (b) bc-plane. The dotted lines indicate the Te–Te contacts (3.71 Å). | ||
We measured the UV absorption spectra of selected Te-bridged heterobiaryls, i.e., compounds 10–15 and 17, and also calculated their HOMO/LUMO levels and transition energies using TD-DFT calculations for reference (Table 3). The compounds 10, 12, and 14, which can be regarded as Te/chalcogen-bridged stilbenes, exhibited a trend of increasing λmax (the longest wavelength absorption maxima) upon changing the extra chalcogen atom from O to Se. This trend is in line with the trends observed for related chalcogen-bridged π-conjugated systems,25,26 and is also consistent with the trend of the lowest transition energies, which are largely represented by the HOMO–LUMO (π–π*) transition (see the ESI† for details). Compound 11, the cross-conjugated isomer of 10, showed a distinctly shorter λmax. Unlike the other compounds, the LUMO of 11 was found to be the C–Te σ*-orbital, with the π*-orbital being located at the LUMO+1 level. The non-tellurophene-type compound 17, which is another structural isomer of 10, exhibited absorption in the visible region, that is in agreement with a much lower transition energy. It is worth noting that none of these compounds were emissive in organic solvents.
| Cmpd | λ max (nm) | ε (104 M−1 cm−1) | E HOMO (eV) | E LUMO (eV) | ΔE (f)c (eV) |
|---|---|---|---|---|---|
| a The longest wavelength absorption maxima from absorption spectra in MeOH. b Calculated using DFT at the level of B3LYP/6-311G* (SDD for Te). c The lowest significant transition energies determined using TD-DFT calculations (f > 0.05; f = oscillator strength). | |||||
| 10 | 348 | 0.51 | −5.67 | −1.55 | 3.65 (0.0992) |
| 11 | 321 | 0.73 | −5.71 | −1.49 | 4.08 (0.1116) |
| 12 | 334 | 0.85 | −5.66 | −1.45 | 3.79 (0.1493) |
| 13 | 348 | 0.68 | −5.23 | −1.21 | 3.56 (0.1878) |
| 14 | 356 | 0.61 | −5.66 | −1.60 | 3.58 (0.0902) |
| 15 | 363 | 0.25 | −5.64 | −1.54 | 3.58 (0.0575) |
| 17 | 425 | 0.31 | −5.10 | −1.71 | 2.98 (0.1492) |
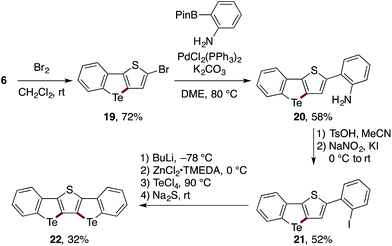
The utility of the present Te-bridging protocol was further demonstrated using a stepwise synthesis of a bis-tellurium-bridged ladder molecule 22 (Scheme 5). The thiophene-fused benzotellurophene 6 was subjected sequentially to bromination, Suzuki–Miyaura coupling with a 2-aminophenylboron reagent, and diazotization–iodination to afford a new 2-iodoheterobiaryl 21. With 21 in hand, the second tellurium bridge was constructed using n-BuLi instead of i-PrMgBr as the metalating agent, thus affording the ladder product 22, albeit in a modest yield.27
Conclusions
In summary, we have established the sequential electrophilic telluration of C(sp2)–Zn and C(sp2)–H bonds as a simple and versatile approach for the construction of tellurium-bridged aromatic systems. The combination of the transition metal-catalyzed (migratory) arylmetalation of alkynes and sequential telluration offers a method for the expedient synthesis of functionalized benzotellurophenes starting from arylmetal reagents and alkynes. The sequential telluration also enables the facile conversion of 2-iodoheterobiaryls into heteroarene-fused benzotellurophenes and other Te-bridged aromatics, most of which are unprecedented in the literature and have been synthesized for the first time. We envision that electrophilic aromatic telluration will open access to an even greater variety of novel Te-containing heterocycles, and that the present study will stimulate further studies on the synthesis, properties, and application of such compounds.Acknowledgements
This work was supported by the Singapore Ministry of Education (RG 114/15), Nanyang Technological University, and JST, CREST. We thank Dr Yongxin Li and Dr Rakesh Ganguly for assistance with the X-ray crystallographic analysis.Notes and references
- (a) A. A. Jahnke and D. S. Seferos, Macromol. Rapid Commun., 2011, 32, 943–951 CrossRef CAS PubMed; (b) E. I. Carrera and D. S. Seferos, Macromolecules, 2015, 48, 297–308 CrossRef CAS; (c) E. Rivard, Chem. Lett., 2015, 44, 730–736 CrossRef CAS; (d) S. M. Parke, M. P. Boone and E. Rivard, Chem. Commun., 2016, 52, 9485–9505 RSC; (e) M. Jeffries-EL, B. M. Kobilka and B. J. Hale, Macromolecules, 2014, 47, 7253–7271 CrossRef CAS.
