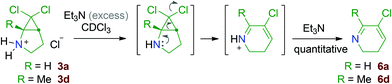Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of functionalised azepanes and piperidines from bicyclic halogenated aminocyclopropane derivatives†
Cheng
Chen
a,
Pullaiah
Kattanguru
a,
Olesya A.
Tomashenko
ab,
Rafał
Karpowicz
ac,
Gabriela
Siemiaszko
a,
Ahanjit
Bhattacharya
a,
Vinícius
Calasans
a and
Yvan
Six
 *a
*a
aLaboratoire de Synthèse Organique (LSO), UMR 7652 CNRS/ENSTA/École Polytechnique, Université Paris-Saclay, 91128 Palaiseau Cedex, France. E-mail: yvan.six@polytechnique.edu
bSaint Petersburg State University, Institute of Chemistry, 7/9 Universitetskaya nab., St Petersburg, 199034 Russia
cDepartment of Organic Chemistry, Faculty of Chemistry, University of Łódź, Tamka 12, Łódź 91-403, Poland
First published on 6th June 2017
Abstract
A series of 6,6-dihalo-2-azabicyclo[3.1.0]hexane and 7,7-dihalo-2-azabicyclo[4.1.0]heptane compounds were prepared by the reaction of dihalocarbene species with N-Boc-2,3-dihydro-1H-pyrroles or -1,2,3,4-tetrahydropyridines. Monochloro substrates were synthesised as well, using a chlorine-to-lithium exchange reaction. The behaviour of several aldehydes and ketones under reductive amination conditions with deprotected halogenated secondary cyclopropylamines was investigated, showing that this transformation typically triggers cyclopropane ring cleavage to give access to interesting nitrogen-containing ring-expanded products.
Introduction
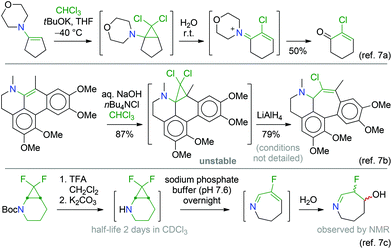
Our long-standing interest in fused bicyclic aminocyclopropanes has led us to develop several transformations of these compounds for the preparation of nitrogen-containing polycyclic systems.1 All these methods involve cyclopropane substrates in which the amino group is the only activating substituent.2 We recently decided to turn our attention to 2,2-dihalo-substituted aminocyclopropane derivatives. Indeed, the presence of a gem-dihalocyclopropane substructure offers extended opportunities for subsequent transformations.3 Of special interest is the thermal ring-opening of halocyclopropanes, proceeding with concomitant departure of a halide ion, to generate allyl cation species.3,4 Starting from fused bicyclic systems, ring-enlarged products can thus be obtained. Many examples can be found in the literature,3 some of which served in the syntheses of natural products.5Nevertheless, reports involving nitrogen-substituted gem-dihalocyclopropane compounds are comparatively scarce.5e,6 Typically, the starting materials are stable amide, carbamate or urea derivatives, i.e. with the lone pair of the nitrogen atom being delocalised into a carbonyl group. These ring-opening reactions (Scheme 1) require heating and/or activation by silver salts (or a Pd catalyst as in the bottom example) and are often low-yielding. Interestingly, sporadic reports show that amines can be more reactive (Scheme 2),7 which can be rationalised by a much more efficient stabilisation of the developing positive charge during the ring-opening process leading to the allyl cation intermediate.
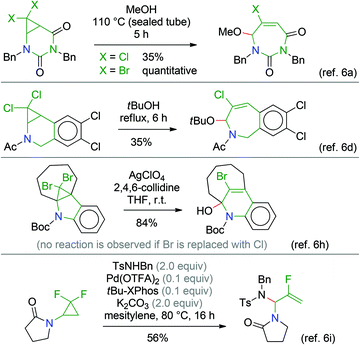 | ||
| Scheme 1 Selected literature examples of cyclopropane ring-opening reactions of nitrogen-substituted gem-dihalocyclopropane derivatives, with departure of a halide ion. | ||
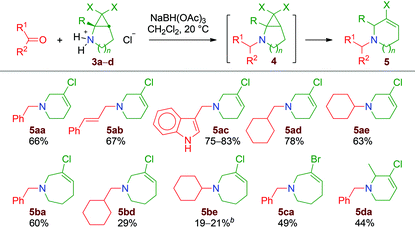
On the basis of these observations, we thought that a fairly general approach could be designed to prepare functionalised halogen-substituted 1,2,3,6-tetrahydropyridine and 2,3,4,7-tetrahydro-1H-azepine derivatives 5 (Scheme 3, n = 1 or 2). Starting from N-Boc-protected cyclic enamine derivatives 1, a dihalocyclopropanation reaction would produce stable N-Boc-bicyclic secondary cyclopropylamines 2. After the removal of the Boc group, reductive amination of aldehydes or ketones would generate thermally unstable dihalogenated aminocyclo-propanes 4. This transformation would thus not only introduce functionality but also trigger the ring-expansion process.
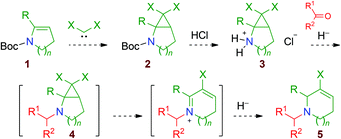 | ||
| Scheme 3 Strategy for the production of functionalised ring-enlarged cyclic amines 5 from dihalogenated bicyclic aminocyclopropanes 2. | ||
Eventually, the corresponding allyl cation adducts would be reduced by the hydride reagent, affording the desired ring-enlarged products 5 (Scheme 3). It is worth noting that 1,2,3,6-tetrahydropyridines and 2,3,4,7-tetrahydro-1H-azepines are important heterocycles, which are present as substructures of many natural products: e.g. vinca alkaloids such as tabersonine, vindoline, vinblastine and vincristine;8 ergot alkaloids such as lysergol and ergotamine;9 pleurostylin,10 didehydrotuberostemonine A,11 huperserines A, B and C;12 and curindolizine.13 Moreover, 2-chlorohuperzine E is an interesting example of a 5-chloro-1,2,3,6-tetrahydropyridine isolated from a plant extract (Fig. 1).14
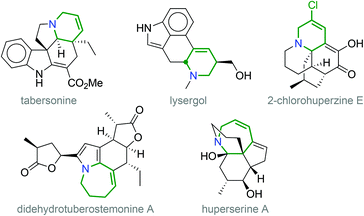 | ||
| Fig. 1 Examples of natural product structures containing 1,2,3,6-tetrahydropyridine and 2,3,4,7-tetrahydro-1H-azepine subunits. | ||
Results and discussion
Synthesis of the substrates
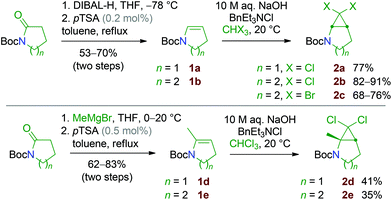
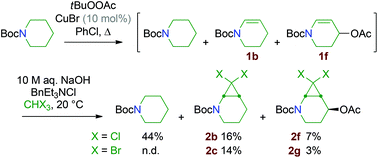
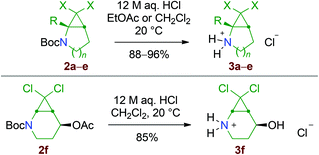
In practice, two routes were investigated for the synthesis of the dihalocyclopropane substrates 2: the first one involved functional group transformation from lactams: installation of the Boc group, nucleophilic addition of a hydride or a methyl carbanion onto the carbonyl group and elimination of water.15 Finally, the cyclic N-Boc enamines 1 thus obtained were reacted with dihalocarbene species generated from chloroform or bromoform, using the phase-transfer catalysis method developed by M. Mąkosza (Scheme 4).16 The second route consisted of applying a Kharasch–Sosnovsky reaction onto N-Boc-piperidine (Scheme 5). This approach was much lower yielding but practical and low cost, since the unreacted starting material could be recycled after the cyclopropanation step.Small amounts of the more complex cyclopropanes 2f and 2g were also isolated, resulting from cyclopropanation of the by-product 1f formed by oxidation of the N-Boc-enamine 1b. Application of the same method to N-Boc-pyrrolidine gave poor results in our hands. The stable N-Boc protected aminocyclopropane substrates 2a–f were then readily converted into secondary amine hydrochlorides by treatment with HCl (Scheme 6). In the process, the acetate group of 2f was hydrolysed into an alcohol function.
Reductive amination – ring enlargement
When a set of aldehydes and ketones were reacted with 2,2-dihalogenated aminocyclopropane salts 3a–e in the presence of NaBH(OAc)3, the reductive amination products 4 were initially formed, as evidenced by NMR after 30–60 minutes of reaction.‡ However, from 3a–d, these were gradually transformed into ring-enlarged products 5, as anticipated (Table 1).§ In particular, the 6,6-dichloro-2-azabicyclo[3.1.0]hexane 3a gives the chloroazacyclohexenes 5aa–ae in moderate to good yields. The presence of a methyl group in 3d is tolerated and the product 5da was isolated in 44% yield. Starting from the homologous 7,7-dihalo-2-azabicyclo[4.1.0]heptane substrates, yields were uniformly lower. This is due, at least in part, to competitive processes leading to by-products (vide infra). Interestingly, halogen-substituted tetrahydroazepine derivatives, with structures closely related to those of 5ba, 5bd–be and 5ca, have been demonstrated to be cycloallene precursors that can be used as intermediates in the preparation of valuable polycyclic building blocks.17 The salt 3f containing a hydroxyl group proved to be unsuitable, affording, with benzaldehyde, the corresponding chloroazepine derivative in 6% yield only. Starting from the better substrate 3b, poor results were also obtained when butyraldehyde and indole-3-carboxaldehyde were employed.‡A much simpler version of the reaction involves the generation of secondary amines by deprotonation of the salts 3 with triethylamine. This was done on a few milligram scale in NMR tubes containing CDCl3 solutions of 3a and 3d. The rearrangement is fast under these conditions and the clean formation of the 5-chloro-2,3-dihydropyridines 6a and 6d is observed after 5 minutes at room temperature (Scheme 7).¶
Interestingly, starting from the methyl-substituted compound 3e and under the reductive amination conditions applied for substrates 3a–d, the reaction takes a different course. Indeed, 3-chloromethylenepiperidine derivatives 7ea and 7eb are isolated as single geometrical isomers (Scheme 8). A similar phenomenon is observed when 3b is reacted with cyclohexanone, with the formation of 7be as the major product.
