Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Chiral phosphine-mediated intramolecular [3 + 2] annulation: enhanced enantioselectivity by achiral Brønsted acid†
Weijun
Yao
 a,
Zhaoyuan
Yu
a,
Zhaoyuan
Yu
 ac,
Shan
Wen
a,
Huanzhen
Ni
a,
Nisar
Ullah
*b,
Yu
Lan
*c and
Yixin
Lu
ac,
Shan
Wen
a,
Huanzhen
Ni
a,
Nisar
Ullah
*b,
Yu
Lan
*c and
Yixin
Lu
 *ad
*ad
aDepartment of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543. E-mail: chmlyx@nus.edu.sg
bChemistry Department, King Fahd University of Petroleum and Materials, Dhahran 31261, Saudi Arabia. E-mail: nullah@kfupm.edu.sa
cSchool of Chemistry and Chemical Engineering, Chongqing University, Chongqing 400030, P. R. China. E-mail: lanyu@cqu.edu.cn
dNational University of Singapore (Suzhou) Research Institute, 377 Lin Quan Street, Suzhou Industrial Park, Suzhou, Jiangsu 215123, P. R. China
First published on 17th May 2017
Abstract
Enantioselective intramolecular [3 + 2] annulation of chalcones bearing an allene moiety has been successfully developed. The reaction was effectively promoted by amino acid-derived phosphines, in combination with achiral Brønsted acids. Dihydrocoumarin architectures were constructed in high yields and with excellent enantiomeric excesses. Theoretical studies via DFT calculations revealed that the hydrogen bonding network induced by achiral Brønsted acids/chiral phosphines could more efficiently distinguish between two enantioselective pathways, thus leading to enhanced enantioselectivity.
In the past decade, asymmetric phosphine catalysis has emerged as an efficient approach for the construction of functionalized chiral carbocyclic structures.1 Allenes are the most commonly investigated substrates in phosphine catalysis, due to their high and versatile reactivities, as well as their ready synthetic accessibility.2 Since Lu’s pioneering report on phosphine-catalyzed [3 + 2] cyclization of allenoates with activated alkenes in 1995,3 a wide variety of asymmetric intermolecular annulation processes between allenes and activated alkenes have been developed, such as [3 + 2],4 [4 + 2],5 and [4 + 1]6 annulations, among others.7 However, phosphine-catalyzed intramolecular annulations are very rare. In 2003, Krische disclosed the first racemic version of intramolecular [3 + 2] annulation between enone and 2-alkynoate moieties, leading to the total synthesis of (±)-hirsutene.8 Subsequently, Kwon reported phosphine-promoted intramolecular [3 + 2] cyclizations of 2-styrenyl allenoates to form functionalized coumarins.9 Very recently, Fu developed an enantioselective intramolecular [3 + 2] cycloaddition of allenes and alkenes to create fused chiral ring scaffolds.10 In the past few years, our group has developed amino acid-based bifunctional phosphine catalysts, and demonstrated their applications in a wide range of enantioselective intermolecular annulation processes.11 Attracted by the great potential of phosphine-catalyzed intramolecular processes for quick access to challenging chiral skeletons, we became interested in such valuable transformations.
Intramolecular reactions proceed more readily than their intermolecular counterparts due to the intrinsic entropy difference. In phosphine catalysis, however, while phosphine-mediated asymmetric intermolecular cyclizations are very common, there is only one reported enantioselective intramolecular annulation to date.10 The paucity of this important reaction type may be due to the crowdedness of the advanced intermediates formed upon phosphine activation. This results in an inherent challenge to distinguish different transition states in a rather crowded and constrained environment. Herein, we document a highly enantioselective intramolecular [3 + 2] annulation of chalcones and allenes, promoted by a catalytic system combining chiral phosphines and achiral Brønsted acids, for highly diastereoselective and enantioselective construction of dihydrocoumarin architectures. We believe that introducing an additive molecule to interact with reaction partners synergistically may represent a novel and general approach to the discovery of asymmetric intramolecular processes in phosphine catalysis.