- (a) A. A. Jahnke, G. W. Howe and D. S. Seferos, Angew. Chem., Int. Ed., 2010, 49, 10140 CrossRef CAS PubMed; (b) A. A. Jahnke, B. Djukic, T. M. McCormick, E. B. Domingo, C. Hellmann, Y. Lee and D. S. Seferos, J. Am. Chem. Soc., 2013, 135, 951 CrossRef CAS PubMed; (c) G. He, L. Kang, W. T. Delgado, O. Shynkaruk, M. J. Ferguson, R. McDonald and E. Rivard, J. Am. Chem. Soc., 2013, 135, 5360 CrossRef CAS PubMed; (d) M. Kaur, D. S. Yang, J. Shin, T. W. Lee, K. Choi, M. J. Cho and D. H. Choi, Chem. Commun., 2013, 49, 5495 RSC; (e) Y. S. Park, Q. Wu, C.-Y. Nam and R. B. Grubbs, Angew. Chem., Int. Ed., 2014, 53, 10691 CrossRef CAS PubMed; (f) E. H. Jung, S. Bae, T. W. Yoo and W. H. Jo, Polym. Chem., 2014, 5, 6545 RSC; (g) M. Planells, B. C. Schroeder and I. McCulloch, Macromolecules, 2014, 47, 5889 CrossRef CAS; (h) P.-F. Li, T. B. Schon and D. S. Seferos, Angew. Chem., Int. Ed., 2015, 54, 9361 CrossRef CAS PubMed; (i) R. S. Ashraf, I. Meager, M. Nikolka, M. Kirkus, M. Planells, B. C. Schroeder, S. Holliday, M. Hurhangee, C. B. Nielsen, H. Sirringhaus and I. McCulloch, J. Am. Chem. Soc., 2015, 137, 1314 CrossRef CAS PubMed; (j) M. Kaur, D. H. Lee, D. S. Yang, H. A. Um, M. J. Cho, J. S. Kang and D. H. Choi, Dyes Pigm., 2015, 123, 317 CrossRef CAS; (k) A. K. Mahrok, E. I. Carrera, A. J. Tilley, S. Y. Ye and D. S. Seferos, Chem. Commun., 2015, 51, 5475 RSC; (l) M. Al-Hashimi, Y. Han, J. Smith, H. S. Bazzi, S. Y. A. Alqaradawi, S. E. Watkins, T. D. Anthopoulos and M. Heeney, Chem. Sci., 2016, 7, 1093 RSC; (m) S. Ye, M. Steube, E. I. Carrera and D. S. Seferos, Macromolecules, 2016, 49, 1704 CrossRef CAS.
- (a) T. M. McCormick, A. A. Jahnke, A. J. Lough and D. S. Seferos, J. Am. Chem. Soc., 2012, 134, 3542 CrossRef CAS PubMed; (b) E. I. Carrera, T. M. McCormick, M. J. Kapp, A. J. Lough and D. S. Seferos, Inorg. Chem., 2013, 52, 13779 CrossRef CAS PubMed; (c) T. M. McCormick, E. I. Carrera, T. B. Schon and D. S. Seferos, Chem. Commun., 2013, 49, 11182 RSC; (d) E. I. Carrera and D. S. Seferos, Dalton Trans., 2015, 44, 2092 RSC; (e) E. I. Carrera, A. E. Lanterna, A. J. Lough, J. C. Scaiano and D. S. Seferos, J. Am. Chem. Soc., 2016, 138, 2678 CrossRef CAS PubMed; (f) P.-F. Li, E. I. Carrera and D. S. Seferos, ChemPlusChem, 2016, 81, 917 CrossRef CAS.