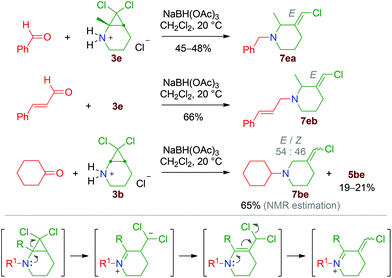 | ||
| Scheme 8 Formation of 3-chloromethylenepiperidine derivatives under reductive amination conditions performed with 3e. | ||
The formation of compounds 7 can be explained by a 2,2-dichloro-1-aminocyclopropane-ring opening proceeding at the C1–C2 bond (Scheme 8, bottom).18 Steric hindrance around the nitrogen atom appears to be a key factor for this process, as well as the size of the nitrogen-containing heterocycle. Indeed, starting from 3a and 3d, which are the lower homologues of 3b and 3e, selectivity is in favour of the ring-expanded molecules 5ae and 5da (Table 1). Nonetheless, careful analysis of the crude products of all the reductive amination experiments revealed, in several cases, the formation of minor amounts of compounds of type 7, as well as of other by-products.‡
Results with monochlorocyclopropane substrates
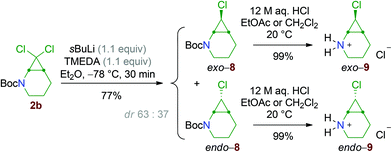
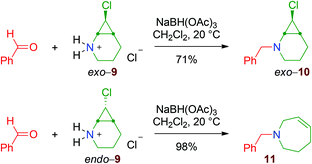
The reactivity of 2-monochloro aminocyclopropane derivatives was next investigated. The dichlorocyclopropane 2b was transformed into the monochloro diastereoisomers 8 by chlorine-to-lithium exchange using sBuLi/TMEDA.|| After separation by flash chromatography, these molecules were converted into the hydrochloride salts, exo- and endo-9 (Scheme 9).Application of reductive amination conditions with benzaldehyde proceeds with high chemoselectivity: while the exo compound gives the tertiary cyclopropylamine exo-10, endo-9 is converted into the ring-expanded tetrahydroazepine 11 (Scheme 10), showing that the endo bicyclic aminocyclopropane endo-10 is not stable and readily undergoes rearrangement, like the dihalo derivatives 4. These divergent results are in agreement with the so-called Woodward–Hoffmann–DePuy rule.19,20 Briefly, the reaction is thought to proceed by a mechanism where the departure of the halide ion and the two-electron electrocyclic cyclopropane-cleavage take place in a concerted fashion. Moreover, the sense of the disrotatory ring-opening is dictated by the relative configurations of the cyclopropane carbon atoms, so that substituents that are trans to the leaving group move outward (Scheme 11, framed). While the substrates having a halogen substituent in the endo relative configuration, i.e. endo-10 and all the dihalo substrates, can be transformed into cyclic cation intermediates 13, exo-10 cannot participate in such a process because the unacceptably strained E cation 12 would be produced (Scheme 11).
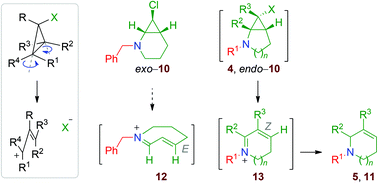 | ||
| Scheme 11 Stereochemical outcome of the transformation of halocyclopropanes into allyl cation species. | ||
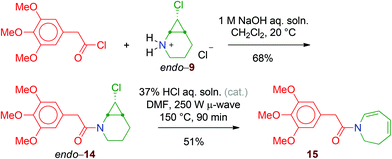
Finally, the successful transformation of endo-9 into the amide endo-14 demonstrates that the reaction of the acyl chloride is fast enough to trap the free secondary amine before it undergoes cyclopropane cleavage. Like the N-Boc derivative endo-8, endo-14 is a stable compound that can be purified by flash chromatography. However, when heated under microwave conditions, it is transformed into the dihydroazepine derivative 15 (Scheme 12).** In contrast, exo-14, prepared in the same way as endo-14, does not react, even in the presence of AgBF4.‡
Conclusions
In summary, endocyclic amine systems fused with a dihalocyclopropane ring undergo cyclopropane cleavage to typically afford ring-expanded products. A minor competitive pathway, which can become predominant with some substrates, is the cleavage of the other cyclopropane bonds adjacent to this atom. Monochloro substrates can also participate in the ring-expansion process, provided that relative configuration of the chlorine substituent is endo. All these transformations readily take place at room temperature, after the amine function is created by a reductive amination reaction or by simple deprotonation of the corresponding hydrochloride salt. In contrast, amides or N-Boc carbamate substrates require activation (e.g. heating) for the ring-expansion to proceed, still on the condition that a halogen substituent is present with the endo relative configuration.Experimental
Selected experimental procedures and data are presented hereafter. Full details can be found in the ESI.†sec-Butyllithium (1.3–1.4 M solution in cyclohexane) was purchased from Sigma-Aldrich or Alfa Aesar and titrated according to a literature method.21 Diethyl ether, and dichloromethane were purified using a MB SPS-800 solvent purification system (MBRAUN). Other solvents and commercial reagents were used as received, without purification. Petroleum ether refers to the 40–60 °C fraction. The microwave-promoted experiments were run using a CEM Discover Microwave Synthesis System with the temperature and time parameters indicated; the reaction vessels were not flushed with an inert gas. All other reactions were carried out under nitrogen or argon. The temperatures mentioned are the temperatures of the cold baths or the oil baths used. Flash column chromatography was performed on VWR Chemicals or Merck silica gel 60 (40–63 μm). Concentration under reduced pressure was carried out using rotary evaporators at 40 °C. NMR spectra were recorded with AM 400 or AVANCE 400 Bruker spectrometers (1H at 400.2 MHz, 13C at 100.6 MHz). Chemical shifts δ are given in ppm, referenced to the peak of tetramethylsilane, defined at δ = 0.00 (1H NMR), or the solvent peak of CDCl3, defined at δ = 77.0 (13C NMR). Multiplicities are abbreviated as follows: s = singlet, d = doublet, t = triplet, q = quadruplet, quint = quintuplet, sext = sextuplet, sept = septuplet, m = multiplet, and br = broad. Coupling constants J are given in Hz and are rounded to the closest multiple of 0.5. Infrared spectra were recorded with a PerkinElmer 2000 or a PerkinElmer Spectrum Two FT-IR spectrometer. Melting points were determined using a Büchi 535 apparatus and were not corrected. Low-resolution mass spectra were recorded on a Hewlett-Packard Quad GC-MS engine spectrometer via direct injection. High-resolution mass spectrometry was performed on a JEOL GC-mate II spectrometer. Underlined m/z values indicate the base peaks.
General procedure A: Cyclopropanation of N-Boc dihydropyrroles and N-Boc tetrahydropyridines with dichlorocarbene22
10 M NaOH aqueous solution (20 mL) was slowly added to a solution of N-Boc cyclic enamine substrate 1 (1.00 equiv., 3.50 mmol) and benzyltriethylammonium chloride (0.63 equiv., 2.20 mmol) in CHCl3 (20 mL). After 90–180 min of vigorous stirring at 20 °C, the aqueous phase was removed. The organic layer was washed with H2O (20 mL) and brine (20 mL), then dried over MgSO4, filtered and concentrated under reduced pressure to afford the crude product, which was then purified by flash column chromatography on silica gel.
