Open Access Article
Open Access ArticleCalcium looping CO2 capture system for back-up power plants
Y. A.
Criado
 *,
B.
Arias
*,
B.
Arias
 and
J. C.
Abanades
and
J. C.
Abanades

Spanish National Research Council (CSIC-INCAR), C/ Francisco Pintado Fe 26, 33011, Oviedo, Spain. E-mail: yolanda.ac@incar.csic.es
First published on 25th August 2017
Abstract
This paper analyses a CO2 capture system based on calcium looping, designed for power plants that operate with very low capacity factors and large load fluctuations, including shut-down and start-up periods. This can be achieved by decoupling the operation of the carbonator and calciner reactors and connecting them to piles filled with CaO or CaCO3. When the power plant enters into operation, calcined solids are fed into the carbonator to provide the necessary flow of CaO for capturing CO2 and storing the carbonated solids. An oxy-CFB calciner designed to have a modest thermal capacity and operate continuously refills the CaO pile. Mass and energy balances of the entire system, combined with state-of-the-art performance criteria for reactor design, have been solved to identify suitable operating windows. An analysis of the effect of the CaO reactivity of the material stored in the piles indicates that temperatures of around 500–600 °C in the carbonator are compatible with the storage of solids at low temperature (<250 °C). This, together with the low inherent cost of the material, allows large piles of stored material. Electricity costs between 0.13–0.15 $ per kWhe are possible for the system proposed in contrast to standard CaL systems where the cost would increase to above 0.19 $ per kWhe when forced to operate at low capacity. The proposed concept could be integrated into existing power plants operating as back-ups in renewable energy systems in preference to other CO2 capture technologies that are heavily penalized when forced to operate under low capacity factors.
Broader contextThe progressive adoption of low-carbon technologies is essential for tackling climate change and achieving the 1.5 °C desirable target as agreed by the COP21 in 2015. In this context, renewable energies and CO2 capture and storage technologies will play an essential role in the power generation sector. Most of the efforts on developing CO2 capture processes assume a base load operation. However, renewable energies are inherently intermittent and CO2 capture systems must be able to treat flue gases from power plants operated as back-up to cover periods of time with no renewable energy production. Thus, the development of flexible CO2 capture systems is increasingly recognized as a key point for the positioning of these technologies. While standard CO2 capture systems developed to date are very limited for their integration in back-up power plants, this work presents a highly flexible CO2 capture system based on calcium looping. This includes low cost solid storage piles within its boundaries allowing the capture of all the CO2 from the existing coal power plants operated under very low capacity factors while maintaining reasonable costs. |
Introduction
The power sector in many countries is rapidly evolving as low-carbon technologies are increasingly deployed to reduce greenhouse emissions.1,2 However, the large share of solar and wind power requires the implementation of energy storage systems3 and/or back-up fossil power generation in order to fill the time periods when no renewable energy is available. The current need for flexible fossil power has already produced a major shift in the operating configurations of many fossil-fired power plants:4 both existing and new fossil fuel power plants are forced to operate with significant load variations, ramping-up-and-down or even having shut-down periods to satisfy the electricity demand. As a result, very low capacity factors are to be expected (leading to significant extra costs) as well as many other technical penalties (e.g. increased wear and tear due to cycling operation).4–7On the other hand, the use of fossil fuels as a back-up power generation system, even for limited periods of time, is incompatible with the possible long-term scenario of a deeply decarbonized energy supply. Consequently, flexible power plants equipped with CO2 capture and storage (CCS) technology are needed to substitute the current fossil infrastructure of back-up power generation. The previously mentioned challenges are exacerbated due to the great complexity of CCS systems and the capital-intensive character of sub-systems originally designed only for base load operation.8–10 This is now recognized as a major weakness of CCS technologies and explains the growing awareness of the need for more flexible CCS processes.8,11–15
In the case of the most developed CO2 capture technologies (i.e. post-combustion, oxy-combustion and pre-combustion), several process alternatives based on different energy and material storage options have been proposed to improve their flexibility. For example, in post-combustion amine-based CO2 capture systems it is possible to reduce the power consumption associated with solvent regeneration and CO2 compression units during peak demand periods.12,16 This can be done by storing a fraction of the rich solvent leaving the absorber and operating the regenerator at lower loads during these periods. As a result, the regeneration of the stored sorbent and the compression of the CO2 captured can be postponed to low power demand periods.17 However, this solution implies economic penalties due to the storage of large masses of relatively costly amine, and the need for an oversized regenerator and CO2 compression unit if the power plant is required to operate continuously at base load.11
For oxy-combustion systems, the use of O2 cryogenic tanks has been proposed in order to operate the air separation unit (ASU) at base load and to store the O2 during low-energy-demand periods (i.e. when the boiler operates at partial load) or by reducing the ASU capacity while the plant load is increased to respond to varying electricity requirements.11,13,18,19 One of the main drawbacks of these approaches is the noticeable increase in O2 production costs as a consequence of the need to liquefy and re-evaporate the stored O2.