- (a) G. He, W. T. Delgado, D. J. Schatz, C. Merten, A. Mohammadpour, L. Mayr, M. J. Ferguson, R. McDonald, A. Brown, K. Shankar and E. Rivard, Angew. Chem., Int. Ed., 2014, 53, 4587 CrossRef CAS PubMed; (b) G. He, B. D. Wiltshire, P. Choi, A. Savin, S. Sun, A. Mohammadpour, M. J. Ferguson, R. McDonald, S. Farsinezhad, A. Brown, K. Shankar and E. Rivard, Chem. Commun., 2015, 51, 5444 RSC; (c) W. T. Delgado, F. Shahin, M. J. Ferguson, R. McDonald, G. He and E. Rivard, Organometallics, 2016, 35, 2140 CrossRef; (d) C. A. Braun, D. Zomerman, I. de Aguiar, Y. Qi, W. T. Delgado, M. J. Ferguson, R. McDonald, G. L. C. de Souza, G. He, A. Brown and E. Rivard, Faraday Discuss., 2017, 196, 255 RSC.
- G. E. Garrett, E. I. Carrera, D. S. Seferos and M. S. Taylor, Chem. Commun., 2016, 52, 9881 RSC.
- (a) C. R. B. Rhoden and G. Zeni, Org. Biomol. Chem., 2011, 9, 1301 RSC; (b) V. I. Minkin and I. D. Sadekov, in Comprehensive Heterocyclic Chemistry III, ed. A. R. Katritzky, C. A. Ramsden, E. F. V. Scriven and R. J. K. Taylor, Elsevier, 2008, pp. 1007–1028 Search PubMed.
- X. Yan and C. Xi, Acc. Chem. Res., 2015, 48, 935 CrossRef CAS PubMed.
- (a) P. M. Beaujuge and J. M. J. Fréchet, J. Am. Chem. Soc., 2011, 133, 20009 CrossRef CAS PubMed; (b) K. Takimiya, S. Shinamura, I. Osaka and E. Miyazaki, Adv. Mater., 2011, 23, 4347 CrossRef CAS PubMed; (c) C. Wang, H. Dong, W. Hu, Y. Liu and D. Zhu, Chem. Rev., 2012, 112, 2208 CrossRef CAS PubMed; (d) J. Mei, Y. Diao, A. L. Appleton, L. Fang and Z. Bao, J. Am. Chem. Soc., 2013, 135, 6724 CrossRef CAS PubMed; (e) K. Takimiya, M. Nakano, M. J. Kang, E. Miyazaki and I. Osaka, Eur. J. Org. Chem., 2013, 217 CrossRef CAS.
- (a) H. Suzuki, T. Nakamura, T. Sakaguchi and K. Ohta, J. Org. Chem., 1995, 60, 5274 CrossRef CAS; (b) K. Takimiya, Y. Konda, H. Ebata, N. Niihara and T. Otsubo, J. Org. Chem., 2005, 70, 10569 CrossRef CAS PubMed; (c) M. Pittelkow, T. K. Reenberg, K. T. Nielsen, M. J. Magnussen, T. I. Sølling, F. C. Krebs and J. B. Christensen, Angew. Chem., Int. Ed., 2006, 45, 5666 CrossRef CAS PubMed; (d) J. Casado, M. M. Oliva, M. C. R. Delgado, R. P. Ortiz, J. J. Quirante, J. T. L. Navarrete, K. Takimiya and T. Otsubo, J. Phys. Chem. A, 2006, 110, 7422 CrossRef CAS PubMed; (e) Y. S. Park, T. S. Kale, C.-Y. Nam, D. Choi and R. B. Grubbs, Chem. Commun., 2014, 50, 7964 RSC.
- B. Wu and N. Yoshikai, Org. Biomol. Chem., 2016, 14, 5402 CAS.
- For an instrumental review on the uniqueness of tellurium among chalcogen elements, see: T. Chivers and R. S. Laitinen, Chem. Soc. Rev., 2015, 44, 1725 RSC.
- H. Sashida, K. Sadamori and T. Tsuchiya, Synth. Commun., 1998, 28, 713 CrossRef CAS.
- (a) L. Engman, J. Heterocycl. Chem., 1984, 21, 413 CrossRef CAS; (b) H. Sashida, M. Kaname and K. Ohyanagi, Heterocycles, 2010, 82, 441 CrossRef CAS.
- B.-H. Tan, J. Dong and N. Yoshikai, Angew. Chem., Int. Ed., 2012, 51, 9610 CrossRef CAS PubMed.
- B. Wu and N. Yoshikai, Angew. Chem., Int. Ed., 2013, 52, 10496 CrossRef CAS PubMed.