2a. Pale yellow solid. M.p. 54.3–55.9 °C. IR (neat) ν 2978 (m), 2934 (w), 2904 (w), 1707 (s, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1478 (w), 1450 (w), 1393 (s), 1368 (m), 1356 (m), 1346 (m), 1288 (w), 1257 (m), 1173 (m), 1127 (m), 1052 (w), 877 (m), 860 (m) cm−1. 1H NMR (CDCl3, 400 MHz),
O), 1478 (w), 1450 (w), 1393 (s), 1368 (m), 1356 (m), 1346 (m), 1288 (w), 1257 (m), 1173 (m), 1127 (m), 1052 (w), 877 (m), 860 (m) cm−1. 1H NMR (CDCl3, 400 MHz), ![[6 with combining low line]](https://www.rsc.org/images/entities/char_0036_0332.gif)
![[3 with combining low line]](https://www.rsc.org/images/entities/char_0033_0332.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 mixture of two rotamers. Major rotamer: δ 1.50 (9 H, s), 2.11–2.36 (3 H, m), 3.37–3.66 (2 H, m), 3.63 (1 H, d, J 7.0). Minor rotamer: δ 1.45 (9 H, s), 2.11–2.36 (3 H, m), 3.28 (1 H, td, J 10.5, 5.5), 3.37–3.66 (1 H, m), 3.82 (1 H, d, J 7.0). 13C NMR (CDCl3, 100.6 MHz),
37 mixture of two rotamers. Major rotamer: δ 1.50 (9 H, s), 2.11–2.36 (3 H, m), 3.37–3.66 (2 H, m), 3.63 (1 H, d, J 7.0). Minor rotamer: δ 1.45 (9 H, s), 2.11–2.36 (3 H, m), 3.28 (1 H, td, J 10.5, 5.5), 3.37–3.66 (1 H, m), 3.82 (1 H, d, J 7.0). 13C NMR (CDCl3, 100.6 MHz), ![[6 with combining low line]](https://www.rsc.org/images/entities/char_0036_0332.gif)
![[3 with combining low line]](https://www.rsc.org/images/entities/char_0033_0332.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 mixture of two rotamers. Major rotamer: δ 24.4, 28.0, 35.9, 48.6, 48.8, 65.1, 80.2, 154.7. Minor rotamer: δ 25.2, 28.0, 34.6, 48.3, 49.0, 64.8, 80.1, 154.7. MS (EI): m/z 114,
37 mixture of two rotamers. Major rotamer: δ 24.4, 28.0, 35.9, 48.6, 48.8, 65.1, 80.2, 154.7. Minor rotamer: δ 25.2, 28.0, 34.6, 48.3, 49.0, 64.8, 80.1, 154.7. MS (EI): m/z 114, ![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif)
![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif)
![[6 with combining low line]](https://www.rsc.org/images/entities/char_0036_0332.gif) , 118, 132, 160, 162, 176, 178 ([M − tBuO]+ with two 35Cl), 180 ([M − tBuO]+ with one 35Cl and one 37Cl), 195, 200, 201, 226, 251 (M+˙ with two 35Cl). HRMS (EI): m/z 251.0474 (M+˙ C10H1535Cl2NO2+˙ requires 251.0475).
, 118, 132, 160, 162, 176, 178 ([M − tBuO]+ with two 35Cl), 180 ([M − tBuO]+ with one 35Cl and one 37Cl), 195, 200, 201, 226, 251 (M+˙ with two 35Cl). HRMS (EI): m/z 251.0474 (M+˙ C10H1535Cl2NO2+˙ requires 251.0475).
2b. Pale yellow oil. IR (neat) ν 2976 (m), 2935 (m), 2873 (w), 2361 (w), 2342 (w), 1710 (s, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1476 (w), 1455 (m), 1403 (s), 1392 (m), 1368 (s), 1354 (m), 1308 (m), 1257 (m), 1168 (s), 1137 (m), 1093 (w), 1024 (w), 839 (w), 824 (w), 772 (w) cm−1. 1H NMR (CDCl3, 400 MHz),
O), 1476 (w), 1455 (m), 1403 (s), 1392 (m), 1368 (s), 1354 (m), 1308 (m), 1257 (m), 1168 (s), 1137 (m), 1093 (w), 1024 (w), 839 (w), 824 (w), 772 (w) cm−1. 1H NMR (CDCl3, 400 MHz), ![[8 with combining low line]](https://www.rsc.org/images/entities/char_0038_0332.gif)
![[5 with combining low line]](https://www.rsc.org/images/entities/char_0035_0332.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 mixture of two rotamers. Major rotamer: δ 1.39–1.51 (1 H, m), 1.53 (9 H, s), 1.61–1.75 (2 H, m), 1.93–2.06 (2 H, m), 2.82 (1 H, ddd, J 12.5, 8.5, 3.5), 3.24 (1 H, d, J 9.0), 3.51 (1 H, ddd, J 12.5, 7.0, 4.0). Minor rotamer, characteristic signals: δ 1.50 (9 H, s), 3.02 (1 H, ddd, J 12.0, 7.5, 4.0), 3.27 (1 H, ddd, J 12.0, 9.5, 4.5), 3.33 (1 H, d, J 9.0). 13C NMR (CDCl3, 100.6 MHz),
15 mixture of two rotamers. Major rotamer: δ 1.39–1.51 (1 H, m), 1.53 (9 H, s), 1.61–1.75 (2 H, m), 1.93–2.06 (2 H, m), 2.82 (1 H, ddd, J 12.5, 8.5, 3.5), 3.24 (1 H, d, J 9.0), 3.51 (1 H, ddd, J 12.5, 7.0, 4.0). Minor rotamer, characteristic signals: δ 1.50 (9 H, s), 3.02 (1 H, ddd, J 12.0, 7.5, 4.0), 3.27 (1 H, ddd, J 12.0, 9.5, 4.5), 3.33 (1 H, d, J 9.0). 13C NMR (CDCl3, 100.6 MHz), ![[8 with combining low line]](https://www.rsc.org/images/entities/char_0038_0332.gif)
![[5 with combining low line]](https://www.rsc.org/images/entities/char_0035_0332.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 mixture of two rotamers. Major rotamer: δ 17.1, 21.2, 28.3, 28.4, 40.0, 40.2, 63.4, 80.4, 156.0. Minor rotamer, characteristic signals: δ 17.0, 21.4, 39.7, 41.6. MS (EI): m/z 128,
15 mixture of two rotamers. Major rotamer: δ 17.1, 21.2, 28.3, 28.4, 40.0, 40.2, 63.4, 80.4, 156.0. Minor rotamer, characteristic signals: δ 17.0, 21.4, 39.7, 41.6. MS (EI): m/z 128, ![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif)
![[3 with combining low line]](https://www.rsc.org/images/entities/char_0033_0332.gif)
![[0 with combining low line]](https://www.rsc.org/images/entities/char_0030_0332.gif) , 132, 135, 174, 192 ([M − tBuO]+ with two 35Cl), 194 ([M − tBuO]+ with one 35Cl and one 37Cl), 209, 211, 232, 234. HRMS (EI): m/z 265.0627 (M+˙ C11H1735Cl2NO2+˙ requires 265.0631).
, 132, 135, 174, 192 ([M − tBuO]+ with two 35Cl), 194 ([M − tBuO]+ with one 35Cl and one 37Cl), 209, 211, 232, 234. HRMS (EI): m/z 265.0627 (M+˙ C11H1735Cl2NO2+˙ requires 265.0631).
2c. White solid. M.p. 46.8–48.0 °C. IR (neat) ν 2971 (m), 2933 (w), 2872 (w), 1704 (s, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1451 (w), 1400 (m), 1392 (m), 1366 (m), 1348 (m), 1315 (w), 1255 (w), 1161 (m), 1135 (m), 1015 (w), 755 (m) cm−1. 1H NMR (CDCl3, 400 MHz),
O), 1451 (w), 1400 (m), 1392 (m), 1366 (m), 1348 (m), 1315 (w), 1255 (w), 1161 (m), 1135 (m), 1015 (w), 755 (m) cm−1. 1H NMR (CDCl3, 400 MHz), ![[8 with combining low line]](https://www.rsc.org/images/entities/char_0038_0332.gif)
![[8 with combining low line]](https://www.rsc.org/images/entities/char_0038_0332.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 mixture of two rotamers. Major rotamer: δ 1.44 (1 H, m), 1.55 (9 H, s), 1.52–1.62 (1 H, m), 1.74 (1 H, m), 2.02–2.13 (2 H, m), 2.90 (1 H, ddd, J 12.5, 8.0, 4.0), 3.29 (1 H, d, J 9.0), 3.43 (1 H, ddd, J 12.5, 8.0, 4.5). Minor rotamer, characteristic signals: δ 1.50 (9 H, s), 3.08 (1 H, ddd, J 12.5, 6.5, 4.0), 3.22 (1 H, ddd, J 12.5, 9.5, 4.0), 3.37 (1 H, d, J 9.0). 13C NMR (CDCl3, 100.6 MHz),
12 mixture of two rotamers. Major rotamer: δ 1.44 (1 H, m), 1.55 (9 H, s), 1.52–1.62 (1 H, m), 1.74 (1 H, m), 2.02–2.13 (2 H, m), 2.90 (1 H, ddd, J 12.5, 8.0, 4.0), 3.29 (1 H, d, J 9.0), 3.43 (1 H, ddd, J 12.5, 8.0, 4.5). Minor rotamer, characteristic signals: δ 1.50 (9 H, s), 3.08 (1 H, ddd, J 12.5, 6.5, 4.0), 3.22 (1 H, ddd, J 12.5, 9.5, 4.0), 3.37 (1 H, d, J 9.0). 13C NMR (CDCl3, 100.6 MHz), ![[8 with combining low line]](https://www.rsc.org/images/entities/char_0038_0332.gif)
![[8 with combining low line]](https://www.rsc.org/images/entities/char_0038_0332.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 mixture of two rotamers. Major rotamer: δ 19.0, 21.1, 28.3, 29.4, 36.7, 40.2, 40.6, 80.4, 155.8. Minor rotamer, characteristic signals: δ 21.2, 28.3, 36.5, 40.1, 41.8, 80.3, 155.9. MS (EI): m/z 94, 95, 119, 146,
12 mixture of two rotamers. Major rotamer: δ 19.0, 21.1, 28.3, 29.4, 36.7, 40.2, 40.6, 80.4, 155.8. Minor rotamer, characteristic signals: δ 21.2, 28.3, 36.5, 40.1, 41.8, 80.3, 155.9. MS (EI): m/z 94, 95, 119, 146, ![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif)
![[7 with combining low line]](https://www.rsc.org/images/entities/char_0037_0332.gif)
![[4 with combining low line]](https://www.rsc.org/images/entities/char_0034_0332.gif) , 175, 176, 199, 218, 220, 255, 280 ([M − tBuO]+ with two 79Br), 282 ([M − tBuO]+ with one 79Br and one 81Br), 284 ([M − tBuO]+ with two 81Br), 297, 299, 301, 353 (M+˙ with two 79Br), 355 (M+˙ with one 79Br and one 81Br), 357 (M+˙ with two 81Br). HRMS (EI): m/z 352.9629 (M+˙ C11H1779Br2NO2+˙ requires 352.9621).