The storage of the H2 produced in the pre-combustion CO2 capture systems has also been proposed8,11 using suitable geological structures due to their relatively low cost and large storage capacity.20,21 The use of syngas in other industries (leading to poly-generation systems) has also been presented as an alternative to the storage of H2.22–24 Both the alternatives increase flexibility through the decoupling of the power generation and hydrogen production blocks.
Other processes have been proposed to adapt the variable power output irrespective of the power input by incorporating a thermal energy storage system within the power plant and/or the CO2 capture system boundaries. This concept has already been considered in previous works25,26 for example by using molten salts as a storage medium to meet intermediate loads in coal-fired power generation. In a similar way, thermal energy storage using solids at high temperature has recently been proposed in order to increase the flexibility of power plants based on oxy-fired circulating fluidized bed (CFB) combustors with CO2 capture taking advantage of the availability of these reactors for the circulation of large flows of high temperature solids between silos.27,28
Another CCS technology that can benefit from the energy storage potential of reacting solids is post-combustion CO2 capture by calcium looping (CaL). A conventional CaL configuration uses two interconnected circulating fluidized bed reactors, a carbonator and a calciner. The flue gas from a power plant is fed to the carbonator in order to capture the CO2 using a stream of CaO particles at temperatures close to 650 °C. The carbonated stream of solids is then separated from the lean-CO2 flue gas leaving the carbonator and sent to the calciner. In this reactor, the CaCO3 is decomposed at temperatures of around 900–920 °C by the combustion of coal under oxy-firing conditions and the regenerated CaO is sent back to the carbonator. Thus, the CO2 captured in the carbonator and the CO2 released during combustion in the calciner are obtained in a concentrated stream. Post-combustion CO2 capture by CaL has developed rapidly in the last five years up to the scale of the MWth with several pilot plants29–35 due to the similarity between its reactors and those of existing circulating fluidized bed power plants and the lower energy penalty compared to that of other CCS systems since extra power can be obtained from the additional heat input required to drive the sorbent regeneration reaction.36,37
The use of CaO/CaCO3 in CaL systems makes it possible to integrate a thermochemical energy storage system by taking advantage of the reversible reaction of CaO with CO2 at high temperature. There is already background knowledge about the use of CaO/CaCO3 to store nuclear38,39 or solar energy40–42 or to increase the flexibility of the integrated gasification combined cycle plants.43 Also, basic schemes of energy storage systems based on CaO/CaCO3 post-combustion CaL have been put forward.44,45 In relation to these concepts of energy storage, a recent European project, FlexiCaL (http://www.flexical.eu), is being carried out to investigate in more detail the viability of flexible CaL systems.
The aim of the present work is to analyze and evaluate the operation variables of a CaL system that incorporates a large-scale energy storage system and exploits the thermo-chemical energy of CaO/CaCO3. Specific reference process schemes for CO2 capture in power plants operating in back-up mode are assessed. The main elements of the resulting CaL CO2 capture system (reactors, solids piles, steam cycle, elements linked to the capture system and auxiliary components for O2 production and CO2 compression and purification) can then be dimensioned and a preliminary estimation of the electricity and CO2 avoidance costs can be calculated to identify the scenarios where the proposed system would be most competitive.