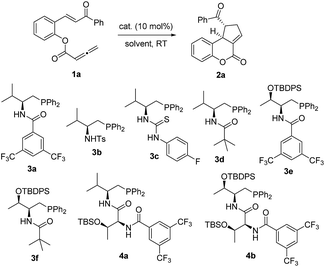
Substrate 1a, which contains both chalcone and allene moieties, was chosen for initial investigation (Table 1). Achiral Ph2PMe effectively promoted the desired [3 + 2] annulation and led to the formation of the racemic tricyclic coumarin122a in 85% yield (entry 1). A series of L-valine-derived bifunctional phosphines were examined, and the amide functionality was found to be superior relative to sulfonamide and thiourea. Pivalamide 3d was the best catalyst (entries 2–5). The threonine core13 again proved to be advantageous; L-threonine-based 3f increased the ee value to 81% (entries 6 and 7). Dipeptide phosphines further enhanced the enantioselectivity when 4b furnished 2a, with an ee value of 87% (entry 9). Recently, Fu observed the beneficial effects of adding a proton donor in asymmetric γ-addition reactions.14 To further improve the enantioselectivity of the annulation reaction, we decided to introduce an achiral Brønsted acid additive as an extra controlling element for asymmetric induction. We hypothesized that the cooperative interplay of the phosphine catalyst, the substrate, and the acidic additive can add in a structural dimension to potentially make the transition states less constrained for such an intramolecular process. To our delight, the addition of benzoic acid (10 mol%) led to a substantial improvement in the enantioselectivity for the reactions catalyzed by mono-amino acid-derived phosphines, despite prolonged reaction times (entries 10–15). Notably, the addition of benzoic acid did not affect the enantioselectivity of this reaction when 4b was employed as the catalyst (entry 16). To provide a more comprehensive picture, more Brønsted acid additives were investigated (entries 17–24). The beneficial effects of the Brønsted acid additives could be correlated to their acidities. Less acidic ethanol (pKa 15.7) had little effect (entry 17) and phenol (pKa 9.95) marginally increased the ee value (entry 18). p-Nitrophenol (pKa 7.10), acetic acid (pKa 4.76), and 3-(trifluoromethyl)benzoic acid (pKa 3.77), which are similar to benzoic acid (pKa 4.20), provided more enantioenriched products (entries 19–21). More acidic diphenylphosphoric acid (pKa 1.90) led to slightly inferior results (entry 22). Too acidic trifluoroacetic acid (pKa −0.25) or methanesulfonic acid (pKa −2.6) inhibited the reaction, and no products were observed (entries 23 and 24). Essentially, the Brønsted acid additive needed to possess sufficient acidity to induce a better enantioselectivity, while a too acidic additive was found to be detrimental to the reaction. A catalytic system consisting of 3f and benzoic acid was then selected, and subsequent solvent screening followed. It was revealed that chlorobenzene was the solvent of choice (entries 25–31). In the presence of the phosphine 3f, with benzoic acid as an additive, the [3 + 2] annulation of 1a in chlorobenzene proceeded smoothly to afford the dihydrocoumarin 2a in 90% yield and with an ee value of 99%.