, 175, 176, 199, 218, 220, 255, 280 ([M − tBuO]+ with two 79Br), 282 ([M − tBuO]+ with one 79Br and one 81Br), 284 ([M − tBuO]+ with two 81Br), 297, 299, 301, 353 (M+˙ with two 79Br), 355 (M+˙ with one 79Br and one 81Br), 357 (M+˙ with two 81Br). HRMS (EI): m/z 352.9629 (M+˙ C11H1779Br2NO2+˙ requires 352.9621).
Monochloro substrates exo-8 and endo-8
sec-Butyllithium solution (0.91 M in cyclohexane, 1.10 equiv., 1.10 mmol, 1.21 mL) was added dropwise, at −78 °C, to a solution of tert-butyl 7,7-dichloro-2-azabicyclo[4.1.0]heptane-2-carboxylate 2b (1.00 equiv., 1.00 mmol, 266 mg) and TMEDA (1.10 equiv., 1.10 mmol, 165 μL) in Et2O (11 mL). After 15 minutes of stirring at −78 °C, H2O (20 mL) was added. The mixture was then extracted with Et2O (3 × 20 mL). The combined organic layers were dried over MgSO4, filtered and concentrated under reduced pressure to afford a pale yellow oil (237 mg). Purification by flash column chromatography on silica gel (EtOAc/petroleum ether, gradient from 2 to 10%) afforded pure exo-8 (105 mg, 453 μmol, 45%), a 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 mixture of the endo and exo diastereoisomers of 8 (64.7 mg, 279 μmol, 28%) and pure endo-8 (7.0 mg, 30 μmol, 3%).
25 mixture of the endo and exo diastereoisomers of 8 (64.7 mg, 279 μmol, 28%) and pure endo-8 (7.0 mg, 30 μmol, 3%).
exo-8. Colourless oil. IR (neat) ν 2977 (m), 2934 (m), 2868 (w), 1702 (s, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1447 (w), 1418 (m), 1384 (m), 1366 (m), 1300 (w), 1269 (w), 1246 (w), 1164 (m), 1131 (m), 1035 (w), 1005 (w), 774 (w) cm−1. 1H NMR (CDCl3, 400 MHz),
O), 1447 (w), 1418 (m), 1384 (m), 1366 (m), 1300 (w), 1269 (w), 1246 (w), 1164 (m), 1131 (m), 1035 (w), 1005 (w), 774 (w) cm−1. 1H NMR (CDCl3, 400 MHz), ![[7 with combining low line]](https://www.rsc.org/images/entities/char_0037_0332.gif)
![[9 with combining low line]](https://www.rsc.org/images/entities/char_0039_0332.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 mixture of two rotamers. Major rotamer: δ 1.12 (1 H, tddd, J 13.0, 12.0, 4.5, 3.5), 1.50 (9 H, s), 1.57 (1 H, br ddd, J 9.5, 6.0, 4.0), 1.61 (1 H, dddd, J 13.0, 6.0, 3.5, 2.0), 1.72 (1 H, ddt, J 13.5, 13.0, 6.0), 1.97 (1 H, br dd, J 13.5, 4.5), 2.43 (1 H, ddd, J 13.0, 12.0, 2.0), 2.65 (1 H, dd, J 4.0, 1.5), 3.03 (1 H, dd, J 9.5, 1.5), 3.76 (1 H, dt, J 13.0, 3.5). Minor rotamer: δ 1.45–1.77 (4 H, m, H2, H3a), 1.46 (9 H, s), 1.97 (1 H, br d, J 13.5), 2.56 (1 H, br t, J 12.5), 2.71 (1 H, br d, J 3.5), 3.15 (1 H, br d, J 9.5), 3.59 (1 H, br d, J 12.5). 13C NMR (CDCl3, 100.6 MHz),
21 mixture of two rotamers. Major rotamer: δ 1.12 (1 H, tddd, J 13.0, 12.0, 4.5, 3.5), 1.50 (9 H, s), 1.57 (1 H, br ddd, J 9.5, 6.0, 4.0), 1.61 (1 H, dddd, J 13.0, 6.0, 3.5, 2.0), 1.72 (1 H, ddt, J 13.5, 13.0, 6.0), 1.97 (1 H, br dd, J 13.5, 4.5), 2.43 (1 H, ddd, J 13.0, 12.0, 2.0), 2.65 (1 H, dd, J 4.0, 1.5), 3.03 (1 H, dd, J 9.5, 1.5), 3.76 (1 H, dt, J 13.0, 3.5). Minor rotamer: δ 1.45–1.77 (4 H, m, H2, H3a), 1.46 (9 H, s), 1.97 (1 H, br d, J 13.5), 2.56 (1 H, br t, J 12.5), 2.71 (1 H, br d, J 3.5), 3.15 (1 H, br d, J 9.5), 3.59 (1 H, br d, J 12.5). 13C NMR (CDCl3, 100.6 MHz), ![[7 with combining low line]](https://www.rsc.org/images/entities/char_0037_0332.gif)
![[9 with combining low line]](https://www.rsc.org/images/entities/char_0039_0332.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 mixture of two rotamers. Major rotamer: δ 19.2, 22.4, 23.1, 28.4, 35.8, 37.5, 39.7, 80.0, 156.0. Minor rotamer: δ 19.2, 22.3, 22.8, 28.4, 35.1, 37.3, 41.3, 80.1, 156.0. MS (EI) m/z
21 mixture of two rotamers. Major rotamer: δ 19.2, 22.4, 23.1, 28.4, 35.8, 37.5, 39.7, 80.0, 156.0. Minor rotamer: δ 19.2, 22.3, 22.8, 28.4, 35.1, 37.3, 41.3, 80.1, 156.0. MS (EI) m/z ![[9 with combining low line]](https://www.rsc.org/images/entities/char_0039_0332.gif)
![[8 with combining low line]](https://www.rsc.org/images/entities/char_0038_0332.gif) , 110, 130, 140, 158 ([M − tBuO]+ with 35Cl), 160 ([M − tBuO]+ with 37Cl), 179, 196 ([M − Cl]+). HRMS m/z (EI) 158.0370 ([M − tBuO]+ C7H9ClNO+ requires 158.0368).
, 110, 130, 140, 158 ([M − tBuO]+ with 35Cl), 160 ([M − tBuO]+ with 37Cl), 179, 196 ([M − Cl]+). HRMS m/z (EI) 158.0370 ([M − tBuO]+ C7H9ClNO+ requires 158.0368).
endo-8. Colourless crystals. M.p. 62–64 °C. IR (neat) ν 2975 (m), 2933 (m), 2869 (w), 1700 (s, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1478 (w), 1454 (w), 1407 (m), 1391 (m), 1365 (m), 1309 (m), 1272 (w), 1256 (w), 1169 (m), 1137 (m), 1066 (w), 963 (w), 774 (m), 712 (w) cm−1. 1H NMR (CDCl3, 400 MHz),
O), 1478 (w), 1454 (w), 1407 (m), 1391 (m), 1365 (m), 1309 (m), 1272 (w), 1256 (w), 1169 (m), 1137 (m), 1066 (w), 963 (w), 774 (m), 712 (w) cm−1. 1H NMR (CDCl3, 400 MHz), ![[7 with combining low line]](https://www.rsc.org/images/entities/char_0037_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 mixture of two rotamers. Major rotamer: δ 1.40–1.73 (4 H, m), 1.48 (9 H, s), 1.94 (1 H, m), 2.86 (1 H, ddd, J 12.0, 8.5, 4.0), 2.89 (1 H, dd, J 9.0, 5.5), 3.14 (1 H, dd, J 8.0, 5.5), 3.53 (1 H, ddd, J 12.0, 7.0, 4.5). Minor rotamer: δ 1.40–1.73 (4 H, m), 1.48 (9 H, s), 1.98 (1 H, m), 2.96 (1 H, dd, J 9.0, 5.5), 3.06 (1 H, ddd, J 12.5, 7.0, 4.0), 3.24 (1 H, dd, J 8.0, 5.5), 3.26 (1 H, ddd, J 12.5, 8.5, 4.0). 13C NMR (CDCl3, 100.6 MHz),
28 mixture of two rotamers. Major rotamer: δ 1.40–1.73 (4 H, m), 1.48 (9 H, s), 1.94 (1 H, m), 2.86 (1 H, ddd, J 12.0, 8.5, 4.0), 2.89 (1 H, dd, J 9.0, 5.5), 3.14 (1 H, dd, J 8.0, 5.5), 3.53 (1 H, ddd, J 12.0, 7.0, 4.5). Minor rotamer: δ 1.40–1.73 (4 H, m), 1.48 (9 H, s), 1.98 (1 H, m), 2.96 (1 H, dd, J 9.0, 5.5), 3.06 (1 H, ddd, J 12.5, 7.0, 4.0), 3.24 (1 H, dd, J 8.0, 5.5), 3.26 (1 H, ddd, J 12.5, 8.5, 4.0). 13C NMR (CDCl3, 100.6 MHz), ![[7 with combining low line]](https://www.rsc.org/images/entities/char_0037_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 mixture of two rotamers. Major rotamer: δ 15.1, 16.2, 22.3, 28.2, 29.5, 37.0, 40.7, 79.4, 156.3. Minor rotamer: δ 15.0, 15.9, 22.3, 28.2, 29.5, 36.7, 42.3, 79.6, 156.4. MS (EI) m/z 96,
28 mixture of two rotamers. Major rotamer: δ 15.1, 16.2, 22.3, 28.2, 29.5, 37.0, 40.7, 79.4, 156.3. Minor rotamer: δ 15.0, 15.9, 22.3, 28.2, 29.5, 36.7, 42.3, 79.6, 156.4. MS (EI) m/z 96, ![[9 with combining low line]](https://www.rsc.org/images/entities/char_0039_0332.gif)
![[8 with combining low line]](https://www.rsc.org/images/entities/char_0038_0332.gif) , 99, 140, 141, 158 ([M − tBuO]+ with 35Cl), 160 ([M − tBuO]+ with 37Cl), 175, 196 ([M − Cl]+). HRMS m/z (EI) 196.1335 ([M − Cl]+ C11H18NO2+ requires 196.1333).