Process description
The flexible post-combustion CO2 capture concept by calcium looping (FlexiCaL), proposed in this work is presented in Fig. 1. It is based on a conventional CaL system46 that connects a CFB carbonator to an existing power plant and includes an oxy-CFB calciner fired with coal using pure oxygen from an air separation unit (ASU). In addition, the proposed system in Fig. 1 includes features designed to allow the integration of the energy storage system: in particular, two solid storage piles to store the solids coming from the carbonator and the calciner (named for the sake of simplicity “CaCO3” and “CaO” respectively in Fig. 1, although the piles include other components as will be discussed later). These allow the circulation of solids between the carbonator and the calciner to be decoupled and the oxy-fired calciner to operate continuously irrespective of the CO2 load to the carbonator.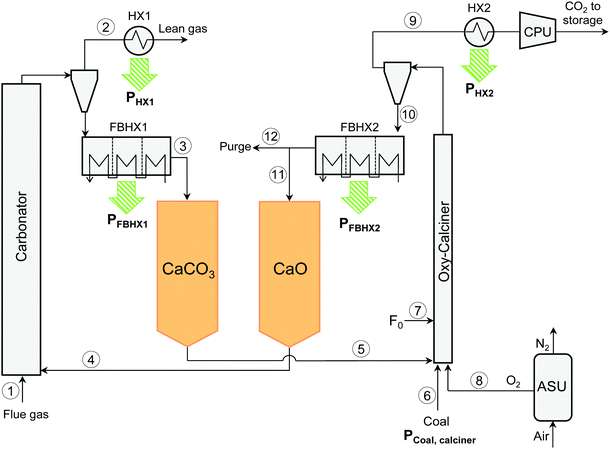 | ||
| Fig. 1 Scheme of the FlexiCaL system including CaO/CaCO3 storage as proposed in this work. Main process streams numbered as 1–12. | ||
At the exit of both the CFBs, the sensible heat from the flue gas depleted in CO2 and from the CO2-rich gas is recovered using convection pass heat exchangers, labelled HX1 and HX2. After the heat has been recovered, the CO2 gas stream leaving the calciner is delivered to the CO2 compression and purification unit (CPU) before transport and storage. The system also includes two series of external fluidized bed heat exchangers (FBHX1 and FBHX2) to cool down the solids coming from the carbonator and the calciner before they are stored at low temperature (TCaCO3 and TCaO, respectively). These FBHXs could be based on a series of fluidized beds in countercurrent flow to the water-steam flows, as suggested by Schwaiger et al.47
In the system depicted in Fig. 1, when the power plant enters into operation and the flue gas is fed into the carbonator, calcined solids are fed from the CaO pile into the carbonator to provide the necessary amount of sorbent during these periods. The carbonated solids leaving this reactor are then cooled down and stored in the CaCO3 pile. This allows the capture of the CO2 and the production of additional power from the HX1 and FBHX1 when the power plant enters into operation during high power demand periods. To fill the CaO pile with calcined solids, the oxy-CFB calciner operates at base load independently of the power plant. Thus, the solids from the CaCO3 pile are fed continuously to the calciner together with a make-up flow of limestone (see F0 in Fig. 1) needed to compensate for the decay of the CO2 carrying capacity of the particles of CaO during cycling and to purge the inerts (ashes and CaSO4) from the inventory of solids. The calcined solids leaving this reactor are stored in the CaO pile after being cooled down. As a result, power is continuously provided by both HX2 and FBHX2. The operation of the oxy-CFB calciner (including the ASU and CPU units) in base-load mode, i.e. disconnected from the main power plant and the carbonator, has inherent advantages in that it is simpler to control and more stable. In addition, the calciner footprint and the associated combustion equipment, which are the main cost components in CaL systems, can be considerably reduced in scale by taking advantage of large and low-cost storage piles of Ca-material.
With respect to the storage conditions of the solid materials, this work mainly focused on operating at low temperatures (<300 °C) and under atmospheric pressure to facilitate very-long duration energy storage. Conventional solid handling and storage equipment, such as that used in the cement industry, is employed to store and handle large masses of this type of material. The main drawback of operating with solid piles at low temperatures is that the storage system will have lower energy storage densities as it can only exploit chemical energy (using the enthalpy of the CaO carbonation reaction, i.e. 168 kJ mol−1 CO2). A storage temperature for the carbonated solids (TCaCO3) of 150 °C has been fixed and a CaO pile temperature (TCaO) objective of 200–250 °C has been targeted. Furthermore, due to the low temperature of the calcined solids entering the carbonator, the latter is assumed to be an adiabatic reactor (in contrast with the boiler-type carbonators usually assumed for standard CaL systems).