| Entry | Catalyst | Additive (pKa in H2O) | Solvent | Yieldb [%] | eec [%] |
|---|---|---|---|---|---|
| a Reactions were performed with 1a (0.15 mmol) and the catalyst (0.015 mmol) in toluene (1.5 mL) at room temperature for 24 h; when an additive (0.015 mmol) was added, the reaction time was 48 h. b Isolated yield for the major regioisomer. c Determined by HPLC analysis on a chiral stationary phase. | |||||
| 1 | MePPh2 | — | Toluene | 85 | — |
| 2 | 3a | — | Toluene | 87 | 43 |
| 3 | 3b | — | Toluene | 73 | 12 |
| 4 | 3c | — | Toluene | 47 | 43 |
| 5 | 3d | — | Toluene | 90 | 65 |
| 6 | 3e | — | Toluene | 88 | 77 |
| 7 | 3f | — | Toluene | 90 | 81 |
| 8 | 4a | — | Toluene | 90 | 58 |
| 9 | 4b | — | Toluene | 90 | 87 |
| 10 | 3a | PhCO2H (4.20) | Toluene | 85 | 66 |
| 11 | 3b | PhCO2H (4.20) | Toluene | 75 | 28 |
| 12 | 3c | PhCO2H (4.20) | Toluene | 45 | 73 |
| 13 | 3d | PhCO2H (4.20) | Toluene | 87 | 84 |
| 14 | 3e | PhCO2H (4.20) | Toluene | 87 | 94 |
| 15 | 3f | PhCO2H (4.20) | Toluene | 90 | 98 |
| 16 | 4b | PhCO2H (4.20) | Toluene | 91 | 86 |
| 17 | 3f | EtOH (15.7) | Toluene | 91 | 82 |
| 18 | 3f | PhOH (9.95) | Toluene | 88 | 84 |
| 19 | 3f | 4-NO2-PhOH (7.10) | Toluene | 90 | 98 |
| 20 | 3f | CH3CO2H (4.76) | Toluene | 88 | 98 |
| 21 | 3f | 3-CF3PhCO2H (3.77) | Toluene | 75 | 95 |
| 22 | 3f | (PhO)2P(O)OH (1.90) | Toluene | 77 | 92 |
| 23 | 3f | TFA (−0.25) | Toluene | — | — |
| 24 | 3f | CH3SO3H (−2.6) | Toluene | — | — |
| 25 | 3f | PhCO2H (4.20) | EtOAc | 72 | 93 |
| 26 | 3f | PhCO2H (4.20) | CH2Cl2 | 38 | 97 |
| 27 | 3f | PhCO2H (4.20) | CHCl3 | 41 | 95 |
| 28 | 3f | PhCO2H (4.20) | Ether | 21 | 91 |
| 29 | 3f | PhCO2H (4.20) | THF | 48 | 88 |
| 30 | 3f | PhCO2H (4.20) | Xylene | 83 | 92 |
| 31 | 3f | PhCO2H (4.20) | PhCl | 90 | 99 |
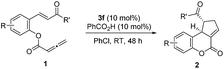
With the optimized reaction conditions in hand, we next investigated the scope of the reaction (Table 2). Both aromatic moieties in chalcone structures could be varied, regardless of the electronic nature and substitution patterns of the aryl structures, and annulation products were obtained in high yields and with near perfect enantioselectivities (entries 1–14). In all of the examples examined, only one diastereomer was detected. The employment of the methyl-substituted enone 1o or γ-methyl substituted allenoate 1p did not lead to the desired product, presumably due to the low reactivity of such substrates. The absolute configurations of the annulation products were assigned on the basis of X-ray structural analysis of crystals of 2a. The annulation product could be manipulated to form more complex ring structures. For instance, the [3 + 2] annulation product 2n underwent intramolecular Mizoroki–Heck coupling to yield a unique coumarin derivative 5 in 76% yield (eqn (1)).