, 99, 140, 141, 158 ([M − tBuO]+ with 35Cl), 160 ([M − tBuO]+ with 37Cl), 175, 196 ([M − Cl]+). HRMS m/z (EI) 196.1335 ([M − Cl]+ C11H18NO2+ requires 196.1333).
Typical procedure for the preparation of the hydrochloride salts
12 M HCl aqueous solution (5.0 equiv., 18.8 mmol, 1.57 mL) was added dropwise to a vigorously stirred solution of 2b (1.00 equiv., 3.76 mmol, 1.00 g) in CH2Cl2 (8.0 mL). After 16 h of stirring at 20 °C, the solution was concentrated under reduced pressure. The residue was washed with a small amount of Et2O (2 × 4.0 mL) and dried under high vacuum to afford pure 3b (724 mg, 3.58 mmol, 95%).
3b. White solid. M.p. 136.5 °C (decomposition). 1H NMR (CDCl3, 400 MHz): δ 1.69 (1 H, m), 1.83 (1 H, br s), 1.97 (1 H, br ddd, J 13.5, 6.5, 5.5), 2.08–2.22 (2 H, m), 2.93 (1 H, br ddd, J 12.5, 9.5, 3.0), 3.35 (1 H, br ddd, J 12.5, 7.0, 3.0), 3.48 (1 H, br d, J 9.5), 9.14 (1 H, br s, NH), 11.60 (1 H, br s, NH). 13C NMR (CDCl3, 100.6 MHz): δ 15.0, 16.5, 25.2, 36.6, 40.5, 59.3. MS (EI): m/z 94, 102, 103, 104, ![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif)
![[3 with combining low line]](https://www.rsc.org/images/entities/char_0033_0332.gif)
![[0 with combining low line]](https://www.rsc.org/images/entities/char_0030_0332.gif) , 131, 132, 164, 166 ([M − Cl]+ with two 35Cl), 167.
, 131, 132, 164, 166 ([M − Cl]+ with two 35Cl), 167.
General procedure B: Reductive amination reactions of aldehydes and ketones with the cyclopropylammonium salts 3 or 9
Sodium triacetoxyborohydride (2.40 equiv., 240 μmol, 50.9 mg) was added at 20 °C to a solution of aldehyde or ketone (1.00 equiv., 100 μmol) and cyclopropylammonium chloride 3 or 9 (1.00 equiv., 100 μmol) in dry CH2Cl2 (1.0 mL). After 15 h of stirring at r.t., saturated NaHCO3 aqueous solution (15 mL) was added. The mixture was extracted with CH2Cl2 (2 × 15 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated under reduced pressure to afford the crude product, which was then purified by flash column chromatography on silica gel (typically, a few drops of Et3N were added to the eluents used).
5aa. Pale yellow oil. IR (neat) ν 3063 (w), 3029 (m), 2919 (s), 2803 (s), 2761 (m), 2724 (w), 1664 (m), 1495 (m), 1455 (m), 1368 (m), 1347 (m), 1149 (m), 1126 (s), 1045 (m), 962 (m), 844 (s), 835 (s), 738 (s) cm−1. 1H NMR (CDCl3, 400 MHz): δ 2.22 (2 H, tdt, J 5.5, 4.0, 2.5), 2.58 (2 H, t, J 5.5), 3.10 (2 H, td, J 2.5, 1.5), 3.62 (2 H, s), 5.85 (1 H, tt, J 4.0, 1.5), 7.24–7.38 (5 H, m). 13C NMR (CDCl3, 100.6 MHz): δ 26.2, 48.4, 57.3, 61.7, 122.5, 127.2, 128.3, 128.8, 129.0, 137.8. MS (EI): m/z 116, 117, 118, 172, ![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif)
![[0 with combining low line]](https://www.rsc.org/images/entities/char_0030_0332.gif)
![[7 with combining low line]](https://www.rsc.org/images/entities/char_0037_0332.gif) (M+˙ with 35Cl), 209 (M+˙ with 35Cl). HRMS (EI): m/z 207.0819 (M+˙ C12H1435ClN+˙ requires 207.0810).
(M+˙ with 35Cl), 209 (M+˙ with 35Cl). HRMS (EI): m/z 207.0819 (M+˙ C12H1435ClN+˙ requires 207.0810).
5ae. Pale yellow oil. IR (neat) ν 2929 (s), 2855 (m), 2802 (w), 2361 (w), 2343 (w), 1665 (w), 1450 (m), 1379 (w), 1138 (w), 987 (w), 958 (w), 839 (w), 772 (w) cm−1. 1H NMR (CDCl3, 400 MHz): δ 1.18–1.30 (1 H, m), 1.24 (2 H, m), 1.25 (2 H, m), 1.64 (1 H, br d, J 12.0), 1.81 (2 H, m), 1.89 (2 H, m), 2.22 (2 H, tdt, J 5.5, 4.0, 2.5), 2.40 (1 H, m), 2.64 (2 H, t, J 5.5), 3.23 (2 H, td, J 2.5, 2.0), 5.84 (1 H, tt, J 4.0, 2.0). 13C NMR (CDCl3, 100.6 MHz): δ 25.8, 26.2, 26.7, 28.8, 44.9, 53.4, 62.7, 122.7, 129.2. MS (EI): m/z 130, 132, ![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif)
![[5 with combining low line]](https://www.rsc.org/images/entities/char_0035_0332.gif)
![[6 with combining low line]](https://www.rsc.org/images/entities/char_0036_0332.gif) , 158, 170, 199 (M+˙ with 35Cl), 201 (M+˙ with 37Cl). HRMS (EI): m/z 199.1134 (M+˙ C11H1835ClN+˙ requires 199.1123).
, 158, 170, 199 (M+˙ with 35Cl), 201 (M+˙ with 37Cl). HRMS (EI): m/z 199.1134 (M+˙ C11H1835ClN+˙ requires 199.1123).
5ba. Pale yellow oil. IR (neat) ν 3063 (w), 3029 (w), 2930 (s), 2839 (m), 2810 (m), 1642 (w), 1494 (m), 1453 (m), 1436 (m), 1361 (w), 1118 (m), 1028 (w), 1015 (w), 969 (w), 951 (w), 822 (w), 757 (m), 733 (s) cm−1. 1H NMR (CDCl3, 400 MHz): δ 1.70 (2 H, tt, J 6.0, 5.5), 2.24 (2 H, dtt, J 6.5, 5.5, 1.0), 2.94 (2 H, t, J 6.0), 3.56 (2 H, t, J 1.0), 3.75 (2 H, s), 6.08 (1 H, t, J 6.5), 7.26 (1 H, distorted tt, J 7.0, 1.5), 7.29–7.38 (4 H, m). 13C NMR (CDCl3, 100.6 MHz): δ 24.2, 26.9, 56.5, 58.2, 60.2, 127.1, 128.3, 128.9, 129.6, 132.6, 138.7. MS (EI): m/z ![[9 with combining low line]](https://www.rsc.org/images/entities/char_0039_0332.gif)
![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif) (Bn+), 92, 120, 121, 130, 186 ([M − Cl]+), 220, 221 (M+˙ with 35Cl), 222, 223 (M+˙ with 37Cl). HRMS (EI): m/z 221.0977 (M+˙ C13H1635ClN+˙ requires 221.0966).
(Bn+), 92, 120, 121, 130, 186 ([M − Cl]+), 220, 221 (M+˙ with 35Cl), 222, 223 (M+˙ with 37Cl). HRMS (EI): m/z 221.0977 (M+˙ C13H1635ClN+˙ requires 221.0966).
5be. Pale yellow oil. IR (neat) ν 2929 (s), 2855 (m), 2806 (w), 2361 (w), 2342 (w), 1698 (w), 1450 (w), 1260 (w), 1125 (w), 1018 (w), 963 (w), 772 (w) cm−1. 1H NMR (CDCl3, 400 MHz): δ 1.04–1.32 (3 H, m), 1.61 (1 H, dm, J 12.5), 1.67 (2 H, tt, J 6.0, 5.5), 1.77 (2 H, dm, J 12.0), 1.91 (2 H, br d, J 11.5), 2.20 (2 H, dt, J 6.0, 5.5), 2.56 (1 H, tt, J 10.5, 3.0), 2.95 (2 H, t, J 6.0), 3.57 (2 H, br s), 5.98 (1 H, br t, J 6.0). 13C NMR (CDCl3, 100.6 MHz): δ 25.2, 25.6, 26.2, 26.7, 29.7, 52.7, 58.0, 60.6, 129.2, 132.3. MS (EI): m/z 112, ![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif)
![[7 with combining low line]](https://www.rsc.org/images/entities/char_0037_0332.gif)
![[0 with combining low line]](https://www.rsc.org/images/entities/char_0030_0332.gif) , 171, 172, 213 (M+˙ with 35Cl), 215 (M+˙ with 37Cl). HRMS (EI): m/z 213.1279 (M+˙ C12H2035ClN+˙ requires 213.1279).