An analysis of the main variables affecting the design of the scheme in Fig. 1 has been carried out by solving mass and energy balances, using in-house CaL process models previously validated against published works that simulate CaL processes using commercial software. This analysis is focused only on steady-state modes. The main variables affecting the process shown in Fig. 1 are the capacity factor (CF), directly related to the fraction of time that the power plant and the CFB carbonator are in operation, the activity of the CaO material (Xave) and the solid storage temperature in the piles. Several reasonable assumptions following the typical values reported in the literature for similar systems have been made as described later. Although these assumptions could introduce uncertainties in the results, a more detailed sensitivity study of other parameters has been considered outside the scope of this first conceptual design.
An average-sized coal-fired power plant with a thermal input (Pcoal,power![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) plant) of 500 MWth has been chosen as the basis for calculations. This produces a total flue gas of 6.8 kmol s−1 with 15.9%v CO2 when operating at full power. For simplification purposes, it has been considered that the flue gas enters the carbonator free of ashes and sulfur after having been cooled down to a temperature of 150 °C. The composition of the coal fed into the oxy-CFB calciner is 75.5% C, 3.0% H, 0.5% S, 8.0% O, 7.0% H2O, 1.0% N and 5.0% ash with a lower heating value (LHV) of 25.0 MJ kg−1. This is burnt in the calciner at 900 °C using pure O2 preheated up to 350 °C with an excess of 6%. Under these conditions, the total calcination of the CaCO3 and a SO2 capture efficiency of 95% in the oxy-CFB calciner can be assumed. It has been considered that 50% of the ashes leave the system through the calciner cyclone with the flue gas while the remaining ashes accumulate with the solids and need to be removed in the solid purge. With respect to the carbonator, the mass and energy balances have been solved assuming a 90% CO2 capture efficiency.
plant) of 500 MWth has been chosen as the basis for calculations. This produces a total flue gas of 6.8 kmol s−1 with 15.9%v CO2 when operating at full power. For simplification purposes, it has been considered that the flue gas enters the carbonator free of ashes and sulfur after having been cooled down to a temperature of 150 °C. The composition of the coal fed into the oxy-CFB calciner is 75.5% C, 3.0% H, 0.5% S, 8.0% O, 7.0% H2O, 1.0% N and 5.0% ash with a lower heating value (LHV) of 25.0 MJ kg−1. This is burnt in the calciner at 900 °C using pure O2 preheated up to 350 °C with an excess of 6%. Under these conditions, the total calcination of the CaCO3 and a SO2 capture efficiency of 95% in the oxy-CFB calciner can be assumed. It has been considered that 50% of the ashes leave the system through the calciner cyclone with the flue gas while the remaining ashes accumulate with the solids and need to be removed in the solid purge. With respect to the carbonator, the mass and energy balances have been solved assuming a 90% CO2 capture efficiency.
The flow of solids needed from the CaO pile is linked to the maximum average CO2 carrying capacity of the particles (Xave), which is directly related to the make-up flow of limestone introduced to the calciner. To estimate the Xave of the inventory of solids the expression proposed by Rodríguez et al.48 has been used.
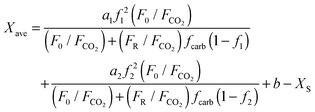 | (1) |
| XN = a1f1N+1 + a2f2N+1 + b | (2) |
Values of a1 = 0.1045, f1 = 0.9822, a2 = 0.7786, f2 = 0.7905 and b = 0.07709 were used in this work as representative fitting parameters of typical high purity lime materials. As mentioned above, it is assumed that CO2 is fed into the carbonator when the power plant enters into operation, while the calciner operates continuously in order to calcine the stored carbonated solids and the required make-up flow of limestone. On the basis of this assumption, F0/FCO2 used in eqn (1) has been calculated as the molar ratio of the limestone fed into the calciner and the CO2 fed into the carbonator during the lifetime of the power plant.