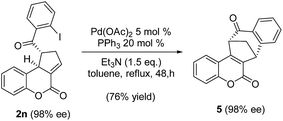 | (1) |
| Entry | Structure | 1 (R or R′) | Yieldb [%] | eec [%] |
|---|---|---|---|---|
| a Reactions were performed with 1 (0.15 mmol) and 3f (0.015 mmol) and benzoic acid (0.015 mmol) in chlorobenzene (1.5 mL) at room temperature for 48 h. b Isolated yield. c Determined by HPLC analysis on a chiral stationary phase. | ||||
| 1 | 1a (R = H) | 90 | 99 | |
| 2 | 1b (R = 4-Me) | 86 | 98 | |
| 3 | 1c (R = 5-OMe) | 87 | 99 | |
| 4 |
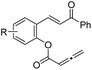
|
1d (R = 4-NO2) | 84 | 95 |
| 5 | 1e (R = 2-Br) | 90 | 98 | |
| 6 | 1f (R = 5-Br) | 92 | 98 | |
| 7 | 1g (R = 4-Br) | 89 | 98 | |
| 8 | 1h (R = 4-Cl) | 91 | 98 | |
| 9 | 1i (R = 2,4-Cl) | 89 | 98 | |
| 10 | 1j (R′ = 4-MePh) | 86 | 98 | |
| 11 |
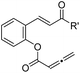
|
1k (R′ = 2-thiophenyl) | 87 | 98 |
| 12 | 1l (R′ = 4-F-Ph) | 86 | 99 | |
| 13 | 1m (R′ = 3-Br-Ph) | 87 | 99.5 | |
| 14 | 1n (R′ = 2-I-Ph) | 85 | 98 | |
| 15 |
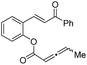
|
1o (R′ = Me) | — | — |
| 16 | 1p | — | — | |
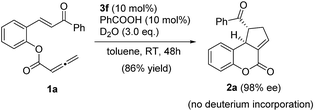
To further understand the role of benzoic acid in this cyclization process, we investigated the reaction with benzoic acid (10 mol%) as an additive in the presence of 3 equivalents of D2O (eqn (2)). The reaction proceeded smoothly to afford the desired product in 86% yield and with an ee value of 98%. 1H NMR showed that there was no deuterium incorporation in the product. This result suggests that benzoic acid is involved in the reaction through hydrogen bonding interactions, rather than facilitating the proton transfer process.
 | (2) |
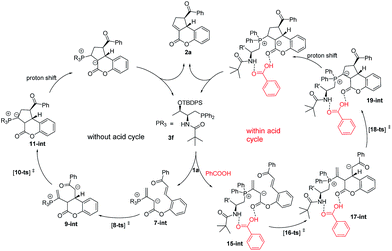
The mechanism of 3f-catalyzed intramolecular [3 + 2] cyclization of chalcone allenoate is proposed in Scheme 1. An initial nucleophilic attack by 3f on the allenoate 1a forms the zwitterionic intermediate 7-int. Without acid as an additive, 7-int undergoes intramolecular Michael addition via the transition state 8-ts to afford the intermediate 9-int. An intramolecular Michael addition of 9-int occurs via the transition state 10-ts to give the intermediate 11-int, followed by proton shift and elimination to yield the product 2a and to release the catalyst 3f. In this case, the ee value of the product is 81%. In the presence of an acidic additive, such as benzoic acid, a hydrogen bond network is formed between the zwitterionic intermediate 7-int and benzoic acid to give complex 15-int, and the subsequent intramolecular Michael addition takes place via the transition state 16-ts to form the complex 17-int. The enantioselectivity was improved to 98% in this pathway.
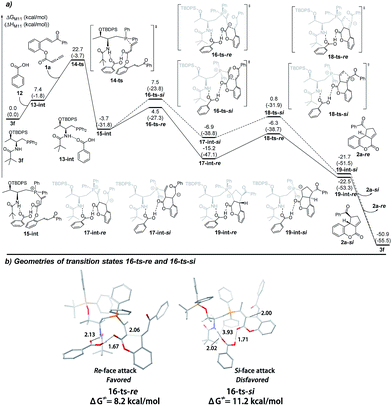
In order to rationalize the origin of the enantioselectivity15 and the effects of the acid additive in the annulation, density functional theory (DFT) calculations were carried out with GAUSSIAN 09 programs.16 The computed Gibbs free energy profiles of the intramolecular [3 + 2] cyclization of 1a catalyzed by 3f are shown in Fig. 1(a), and optimized structures of selected transition states are shown in Fig. 1(b). This multi-step cyclization process starts from the nucleophilic attack by 3f on the allenoate 1avia the transition state 6-ts, which has an 18.6 kcal mol−1 energy barrier to form the zwitterionic intermediate 7-int. The key enantio-differentiated cyclization then occurs via two possible pathways. The first pathway is the Si-face attack that occurs through 8-ts-Si, with a 2.5 kcal mol−1 barrier, generating the intermediate 9-int-Si. The subsequent ring closure occurs via the transition state 10-ts-Si to form the intermediate 11-int-Si. The relative free energy of 10-ts-Si is 11.3 kcal mol−1 lower than that of 8-ts-Si. The alternative Re-face attack proceeds via the transition state 8-ts-Re with a barrier of 1.5 kcal mol−1, which is 1.0 kcal mol−1 lower than that of 8-ts-Si. The above calculations suggest that the enantioselectivity is determined by the cyclization step and predict an ee value of 69%, based on the energy difference between transition states 8-ts-Re and 8-ts-Si. This is in good agreement with the experimental result, where the product 2a-Re was formed preferentially. The analysis of the two transition states in Fig. 1(b) reveals the origin of the enantioselectivity; in the geometry of 8-ts-Si, the C⋯C distance of 3.86 Å suggests repulsion between the phenyl group of the reactant and the tert-butyl moiety of the phosphine catalyst, resulting in a higher transition state barrier.