, 171, 172, 213 (M+˙ with 35Cl), 215 (M+˙ with 37Cl). HRMS (EI): m/z 213.1279 (M+˙ C12H2035ClN+˙ requires 213.1279).
5ca. Pale yellow oil. IR (neat) ν 3029 (w), 2928 (s), 2850 (m), 2811 (m), 2361 (w), 2342 (w), 1495 (w), 1454 (m), 1436 (w), 1362 (w), 1118 (m), 728 (m) cm−1. 1H NMR (CDCl3, 400 MHz): δ 1.69 (2 H, quint, J 5.5), 2.21 (2 H, dt, J 6.5, 5.5), 2.95 (2 H, t, J 5.5), 3.67 (2 H, s), 3.76 (2 H, s), 6.33 (1 H, t, J 6.5), 7.22–7.40 (5 H, m). 13C NMR (CDCl3, 100.6 MHz): δ 24.1, 28.5, 56.4, 57.9, 62.1, 123.0, 127.1, 128.3, 128.9, 134.0, 138.8. MS (EI): m/z ![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif)
![[0 with combining low line]](https://www.rsc.org/images/entities/char_0030_0332.gif) , 121, 130, 186 ([M − Br]+), 265 (M+˙ with 79Br), 267 (M+˙ with 81Br). HRMS (EI): m/z 265.0468 (M+˙ C13H1679BrN+˙ requires 265.0461).
, 121, 130, 186 ([M − Br]+), 265 (M+˙ with 79Br), 267 (M+˙ with 81Br). HRMS (EI): m/z 265.0468 (M+˙ C13H1679BrN+˙ requires 265.0461).
5da. Pale yellow oil. IR (neat) ν 3028 (w), 2976 (m), 2935 (m), 2836 (m), 2809 (m), 2361 (w), 2342 (w), 1495 (w), 1453 (m), 1368 (m), 1120 (m), 970 (m), 801 (m), 734 (s) cm−1. 1H NMR (CDCl3, 400 MHz): δ 1.31 (3 H, d, J 6.5), 1.99 (1 H, dddd, J 17.0, 5.5, 5.0, 3.0), 2.34 (1 H, dddd, J 17.0, 10.0, 3.0, 2.5), 2.62 (1 H, ddd, J 13.0, 5.5, 2.5), 2.94 (1 H, ddd, J 13.0, 10.0, 5.0), 3.17 (1 H, q, J 6.5), 3.72 (2 H, AB system, δA 3.68, δB 3.77, JAB 13.5), 5.87 (1 H, t, J 4.0), 7.22–7.39 (5 H, m). 13C NMR (CDCl3, 100.6 MHz): δ 16.6, 24.2, 42.1, 57.6, 58.3, 122.5, 127.0, 128.3, 128.7, 135.0, 139.0. MS (EI): m/z 120, ![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif)
![[0 with combining low line]](https://www.rsc.org/images/entities/char_0030_0332.gif)
![[6 with combining low line]](https://www.rsc.org/images/entities/char_0036_0332.gif) ([M − Me]+ with 35Cl), 207, 208 ([M − Me]+ with 37Cl), 221 (M+˙ with 35Cl). HRMS (EI): m/z 221.0971 (M+˙ C13H1635ClN+˙ requires 221.0966).
([M − Me]+ with 35Cl), 207, 208 ([M − Me]+ with 37Cl), 221 (M+˙ with 35Cl). HRMS (EI): m/z 221.0971 (M+˙ C13H1635ClN+˙ requires 221.0966).
7ea. Pale yellow oil. IR (neat) ν 3063 (w), 3028 (w), 2935 (s), 2854 (m), 2811 (m), 1731 (w), 1637 (w), 1494 (w), 1455 (m), 1366 (m), 1291 (m), 1125 (m), 1028 (m), 775 (m), 732 (m) cm−1. 1H NMR (CDCl3, 400 MHz): δ 1.21 (3 H, d, J 7.0), 1.55–1.74 (2 H, m), 2.19 (1 H, dddd, J 14.0, 12.0, 5.5, 1.5), 2.61 (1 H, dt, J 13.0, 3.5), 2.70 (1 H, dt, J 14.0, 4.0), 2.89 (1 H, ddd, J 13.0, 11.0, 3.5), 3.32 (1 H, qdd, J 7.0, 1.5, 1.0), 3.63 (2 H, AB system, δA 3.62, δB 3.64, JAB 14.0), 5.87 (1 H, d, J 1.0), 7.24 (1 H, br t, J 7.0), 7.31 (2 H, br dd, J 7.5, 7.0), 7.34 (2 H, br d, J 7.5). 13C NMR (CDCl3, 100.6 MHz): δ 14.3, 23.2, 23.5, 46.0, 57.4, 58.5, 110.9, 126.9, 128.3, 128.7, 139.0, 141.2. MS (EI): m/z 117, 149, ![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif)
![[0 with combining low line]](https://www.rsc.org/images/entities/char_0030_0332.gif) ([M − Me]+ with 35Cl), 221, 222 ([M − Me]+ with 37Cl), 235 (M+˙ with 35Cl). HRMS (EI): m/z 235.1131 (M+˙ C14H1835ClN+˙ requires 235.1123).
([M − Me]+ with 35Cl), 221, 222 ([M − Me]+ with 37Cl), 235 (M+˙ with 35Cl). HRMS (EI): m/z 235.1131 (M+˙ C14H1835ClN+˙ requires 235.1123).
exo-10. Colourless oil. IR (neat) ν 3029 (m), 2939 (s), 2859 (m), 2800 (m), 1494 (m), 1453 (s), 1349 (m), 1315 (m), 1247 (m), 1155 (m), 1061 (m), 1029 (m), 762 (s), 737 (s) cm−1. 1H NMR (CDCl3, 400 MHz) δ 1.28 (1 H, ddtd, J 13.0, 6.5, 5.5, 2.5), 1.35 (1 H, dddd, J 9.5, 9.0, 3.5, 2.5), 1.42 (1 H, ddddd, J 13.0, 9.5, 9.0, 5.5, 3.0), 1.51 (1 H, dddd, J 14.0, 9.0, 5.5, 2.5), 1.93 (1 H, ddt, J 14.0, 9.0, 5.5), 2.02 (1 H, ddd, J 11.5, 9.5, 2.5), 2.40 (1 H, dd, J 9.5, 2.5), 2.42 (1 H, ddd, J 11.5, 6.5, 3.0), 2.76 (1 H, dd, J 3.5, 2.5), 3.69 (2 H, AB system, δA 3.65, δB 3.73, JAB 13.0), 7.19 (1 H, br t, J 7.0), 7.23–7.32 (4 H, m). 13C NMR (CDCl3, 100.6 MHz) δ 20.6, 21.7, 22.3, 33.9, 44.4, 47.6, 61.4, 127.1, 128.2, 129.2, 138.0. MS (EI): m/z 91 (Bn+), 92, 94, 95, 102, ![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif)
![[8 with combining low line]](https://www.rsc.org/images/entities/char_0038_0332.gif)
![[6 with combining low line]](https://www.rsc.org/images/entities/char_0036_0332.gif) ([M − Cl]+), 187, 220, 221 (M+˙ with 35Cl), 222. HRMS (EI): m/z 186.1273 ([M − Cl]+ C13H16N+ requires 186.1278), 221.0989 (M+˙ C13H1635ClN+˙ requires 221.0966).
([M − Cl]+), 187, 220, 221 (M+˙ with 35Cl), 222. HRMS (EI): m/z 186.1273 ([M − Cl]+ C13H16N+ requires 186.1278), 221.0989 (M+˙ C13H1635ClN+˙ requires 221.0966).
11. Colourless oil. IR (neat) ν 3023 (m), 2928 (s), 2837 (m), 2803 (m), 2760 (m), 1652 (w), 1495 (m), 1453 (m), 1354 (m), 1153 (m), 1115 (m), 1028 (w), 741 (m) cm−1. 1H NMR (CDCl3, 400 MHz): δ 1.61 (2 H, distorted quint, J 5.5), 2.18 (2 H, br qd, J 5.5, 1.0), 2.80 (2 H, distorted t, J 5.5), 3.10 (2 H, dd, J 5.5, 1.0), 3.58 (2 H, s), 5.57 (1 H, dtt, J 11.0, 5.5, 1.0), 5.85 (1 H, dtt, J 11.0, 5.5, 1.0), 7.14–7.30 (5 H, m). 13C NMR (CDCl3, 100.6 MHz): δ 25.8, 28.2, 53.4, 58.1, 60.5, 126.8, 128.2, 128.9, 129.3, 133.5, 139.4. MS (EI): m/z ![[9 with combining low line]](https://www.rsc.org/images/entities/char_0039_0332.gif)
![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif) (Bn+), 92, 96, 110, 120, 131, 172, 186, 187 (M+˙), 188. HRMS (EI): m/z 187.1354 (M+˙ C13H17N+˙ requires 187.1356).