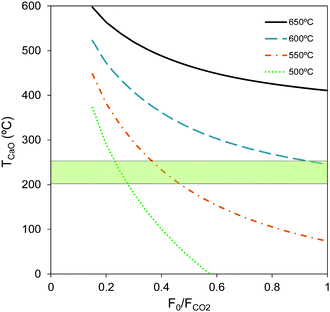
As the carbonator is adiabatic, the reactor operation temperature and TCaO are directly linked by the activity of the solids. Thus, in order to find feasible operation windows, the effect of sorbent activity (i.e. different F0/FCO2 ratios) on the CaO storage temperature was analyzed. A minimum operation temperature in the carbonator of 500 °C was chosen on the basis of previous experimental studies that indicate that temperature has only a modest effect on the carbonation reaction rates in the range of 500–650 °C due to the low activation energy.50–54 From the heat recovery point of view, lower than usual (650 °C) temperatures in the carbonator (i.e. 500–600 °C) are possible at the expense of modest efficiencies and larger heat transfer areas in HX1 and FBHX1.
Fig. 2 shows the effect of the F0/FCO2 ratio on the temperature of the storage pile of CaO (TCaO). Mass and energy balances have been solved for four different carbonator temperatures (500/550/600/650 °C). As can be appreciated in this figure, a carbonator operation temperature of 650 °C is only possible for CaO storage temperatures well above 400 °C. This high TCaO is compatible for process schemes with lower storage capacity requirements if refractory silos are used (i.e. for the capture of CO2 from power plants operating continuously but with load changes44,45), but it is considered less feasible for the storage and handling of very large masses of solids.
On the other hand, it is possible to store the CaO at temperatures around 250 °C and operate the carbonator at 600 °C if large ratios of F0/FCO2 (>0.9) are allowed. This option is possible despite the large F0/FCO2 ratios shown in Fig. 2 since the total consumption of fresh limestone and purge produced is reasonable and in the range of that required in cement plants, and furthermore when taking into account the moderate FCO2 fed into the carbonator if the power plant operates with a low capacity factor. However, such large F0/FCO2 scenarios need to be analyzed by making better use of the potential synergy between the FlexiCaL system and a cement plant (or any other large scale CaO user) which is beyond the scope of this study. If synergy with a cement plant is not feasible, lower F0/FCO2 ratios are needed (0.25–0.35) to reduce the consumption of limestone in the CO2 capture system. In this case, the carbonator must operate at temperatures below 550 °C to ensure the CaO storage temperatures stay within the targeted range (see Fig. 2).
Another important parameter affecting the process depicted in Fig. 1 is the capacity factor of the existing power plant. Fig. 3 shows the fraction of thermal input to the calciner with respect to the total thermal input for different capacity factors as a function of the F0/FCO2 ratio. In standard CaL systems, approximately 45–50% of the thermal input into the entire system (power plant and CaL) is introduced in the oxy-fired CFB calciner.37 As can be seen in Fig. 3, the thermal input to the calciner can be drastically reduced by integrating the energy storage system. Thus, the fraction of the thermal input to the calciner could be lower than 0.20–0.25 if the existing power plant is operated with a capacity factor of 0.2. Other improved configurations for further minimizing the energy demand by preheating the carbonated solids entering the calciner could be implemented to further reduce the thermal input to the calciner.44
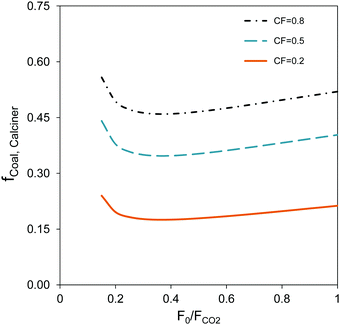 | ||
| Fig. 3 Fraction of thermal input to the oxy-CFB calciner (fcoal,calciner) as a function of the F0/FCO2 molar ratio for different capacity factors (CF) and a carbonator temperature of 600 °C. | ||
In order to illustrate the operation of the conceptual design of the system proposed in this work, a capacity factor of 0.2 for the existing power plant has been chosen. This low capacity factor would be extremely challenging for any CO2 capture system, as the capital cost component of electricity is inversely proportional to the capacity factor. However, it can be considered a reasonable mid-term assumption for fossil plants without capture, considering the trend observed in the operation hours of existing coal power plants connected to electrical markets with a large share of renewable energy (e.g. in the Spanish electricity market, where there has been a significant increase in the share of renewable energy during the last decade from 20% of the total electricity produced in 2007 to 40% in 2016 and where transnational network connections are limited, the fraction of operation time of fossil fuel power plants has dropped from 0.85 in 2007 to 0.53 in 2016, see http://www.ree.es).