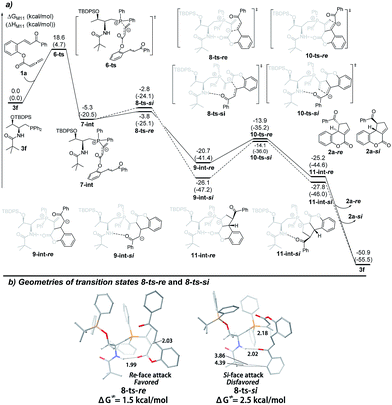 | ||
| Fig. 1 (a) The energy surface of intramolecular [3 + 2] cyclization catalyzed by the phosphine catalyst 3f, and (b) the geometries of the transition states 8-ts-Re and 8-ts-Re. | ||
We next set out to understand the enhancement of enantioselectivity with the addition of benzoic acid. As the proton transfer process takes place after the key C–C bond formation, we were thus focusing on the involvement of benzoic acid in hydrogen bonding interactions to understand the observed enhancement of enantioselectivity. As shown in Fig. 2(a), a hydrogen-bonding complex 13-int is formed from the active catalyst 3f and benzoic acid 12 with a free energy increase of 7.4 kcal mol−1. The nucleophilic addition of phosphine takes place via the transition state 14-ts with an overall barrier of 22.7 kcal mol−1, and generates the intermediate 15-int. Similarly, from the intermediate 15-int, the intramolecular Michael addition can then occur via two possible pathways: the Si-face attack pathway leads to the formation of 2a-Si and the Re-face attack pathway gives the product 2a-Re. In both pathways, the benzoic acid forms two hydrogen bonds with the amide moiety of the catalyst and the ester group. In the presence of benzoic acid and two induced hydrogen bonding interactions, the two possible transition states, i.e. the Si-face attack pathway via16-ts-Si and the Re-face attack pathway via16-ts-Re, are better differentiated. The calculated Gibbs free energy difference of 3.0 kcal mol−1 predicts an enantiomeric excess of 99%, fully consistent with the experimental results. The geometries of the transition states for the Re-face and Si-face attack involving benzoic acid are illustrated in Fig. 2(b). In the transition state 16-ts-Si, when the hydrogen bonds are formed the phosphorus bearing two phenyl groups is rotated and gets closer to the tert-butyl moiety of the phosphine catalyst. The C⋯C distance of 3.93 Å indicates that steric repulsion occurs, resulting in a higher transition state barrier.