(Bn+), 92, 96, 110, 120, 131, 172, 186, 187 (M+˙), 188. HRMS (EI): m/z 187.1354 (M+˙ C13H17N+˙ requires 187.1356).
Amide endo-14
1 M NaOH aqueous solution (10 mL) was added to a solution of 2-(3,4,5-trimethoxyphenyl)acetyl chloride (1.10 equiv., 274 μmol, 67.0 mg) and endo-9 (1.00 equiv., 249 μmol, 41.6 mg) in CH2Cl2 (10 mL). After 2 h of stirring at 20 °C, the organic layer was separated and the aqueous phase was extracted with CH2Cl2 (2 × 10 mL). The combined organic layers were dried over MgSO4, filtered and concentrated under reduced pressure to afford a thick pale yellow oil (74.0 mg). Purification by flash column chromatography on silica gel (EtOAc/petroleum ether, gradient from 30 to 100%) afforded pure endo-14 (57.6 mg, 218 μmol, 68%).
endo-14. Thick pale yellow oil. IR (neat) ν 2996 (w), 2939 (m), 2871 (w), 2838 (w), 1651 (s, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1590 (m), 1508 (m), 1457 (m), 1424 (m), 1334 (m), 1240 (m), 1125 (s), 1008 (m), 789 (w) cm−1. 1H NMR (CDCl3, 400 MHz),
O), 1590 (m), 1508 (m), 1457 (m), 1424 (m), 1334 (m), 1240 (m), 1125 (s), 1008 (m), 789 (w) cm−1. 1H NMR (CDCl3, 400 MHz), ![[8 with combining low line]](https://www.rsc.org/images/entities/char_0038_0332.gif)
![[4 with combining low line]](https://www.rsc.org/images/entities/char_0034_0332.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 mixture of two rotamers. Major rotamer: δ 1.50–1.75 (4H, m), 2.02 (1H, m), 2.83 (1H, ddd, J 13.0, 8.5, 3.5), 2.98 (1H, dd, J 9.5, 5.0), 3.34 (1H, dd, J 8.0, 5.0), 3.74 (2 H, AB system, δA 3.71, δB 3.77, JAB 15.0), 3.83 (3H, s), 3.85 (6H, s), 3.93 (1H, ddd, J 13.0, 6.5, 4.0), 6.51 (2H, s). Minor rotamer, characteristic signals: δ 3.15 (1H, ddd, J 12.0, 8.0, 4.0), 3.21 (1H, dd, J 9.0, 6.0), 3.35 (1 H, m), 6.51 (2H, s). 13C NMR (CDCl3, 100.6 MHz),
16 mixture of two rotamers. Major rotamer: δ 1.50–1.75 (4H, m), 2.02 (1H, m), 2.83 (1H, ddd, J 13.0, 8.5, 3.5), 2.98 (1H, dd, J 9.5, 5.0), 3.34 (1H, dd, J 8.0, 5.0), 3.74 (2 H, AB system, δA 3.71, δB 3.77, JAB 15.0), 3.83 (3H, s), 3.85 (6H, s), 3.93 (1H, ddd, J 13.0, 6.5, 4.0), 6.51 (2H, s). Minor rotamer, characteristic signals: δ 3.15 (1H, ddd, J 12.0, 8.0, 4.0), 3.21 (1H, dd, J 9.0, 6.0), 3.35 (1 H, m), 6.51 (2H, s). 13C NMR (CDCl3, 100.6 MHz), ![[8 with combining low line]](https://www.rsc.org/images/entities/char_0038_0332.gif)
![[4 with combining low line]](https://www.rsc.org/images/entities/char_0034_0332.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 mixture of two rotamers. Major rotamer: δ 16.2, 16.3, 21.8, 30.5, 37.2, 39.9, 40.9, 56.0, 60.7, 106.3, 130.5, 136.6, 153.0, 172.8. Minor rotamer: δ 14.7, 16.0, 22.6, 29.9, 36.4, 41.3, 43.8, 56.0, 60.7, 105.6, 130.3, 136.5, 153.1, 172.3. MS (EI): m/z 96, 97,
16 mixture of two rotamers. Major rotamer: δ 16.2, 16.3, 21.8, 30.5, 37.2, 39.9, 40.9, 56.0, 60.7, 106.3, 130.5, 136.6, 153.0, 172.8. Minor rotamer: δ 14.7, 16.0, 22.6, 29.9, 36.4, 41.3, 43.8, 56.0, 60.7, 105.6, 130.3, 136.5, 153.1, 172.3. MS (EI): m/z 96, 97, ![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif)
![[8 with combining low line]](https://www.rsc.org/images/entities/char_0038_0332.gif)
![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif) , 182, 208, 244, 246, 304 ([M − Cl]+), 339 (M+˙ with 35Cl), 341 (M+˙ with 37Cl). HRMS (EI): m/z 339.1228 (M+˙ C17H2235ClNO4+˙ requires 339.1232).
, 182, 208, 244, 246, 304 ([M − Cl]+), 339 (M+˙ with 35Cl), 341 (M+˙ with 37Cl). HRMS (EI): m/z 339.1228 (M+˙ C17H2235ClNO4+˙ requires 339.1232).
Amide 15
37% HCl aqueous solution (1 drop) was added, at 20 °C, to a solution of endo-14 (1.00 equiv., 64.7 μmol, 22.0 mg) in DMF (1.0 mL). The mixture was heated at 150 °C for 90 minutes in a microwave reactor (power 250 W). After cooling, H2O (15 mL) was added and the mixture was extracted with EtOAc (3 × 10 mL). The combined organic phases were dried over MgSO4, filtered and concentrated under reduced pressure to afford a sticky orange oil (14.0 mg). Purification by flash column chromatography on silica gel (EtOAc/petroleum ether, gradient from 5 to 50%) gave pure 15 (10.0 mg, 33.0 μmol, 51%).
15. Pale yellow oil. IR (neat) ν 2996 (w), 2937 (w), 2838 (w), 1666 (m, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1634 (m), 1599 (m), 1591 (m), 1508 (m), 1461 (m), 1425 (m), 1332 (m), 1240 (m), 1126 (s), 1007 (w), 715 (w) cm−1. 1H NMR (CDCl3, 400 MHz) δ 2.51 (2H, qd, J 5.0, 1.5), 3.75 (2H, s), 3.77 (2H, t, J 5.0), 3.83 (3H, s), 3.84 (6H, s), 5.30 (1H, dd, J 9.0, 7.5), 5.86 (1H, ddt, J 11.5, 7.5, 1.5), 5.98 (1H, dt, J 11.5, 5.0), 6.45 (2H, s), 6.62 (1H, d, J 9.0). 13C NMR (CDCl3, 100.6 MHz) δ 32.6, 41.7, 42.2, 56.1, 60.8, 105.9, 111.1, 122.7, 129.2, 129.9, 133.6, 136.9, 153.3, 169.1. MS (EI): m/z
O), 1634 (m), 1599 (m), 1591 (m), 1508 (m), 1461 (m), 1425 (m), 1332 (m), 1240 (m), 1126 (s), 1007 (w), 715 (w) cm−1. 1H NMR (CDCl3, 400 MHz) δ 2.51 (2H, qd, J 5.0, 1.5), 3.75 (2H, s), 3.77 (2H, t, J 5.0), 3.83 (3H, s), 3.84 (6H, s), 5.30 (1H, dd, J 9.0, 7.5), 5.86 (1H, ddt, J 11.5, 7.5, 1.5), 5.98 (1H, dt, J 11.5, 5.0), 6.45 (2H, s), 6.62 (1H, d, J 9.0). 13C NMR (CDCl3, 100.6 MHz) δ 32.6, 41.7, 42.2, 56.1, 60.8, 105.9, 111.1, 122.7, 129.2, 129.9, 133.6, 136.9, 153.3, 169.1. MS (EI): m/z ![[9 with combining low line]](https://www.rsc.org/images/entities/char_0039_0332.gif)
![[5 with combining low line]](https://www.rsc.org/images/entities/char_0035_0332.gif) , 97, 109, 111, 121, 123, 125, 135, 147, 193, 208, 221, 303 (M+˙).
, 97, 109, 111, 121, 123, 125, 135, 147, 193, 208, 221, 303 (M+˙).
Acknowledgements
We wish to thank Vincent Jactel and Michel Levart for MS analyses and the following institutions for funding: École Polytechnique for grants attributed to P. K., G. S. and A. B.; the French Embassy in Russia for a 3-month Metchnikov fellowship awarded to O. A. T. and the Agence Nationale de la Recherche (ANR) for the grants of C. C. and R. K. (ANR-12-BS07-0013 “ACTIMAC”). Finally, Y. S. wishes to thank Prof. S. Z. Zard for his continuous encouragement and invaluable support.Notes and references
- (a) Selected papers: L. Larquetoux, J. A. Kowalska and Y. Six, Eur. J. Org. Chem., 2004, 3517–3525 CrossRef CAS; (b) L. Larquetoux, N. Ouhamou, A. Chiaroni and Y. Six, Eur. J. Org. Chem., 2005, 4654–4662 CrossRef CAS; (c) C. Madelaine, O. Buriez, B. Crousse, I. Florent, P. Grellier, P. Retailleau and Y. Six, Org. Biomol. Chem., 2010, 8, 5591–5601 RSC; (d) A. Wasilewska, B. A. Woźniak, G. Doridot, K. Piotrowska, N. Witkowska, P. Retailleau and Y. Six, Chem. – Eur. J., 2013, 19, 11759–11567 CrossRef CAS PubMed.