A F0/FCO2 of 0.95 has been adopted on the assumption that the purge can be used in a cement plant. This results in a maximum CO2 carrying capacity of the solids of 0.52 (configuration FlexiCaL/Xave = 0.52). In accordance with the discussion above, a carbonator temperature of 600 °C could be achieved by allowing a CaO solid storage temperature of 250 °C. The main mass flow streams, temperatures and power available from the different heat exchangers are summarized in Table 1.
| Stream | FlexiCaL/Xave = 0.52 | FlexiCaL/Xave = 0.26 | ||
|---|---|---|---|---|
| Mass flow (kg s−1) | Temperature (°C) | Mass flow (kg s−1) | Temperature (°C) | |
| a Refers to the volume of solids stored during one day with the power plant operating at full load and assuming a solid bulk density of 1000 kg m−3. b Available heat after the preheating of the stream (8). | ||||
| 1 | 204.5 | 150 | 204.5 | 150 |
| 2 | 161.6 | 600 | 161.6 | 500 |
| 3 | 176.6 | 600 | 320.0 | 500 |
| 4 | 133.7 | 250 | 277.1 | 230 |
| 5 | 35.3 | 150 | 64.0 | 150 |
| 6 | 5.3 | 20 | 4.5 | 20 |
| 7 | 20.6 | 20 | 5.4 | 20 |
| 8 | 12.2 | 350 | 10.3 | 350 |
| 9 | 34.8 | 900 | 25.5 | 900 |
| 10 | 38.5 | 900 | 58.6 | 900 |
| 11 | 26.8 | 250 | 55.4 | 230 |
| 12 | 11.7 | 250 | 3.2 | 230 |
| Parameter | ||
|---|---|---|
P
coal,power![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) plant (MWth) plant (MWth) |
500 | 500 |
| P coal,calciner(MWth) | 132 | 112 |
| T CaCO3 (°C) | 150 | 150 |
| T CaO (°C) | 250 | 230 |
| F 0/FCO2 | 0.95 | 0.25 |
| X ave (eqn (1)) | 0.52 | 0.26 |
| CaO storeda (m3 per day full load) | 9243 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 155 155 |
| CaCO3 storeda (m3 per day full load) | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 209 209 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 121 121 |
| F 0,mass (ton per year) | 649![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 116 116 |
170![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 820 820 |
| Purge (ton per year) | 369![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 658 658 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 854 854 |
| P carbonator (MWth) | 194.2 | 201.5 |
| P HX1 (MWth) | 107.6 | 87.7 |
| P FBHX1 (MWth) | 86.6 | 113.8 |
| P calciner (MWth) | 60.9 | 65.4 |
| P HX2 (MWth)b | 36.5 | 26.4 |
| P FBHX2 (MWth) | 24.4 | 39.0 |
When the power plant is in operation, the flow of calcined solids is fed into the carbonator at 133.7 kg s−1 while the flow of carbonated solids sent to the CaCO3 pile is 176.6 kg s−1. Assuming a bulk density for the CaO solids of 1000 kg m−3, a total of 9243 m3 of calcined solids are needed in the CaO pile per day of power plant operation. In the case of the operation conditions in the calciner, a thermal input of 132 MWth is supplied continuously to calcine a 20.6 kg s−1 flow of fresh limestone and a 35.3 kg s−1 flow of carbonated solids coming from the CaCO3 pile. In order to ensure that the inventory of solids in the storage system is kept constant, a 26.8 kg s−1 flow of calcined solids is sent to the CaO pile. As a result, a 11.7 kg s−1 purge of rich CaO solids is produced continuously. This translates into an annual production of around 0.37 Mton which represents approximately half of the CaO requirements of a typical cement plant with a clinker capacity of 1 Mtonclinker per year assuming 0.63 kgCaO kg−1cement.