Conclusions
In summary, we have developed an enantioselective intramolecular [3 + 2] annulation of chalcones with allenes by employing a catalytic system combining chiral bifunctional phosphines and achiral Brønsted acids. Highly functionalized dihydrocoumarin scaffolds were obtained in high yields and with excellent enantioselectivities. Our DFT calculations revealed that the key hydrogen bonding network introduced by the achiral Brønsted acid additives was crucial for the observed enantioselectivity. The method described in this report may represent a general approach for the discovery of more phosphine-catalyzed enantioselective intramolecular processes. We are currently investigating in this direction, and our discoveries will be reported in due course.Acknowledgements
Yixin Lu thanks the National University of Singapore (R-143-000-599-112) and the National Natural Science Foundation of China (21672158) for generous financial support. N. U. and Yixin Lu are grateful for the KFUPM-NUS Collaborative Fund support (NUS15103, R-143-004-617-597).References
- For reviews on phosphine catalysis, see: (a) X. Lu, C. Zhang and Z. Xu, Acc. Chem. Res., 2001, 34, 535 CrossRef CAS PubMed; (b) J. L. Methot and W. R. Roush, Adv. Synth. Catal., 2004, 346, 1035 CrossRef CAS; (c) L.-W. Ye, J. Zhou and Y. Tang, Chem. Soc. Rev., 2008, 37, 1140 RSC; (d) A. Marinetti and A. Voituriez, Synlett, 2010, 174 CrossRef CAS; (e) S.-X. Wang, X. Han, F. Zhong, Y. Wang and Y. Lu, Synlett, 2011, 2766 CAS; (f) Q.-Y. Zhao, Z. Lian, Y. Wei and M. Shi, Chem. Commun., 2012, 48, 1724 RSC; (g) Y. C. Fan and O. Kwon, Chem. Commun., 2013, 49, 11588 RSC; (h) Z. Wang, X. Xu and O. Kwon, Chem. Soc. Rev., 2014, 43, 2927 RSC; (i) Y. Xiao, Z. Sun, H. Guo and O. Kwon, Beilstein J. Org. Chem., 2014, 10, 2089 CrossRef PubMed; (j) Y. Wei and M. Shi, Chem.–Asian J., 2014, 9, 2720 CrossRef CAS PubMed; (k) P. Xie and Y. Huang, Org. Biomol. Chem., 2015, 13, 8578 RSC; (l) W. Li and J. Zhang, Chem. Soc. Rev., 2016, 45, 1657 RSC; (m) T. Wang, X. Han, F. Zhong, W. Yao and Y. Lu, Acc. Chem. Res., 2016, 49, 1369 CrossRef CAS PubMed.
- B. J. Cowen and S. J. Miller, Chem. Soc. Rev., 2009, 38, 3102 RSC.
- C. Zhang and X. Lu, J. Org. Chem., 1995, 60, 2906 CrossRef CAS.
- For selected examples of enantioselective [3 + 2] annulations, see: (a) G. Zhu, Z. Chen, Q. Jiang, D. Xiao, P. Cao and X. Zhang, J. Am. Chem. Soc., 1997, 119, 3836 CrossRef CAS; (b) J. E. Wilson and G. C. Fu, Angew. Chem., Int. Ed., 2006, 45, 1426 CrossRef CAS PubMed; (c) B. J. Cowen and S. J. Miller, J. Am. Chem. Soc., 2007, 129, 10988 CrossRef CAS PubMed; (d) A. Voituriez, A. Panossian, N. Fleury-Brégeot, P. Retailleau and A. Marinetti, J. Am. Chem. Soc., 2008, 130, 14030 CrossRef CAS PubMed; (e) A. Voituriez, N. Pinto, M. Neel, P. Retailleau and A. Marinetti, Chem.–Eur. J., 2010, 16, 12541 CrossRef CAS PubMed; (f) H. Xiao, Z. Chai, C.