- The +M effect exercised by this group is relatively small because of the limited π-acceptor ability of the cyclopropane ring.
- (a) Selected reviews: R. R. Kostikov, A. P. Motchanov and H. Hopf, Top. Curr. Chem., 1990, 155, 41–80 CrossRef CAS; (b) M. Fedoryński, Chem. Rev., 2003, 103, 1099–1132 CrossRef PubMed; (c) B. Halton and J. Harvey, Synlett, 2006, 1975–2000 CAS; (d) A. P. Thankachan, K. S. Sindhu, K. K. Krishnan and G. Anilkumar, Org. Biomol. Chem., 2015, 13, 8780–8802 RSC.
- (a) Selected early reports: J. D. Roberts and V. C. Chambers, J. Am. Chem. Soc., 1951, 73, 5034–5040 CrossRef CAS; (b) W. E. Parham and H. E. Reiff, J. Am. Chem. Soc., 1955, 77, 1177–1178 CrossRef CAS; (c) P. S. Skell and S. R. Sandler, J. Am. Chem. Soc., 1958, 80, 2024–2025 CrossRef CAS; (d) Early review: W. E. Parham and E. E. Schweizer, Org. React., 1963, 13, 55–90 CAS.
- (a) Selected articles: C. H. Heathcock, C. M. Tice and T. C. Germroth, J. Am. Chem. Soc., 1982, 104, 6081–6091 CrossRef CAS; (b) R. L. Danheiser, J. M. Morin Jr. and E. J. Salaski, J. Am. Chem. Soc., 1985, 107, 8066–8073 CrossRef CAS; (c) M. G. Banwell, J. E. Harvey, D. C. R. Hockless and A. W. Wu, J. Org. Chem., 2000, 65, 4241–4250 CrossRef CAS PubMed; (d) C. Fu, Y. Zhang, J. Xuan, C. Zhu, B. Wang and H. Ding, Org. Lett., 2014, 16, 3376–3379 CrossRef CAS PubMed; (e) H. Yuan, Z. Guo and T. Luo, Org. Lett., 2017, 29, 624–627 CrossRef PubMed.
- (a) H. P. M. Thiellier, G. J. Koomen and U. K. Pandit, Tetrahedron, 1977, 33, 2603–2607 CrossRef CAS; (b) H. P. M. Thiellier, G. J. Koomen and U. K. Pandit, Tetrahedron, 1977, 33, 2609–2612 CrossRef CAS; (c) H. P. Soetens and U. K. Pandit, Heterocycles, 1978, 11, 75–82 CrossRef CAS; (d) C. D. Perchonock, I. Lantos, J. A. Finkelstein and K. G. Holden, J. Org. Chem., 1980, 45, 1950–1953 CrossRef CAS; (e) H. P. Soetens and U. K. Pandit, Recl.: J. R. Neth. Chem. Soc., 1980, 99, 271–274 CAS; (f) G. Manikumar and M. Shamma, J. Org. Chem., 1981, 46, 386–389 CrossRef CAS; (g) J. L. Castro, L. Castedo and R. Riguera, J. Org. Chem., 1987, 52, 3579–3584 CrossRef CAS; (h) D. Dhanak, R. Kuroda and C. B. Reese, Tetrahedron Lett., 1987, 28, 1827–1830 CrossRef CAS; (i) J. Xu, E.-A. Ahmed, B. Xiao, Q.-Q. Lu, Y.-L. Wang, C.-G. Yu and Y. Fu, Angew. Chem., 2015, 127, 8349–8353 ( Angew. Chem. Int. Ed. , 2015 , 54 , 8231–8235 ) CrossRef.
- (a) M. Ohno, Tetrahedron Lett., 1963, 1753–1755 CrossRef CAS; (b) J. L. Castro, L. Castedo and R. Riguera, Tetrahedron Lett., 1985, 26, 1561–1564 CrossRef CAS; (c) V. Kubyshkin, Y. Kheylik and P. K. Mykhailiuk, J. Fluorine Chem., 2015, 175, 73–83 CrossRef CAS.
- (a) Selected articles: N. Langlois, F. Guéritte, Y. Langlois and P. Potier, J. Am. Chem. Soc., 1976, 98, 7017–7024 CrossRef CAS PubMed; (b) M. Ishikura, K. Yamada and T. Abe, Nat. Prod. Rep., 2010, 27, 1630–1680 RSC; (c) M. Ishikura, T. Abe, T. Choshi and S. Hibino, Nat. Prod. Rep., 2013, 30, 694–752 RSC; (d) M. Ishikura, T. Abe, T. Choshi and S. Hibino, Nat. Prod. Rep., 2015, 32, 1389–1471 RSC.
- Recent review: H. Liu and Y. Jia, Nat. Prod. Rep., 2017, 34, 411–432 RSC.
- H. Wagner and J. Burghart, Helv. Chim. Acta, 1981, 64, 283–296 CrossRef CAS.
- J.-P. Hu, D.-H. Yang, W.-H. Lin and S.-Q. Cai, Helv. Chim. Acta, 2009, 92, 2125–2133 CrossRef CAS.
- W.-W. Jiang, F. Liu, X. Gao, J. He, X. Cheng, L.-Y. Peng, X.-D. Wu and Q.-S. Zhao, Phitoterapia, 2014, 99, 72–77 CAS.
- W. B. Han, A. H. Zhang, X. Z. Deng, X. Lei and R. X. Tan, Org. Lett., 2016, 18, 1816–1819 CrossRef CAS PubMed.
- H.-B. Wang, C.-H. Tan, J.-J. Tan, M.-Y. Gao, Y.-M. Li, S.-H. Jiang and D.-Y. Zhu, Helv. Chim. Acta, 2007, 90, 153–157 CrossRef CAS.
- R. K. Dieter and R. R. Sharma, J. Org. Chem., 1996, 61, 4180–4184 CrossRef CAS PubMed.
- (a) M. Mąkosza and M. Wawrzyniewicz, Tetrahedron Lett., 1969, 4659–4662 CrossRef; (b) M. Mąkosza and M. Fedoryński, Russ. Chem. Bull., 2011, 60, 2141–2146 CrossRef.
- B. Schurgers, B. Brigou, Z. Urbanczyk-Lipkowska, D. Tourwé, S. Ballet, F. De Proft, G. Van Lommen and G. Verniest, Org. Lett., 2014, 16, 3712–3715 CrossRef CAS PubMed.
- (a) For other examples of such selectivity in the cyclopropane-ring cleavage, see ref. 6a,b and: V. E. Marquez, K. V. B. Rao, J. V. Silverton and J. A. Kelley, J. Org. Chem., 1984, 49, 912–919 CrossRef CAS; (b) I. Lantos and H. E. Katerinopoulos, Can. J. Chem., 1991, 69, 1033–1037 CrossRef CAS; (c) A. Padwa, P. Rashatasakhon, A. D. Ozdemir and J. Willis, J. Org. Chem., 2005, 70, 519–528 CrossRef CAS PubMed.
- (a) Examples with other bicyclic halocyclopropanes: C. H. De Puy, L. G. Schnack, J. W. Hawser and W. Wiedemann, J. Am. Chem. Soc., 1965, 87, 4006–4006 CrossRef CAS; (b) S. J. Cristol, R. M. Sequeira and C. H. DePuy, J. Am. Chem. Soc., 1965, 87, 4007–4008 CrossRef CAS; (c) T. Ando, H. Hosaka, H. Yamanaka and W. Funasaka, Bull. Chem. Soc. Jpn., 1969, 42, 2013–2021 CrossRef CAS; (d) U. K. Pandit and S. A. G. de Graaf, J. Chem. Soc., Chem. Commun., 1972, 659–660 RSC . See also ref. 6a and b.
- (a) Mechanistic considerations: R. B. Woodward and R. Hoffmann, J. Am. Chem. Soc., 1965, 87, 395–397 CrossRef CAS; (b) R. Hoffmann and R. B. Woodward, Acc. Chem. Res., 1968, 1, 17–22 CrossRef CAS; (c) C. H. DePuy, Acc. Chem. Res., 1968, 1, 33–41 CrossRef CAS; (d) R. B. Woodward and R. Hoffmann, Angew. Chem., 1969, 81, 797–869 ( Angew. Chem. Int. Ed. , 1969 , 8 , 781–853 ) CrossRef; (e) O. N. Faza, C. S. López, R. Álvarez and Á. R. de Lera, J. Org. Chem., 2004, 69, 9002–9010 CrossRef PubMed.
- W. G. Kofron and L. M. Baclawski, J. Org. Chem., 1976, 41, 1879–1880 CrossRef CAS.
- Adapted from: I. Lantos, D. Bhattacharjee and D. S. Eggleston, J. Org. Chem., 1986, 51, 4147–4150 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ob01238a |
| ‡ See the ESI† for details. |
| § The quality of the hydride reagent proved important. See the ESI† for a short study in the case of the reaction of benzaldehyde with 3b. |
| ¶ Using K2CO3 as the base, no reaction was observed after several hours, most certainly because of the very low solubility of this reagent in chloroform. With NaOH, complex mixtures of products were generated after 5 minutes. |
| || Without TMEDA, yields are significantly lower. Using nBuLi/TMEDA instead of sBuLi/TMEDA, no Cl–Li exchange is observed. |
| ** The production of 15 can be explained by the loss of a proton from the iminium intermediate 13, which we were hoping would cyclise onto the electron-rich aromatic ring. |
| This journal is © The Royal Society of Chemistry 2017 |