Table 1 also includes another example of a conceptual design (FlexiCaL/Xave = 0.26) where synergy with a cement plant is not possible. In this case, a F0/FCO2 of 0.25 has been fixed to reduce limestone consumption to 5.4 kg s−1. The average CO2 carrying capacity of the solids under these conditions is 0.26. As a result, the carbonator can be operated at a lower temperature (500 °C) to allow the storage of the CaO solids at 230 °C. Due to the lower activity of the solids, the flow of solids required to be fed into the carbonator increases up to 277.1 kg s−1 while the storage capacity needed per day of operation increases up to 19155 m3. In this case, the calciner's capacity decreases slightly and a thermal input of 112 MWth is needed.
A detailed integration of the heat sources of the system depicted in Fig. 1 into a steam cycle is considered to be outside the scope of this work. However, since HX1 and FBHX1 only enter into operation with the power plant, the use of two different steam cycles will be required (e.g. one associated with the carbonator and one linked to the calciner). The steam cycle associated with the calciner must be able to satisfy the power requirements of the calciner (including the ASU, CPU and auxiliaries). In order to estimate the power needed, average specific consumptions of 200 kWhe per tO2 and 120 kWhe per tCO2 for the ASU and CPU respectively have been adopted on the basis of the values reported in the literature.55 It is assumed that 5% of the gross electric power output is consumed in the auxiliaries.56 Assuming a reasonable efficiency value of 45% for the steam cycle associated with the oxy-calciner, the power produced would be 27.4 and 29.4 MWe for the FlexiCaL configurations with values of Xave = 0.52 and Xave = 0.26 respectively. Thus, after accounting for the ASU, CPU and auxiliaries consumptions, the power available for export from the system proposed could be drastically reduced to 2 and 9 MWe during the power plant shut down periods (i.e. when no electricity is required). On the other hand, additional power of up to 194 and 202 MWth is available from HX1 and FBHX1 (Pcarbonator) for Xave = 0.52 and Xave = 0.26 respectively. By assuming a net efficiency of 0.39 for the operation conditions of the heat sources in the CFB carbonator (500–600 °C) as in similar steam cycles integration schemes,57,58 74.1 and 84.2 MWe of extra power can be produced when the power plant enters into operation.
Costs analysis
A basic economic study has been carried out to estimate the impact of retrofitting the CO2 capture system of Fig. 1 into an existing power plant. The economic parameters used in this analysis are the cost of the electricity (COE) and the CO2 avoidance costs (AC). The COE can be calculated as follows: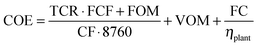 | (3) |
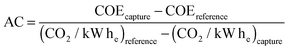 | (4) |
| Parameter | Units | New coal power plant | Amortized coal power plant | Standard CaLa |
|---|---|---|---|---|
| a For a make-up flow consumption of (F0/FCO2 = 0.1) and a thermal input into the calciner of 410 MWth36 assuming that it retrofits an amortized coal power plant. b Including a make-up limestone cost of 10 $ per ton. | ||||
| TCR | $ per kWth | 855 | 0 | 810 |
| ($ per kWe) | (1900) | (0) | (2455) | |
| η plant | kWe kWth−1 | 0.45 | 0.35 | 0.33 |
| FOM | $ per kWe | 40 | 40 | 40 |
| FCF | year | 0.1 | 0.1 | 0.1 |
| VOM | $ per kWhe | 0.005 | 0.005 | 0.007b |
| Fuel cost | $ per GJ | 2 | 2 | 2 |
| CO2 emission factor | kgCO2 MW he−1 | — | — | 67 |
| COE(CF=0.9) | $ per kWhe | 0.050 | 0.031 | 0.065 |
| COE(CF=0.2) | 0.152 | 0.048 | 0.192 | |
| AC(CF=0.9) | $ per tCO2 | — | — | 56 |
| AC(CF=0.2) | 251 | |||
The calculated costs of the electricity for the new and the amortized coal power plant operating at base load (CF = 0.9) are 0.050 and 0.031 $ per kWhe respectively which is in agreement with the data reported in the literature.59 However, if the new power plant is operating as a back-up system with a low capacity factor (CF = 0.2), the COE increases up to the uneconomic value of 0.152 $ per kWhe. Using large coal power plants as back-ups seems a more reasonable strategy for amortized systems, as the cost of the electricity is mainly dependent on the operation and fuel costs. Thus, as shown in Table 2, the COE of an amortized power plant operating with a capacity factor of 0.2 could be as low as 0.048 $ per kWhe. Accordingly, the analysis that follows will be focused only on the integration of the CO2 capture system of Fig. 1 into an existing power plant whose initial capital cost expenditure has been fully amortized. For comparison purposes, a conventional CaL system has been also included in this analysis assuming that it retrofits an amortized coal power plant (Standard CaL in Table 2).