- B. Wu, M. Santra and N. Yoshikai, Angew. Chem., Int. Ed., 2014, 53, 7543 CrossRef CAS PubMed.
- F. Xue, J. Zhao and T. S. A. Hor, Chem. Commun., 2013, 49, 10121 RSC.
- SCl2 was not examined as it was not available in Singapore.
- ESI.†.
- B. Wu, R. Chopra and N. Yoshikai, Org. Lett., 2015, 17, 5666 CrossRef CAS PubMed.
- A. Maercker, H. Bodenstedt and L. Brandsma, Angew. Chem., Int. Ed., 1992, 31, 1339 CrossRef.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718 CrossRef CAS PubMed.
- (a) L. Ilies, H. Tsuji, Y. Sato and B. Nakamura, J. Am. Chem. Soc., 2008, 130, 4240 CrossRef CAS PubMed; (b) L. Ilies, H. Tsuji and E. Nakamura, Org. Lett., 2009, 11, 3966 CrossRef CAS PubMed; (c) M. Tobisu, M. Onoe, Y. Kita and N. Chatani, J. Am. Chem. Soc., 2009, 131, 7506 CrossRef CAS PubMed; (d) E. Shirakawa, S. Masui, R. Narui, R. Watabe, D. Ikeda and T. Hayashi, Chem. Commun., 2011, 47, 9714 RSC; (e) Y. Liang, W. Geng, J. Wei and Z. Xi, Angew. Chem., Int. Ed., 2012, 51, 1934 CrossRef CAS PubMed; (f) K. Ouyang, Y. Liang and Z. Xi, Org. Lett., 2012, 14, 4572 CrossRef CAS PubMed; (g) L. Xu, S. Zhang and P. Li, Org. Chem. Front., 2015, 2, 459 RSC.
- (a) T. Sanji, K. Shiraishi, T. Kashiwabara and M. Tanaka, Org. Lett., 2008, 10, 2689 CrossRef CAS PubMed; (b) H. Tsuji, K. Sato, L. Ilies, Y. Itoh, Y. Sato and E. Nakamura, Org. Lett., 2008, 10, 2263 CrossRef CAS PubMed; (c) A. Fukazawa, Y. Ichihashi, Y. Kosaka and S. Yamaguchi, Chem.–Asian J., 2009, 4, 1729 CrossRef CAS PubMed; (d) Y.-R. Chen and W.-L. Duan, J. Am. Chem. Soc., 2013, 135, 16754 CrossRef CAS PubMed; (e) Y. Unoh, K. Hirano, T. Satoh and M. Miura, Angew. Chem., Int. Ed., 2013, 52, 12975 CrossRef CAS PubMed; (f) W. Ma and L. Ackermann, Synthesis, 2014, 46, 2297 CrossRef CAS; (g) Y. Xu, Z. Wang, Z. Gan, Q. Xi, Z. Duan and F. Mathey, Org. Lett., 2015, 17, 1732 CrossRef CAS PubMed; (h) Y. Zhou, Z. Gan, B. Su, J. Li, Z. Duan and F. Mathey, Org. Lett., 2015, 17, 5722 CrossRef CAS PubMed; (i) V. Quint, F. Morlet-Savary, J. F. Lohier, J. Lalevee, A. C. Gaumont and S. Lakhdar, J. Am. Chem. Soc., 2016, 138, 7436 CrossRef CAS PubMed; (j) Y. Unoh, Y. Yokoyama, T. Satoh, K. Hirano and M. Miura, Org. Lett., 2016, 18, 5436 CrossRef CAS PubMed.
- M. Matsumura, A. Muranaka, R. Kurihara, M. Kanai, K. Yoshida, N. Kakusawa, D. Hashizume, M. Uchiyama and S. Yasuike, Tetrahedron, 2016, 72, 8085 CrossRef CAS.
- A. Muranaka, S. Yasuike, C.-Y. Liu, J. Kurita, N. Kakusawa, T. Tsuchiya, M. Okuda, N. Kobayashi, Y. Matsumoto, K. Yoshida, D. Hashizume and M. Uchiyama, J. Phys. Chem. A, 2009, 113, 464 CrossRef CAS PubMed.
- Attempts to simultaneously construct the two tellurium bridges of 22 starting from a teraryl diiodide precursor have not been successful.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1523262–1523264. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc01162h |
| This journal is © The Royal Society of Chemistry 2017 |