-W. Zheng, Y.-Q. Yang, W. Liu, J.-K. Zhang and G. Zhao, Angew. Chem., Int. Ed., 2010, 49, 4467 CrossRef CAS PubMed; (g) Y. Fujiwara and G. C. Fu, J. Am. Chem. Soc., 2011, 133, 12293 CrossRef CAS PubMed; (h) X. Han, Y. Wang, F. Zhong and Y. Lu, J. Am. Chem. Soc., 2011, 133, 1726 CrossRef CAS PubMed; (i) F. Zhong, X. Han, Y. Wang and Y. Lu, Angew. Chem., Int. Ed., 2011, 50, 7837 CrossRef CAS PubMed; (j) Q. Zhao, X. Han, Y. Wei, M. Shi and Y. Lu, Chem. Commun., 2012, 48, 970 RSC; (k) M. Steurer, K. L. Jensen, D. Worgull and K. A. Jørgensen, Chem.–Eur. J., 2012, 18, 76 CrossRef CAS PubMed; (l) P.-Y. Dakas, J. A. Parga, S. Höing, H. R. Schöler, J. Sterneckert, K. Kumar and H. Waldmann, Angew. Chem., Int. Ed., 2013, 52, 9576 CrossRef CAS PubMed; (m) J. Marco-Martínez, V. Marcos, S. Reboredo, S. Filippone and N. Martín, Angew. Chem., Int. Ed., 2013, 52, 5115 CrossRef PubMed; (n) S. Y. Lee, Y. Fujiwara, A. Nishiguchi, M. Kalek and G. C. Fu, J. Am. Chem. Soc., 2015, 137, 4587 CrossRef CAS PubMed; (o) D. Wang, G.-P. Wang, Y.-L. Sun, S.-F. Zhu, Y. Wei, Q.-L. Zhou and M. Shi, Chem. Sci., 2015, 6, 7319 RSC; (p) H. Ni, W. Yao and Y. Lu, Beilstein J. Org. Chem., 2016, 12, 343 CrossRef CAS PubMed.
- For selected examples of phosphine catalyzed [4 + 2]-annulations, see: (a) Y. S. Tran and O. Kwon, J. Am. Chem. Soc., 2007, 129, 12632 CrossRef CAS PubMed; (b) B. Baskar, P.-Y. Dakas and K. Kumar, Org. Lett., 2011, 13, 1988 CrossRef CAS PubMed; (c) Y. S. Tran, T. J. Martin and O. Kwon, Chem.–Asian J., 2011, 6, 2101 CrossRef CAS PubMed; (d) F. Zhong, X. Han, Y. Wang and Y. Lu, Chem. Sci., 2012, 3, 1231 RSC; (e) H. Xiao, Z. Chai, D. Cao, H. Wang, J. Chen and G. Zhao, Org. Biomol. Chem., 2012, 10, 3195 RSC; (f) W. Yang, Y. Zhang, S. Qiu, C. Zhao, L. Zhang, H. Liu, L. Zhou, Y. Xiao and H. Guo, RSC Adv., 2015, 5, 62343 RSC; (g) A. Danda, N. Kesava-Reddy, C. Golz, C. Strohmann and K. Kumar, Org. Lett., 2016, 18, 2632 CrossRef CAS PubMed; (h) H. Liu, Y. Liu, C. Yuan, G.-P. Wang, S.-F. Zhu, Y. Wu, B. Wang, Z. Sun, Y. Xiao, Q.-L. Zhou and H. Guo, Org. Lett., 2016, 18, 1302 CrossRef CAS PubMed; (i) E. Li, Y. Huang, L. Liang and P. Xie, Org. Lett., 2013, 15, 3138 CrossRef CAS PubMed; (j) M. Gicquel, C. Gomez, P. Retailleau, A. Voituriez and A. Marinetti, Org. Lett., 2013, 15, 4002 CrossRef CAS PubMed; (k) Y. Jia, X. Tang, G. Cai, R. Jia, B. Wang and Z. Miao, Eur. J. Org. Chem., 2015, 2015, 4720 CrossRef CAS.
- (a) Q. Zhang, L. Yang and X. Tong, J. Am. Chem. Soc., 2010, 132, 2550 CrossRef CAS PubMed; (b) X. Han, W. Yao, T. Wang, Y. R. Tan, Z. Yan, J. Kwiatkowski and Y. Lu, Angew. Chem., Int. Ed., 2014, 53, 5643 CrossRef CAS PubMed; (c) D. T. Ziegler, L. Riesgo, T. Ikeda, Y. Fujiwara and G. C. Fu, Angew. Chem., Int. Ed., 2014, 53, 13183 CrossRef CAS PubMed; (d) S. Kramer and G. C. Fu, J. Am. Chem. Soc., 2015, 137, 3803 CrossRef CAS PubMed.
- S. Takizawa, K. Kishi, Y. Yoshida, S. Mader, F. A. Arteaga, S. Lee, M. Hoshino, M. Rueping, M. Fujita and H. Sasai, Angew. Chem., Int. Ed., 2015, 54, 15511 CrossRef CAS PubMed.
- (a) J.-C. Wang, S.-S. Ng and M. J. Krische, J. Am. Chem. Soc., 2003, 125, 3682 CrossRef CAS PubMed; (b) J.-C. Wang and M. J. Krische, Angew. Chem., Int. Ed., 2003, 42, 5855 CrossRef CAS PubMed. For an elegant example of phosphine-catalyzed intramolecular RC reaction, see: (c) L.-C. Wang, A. L. Luis, K. Agapiou, H.-Y. Jang and M. J. Krische, J. Am. Chem. Soc., 2002, 124, 2402 CrossRef CAS PubMed.
- C. E. Henry and O. Kwon, Org. Lett., 2007, 9, 3069 CrossRef CAS PubMed.
- S. Y. Lee, Y. Fujiwara, A. Nishiguchi, M. Kalek and G. C. Fu, J. Am. Chem. Soc., 2015, 137, 4587 CrossRef CAS PubMed.
- (a) X. Han, W.-L. Chan, W. Yao, Y. Wang and Y. Lu, Angew. Chem., Int. Ed., 2016, 55, 6492 CrossRef CAS PubMed; (b) H. Ni, W. Yao, A. Waheed, N. Ullah and Y. Lu, Org. Lett., 2016, 18, 2138 CrossRef CAS PubMed; (c) W. Yao, X. Dou and Y. Lu, J. Am. Chem. Soc., 2015, 137, 54 CrossRef CAS PubMed; (d) X. Han, F. Zhong, Y. Wang and Y. Lu, Angew. Chem., Int. Ed., 2012, 51, 767 CrossRef CAS PubMed.
- (a) W. Kozak, M. Dásko, M. Masłyk, J. S. Pieczykolan, B. Gielniewski, J. Rachona and S. Demkowicz, RSC Adv., 2014, 4, 44350 RSC; (b) A. Purohit, L. W. L. Woo, B. V. L. Potter and M. J. Reed, Cancer Res., 2000, 60, 3394 CAS.
- (a) L. Cheng, X. Han, H. Huang, M. W. Wong and Y. Lu, Chem. Commun., 2007, 4143 RSC; (b) X. Wu, Z. Jiang, H.-M. Shen and Y. Lu, Adv. Synth. Catal., 2007, 349, 812 CrossRef CAS.
- (a) M. Kalek and G. C. Fu, J. Am. Chem. Soc., 2015, 137, 9438 CrossRef CAS PubMed; (b) R. Sinisi, J. Sun and G. C. Fu, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 20652 CrossRef CAS PubMed; (c) S. W. Smith and G. C. Fu, J. Am. Chem. Soc., 2009, 131, 14231 CrossRef CAS PubMed; (d) Y. K. Chung and G. C. Fu, Angew. Chem., Int. Ed., 2009, 48, 2225 CrossRef CAS PubMed.
- (a) Y. Xia, Y. Liang, Y. Chen, M. Wang, L. Jiao, F. Huang, S. Liu, Y. Li and Z.-X. Yu, J. Am. Chem. Soc., 2007, 129, 3470 CrossRef CAS PubMed; (b) T. Dudding, O. Kwon and E. Mercier, Org. Lett., 2006, 8, 3643 CrossRef CAS PubMed; (c) P. Xie, W. Lai, Z. Geng, Y. Huang and R. Chen, Chem.–Asian J., 2012, 7, 1533 CrossRef CAS PubMed.
- See the ESI† for the details of DFT calculations.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7sc00952f |
| This journal is © The Royal Society of Chemistry 2017 |