In order to calculate the capital cost of the equipment, the specific cost of the equipment is expressed in terms of unit of thermal input as explained in similar works for other CaL systems.36 In the case of the FlexiCaL system in Fig. 1, we have attempted to estimate the specific costs by exploiting similarities with the elements already developed at the commercial level. Four major components can be identified for the cost analysis: the power plant, the CFB carbonator, the oxy-CFB calciner, and the storage system. In order to facilitate discussion, the specific costs of the equipment, TCRTotal, have been defined as per unit of thermal power. Thus, the total cost of the whole system (TCR) can be estimated as
TCR = TCRtotalPtotal = TCRtotal(Pcoal,power![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) plant + Pcarbonator + Pcoal,calciner) plant + Pcarbonator + Pcoal,calciner) | (5) |
Regarding the cost of the existing power plant, we considered that no additional modifications are needed to satisfy the requirements of the CO2 capture step. Therefore, the specific cost of the power plant in Fig. 1 can be assumed to be zero. The CFB carbonator is linked to the thermal input to the power plant. This component can be considered as an adiabatic combustor whose TCRrefractory is assumed to be 125 $ per kWth based on the cost of pre-calciners in cement plants.60 Another element that is related to the thermal input of the power plant is the fraction of CPU that treats the flue gas captured in the carbonator. A specific cost of 80 $ per kWth has been assumed for the CPU unit61 considering that the CO2 captured as CaCO3 from the power plant is then released from the calciner operating at base load.
The process of Fig. 1 also includes the equipment needed to extract the thermal power associated with HX1 and FBHX1 (Pcarbonator) and to generate power. A TCRPcarbonator of 450 $ per kWth has been adopted assuming that these elements represent around 50% of the TCR of a conventional power plant.59 In the case of the energy storage system, only the cost associated with the inventory of solids in the solids piles has been included in the specific capital cost of the solid storage system, TCRstorage, as the handling and transport of solids between the solid piles has already been included in the cost of the carbonator and calciner circulating fluidized reactor. TCRstorage has been calculated assuming 10 $ per ton of limestone and taking into account the fact that the CO2 carrying capacity of the solids and the enthalpy of the carbonation reaction (467 kWhth per ton of active sorbent) will result in 0.05–0.10 $ per kWhth depending on the value of Xave in eqn (1).
Finally, the oxy-CFB calciner in Fig. 1 resembles an oxy-CFB power plant. According to the data available in the literature,61 a total capital requirement for an oxy-CFB power plant of 3700 $ per kWe is assumed, which results in 1296 $ per kWth by assuming a thermal efficiency of 0.35. Approximately 60% of this cost corresponds to the combustion equipment (i.e. coal feeding system, ash removal systems, flue gas cleaning, ASU and CPU units) while the rest can be associated with the power block (including the heat exchangers HX2 and FBHX2 and the corresponding entire steam cycle). The thermal power transferred to the power block in the oxy-CFB calciner (Pcalciner) in Fig. 1 represents only around 50% of the thermal input fed into the calciner (Pcoal,calciner) when compared with conventional oxy-fired CFB power plants. Therefore, the cost of the TCRcalciner is assumed to be 1037 $ per kWth.
Once the cost associated with the different components has been defined, the total capital requirement of the system of Fig. 1 can be calculated as follows:
TCRtotal = (TCRpower![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) plant + TCRCPU·CF + TCRrefractory)·fpower plant + TCRCPU·CF + TCRrefractory)·fpower![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) plant + (TCRPcarbonator + 2·TCRstorage·tmax)·fcarbonator + TCRcalciner·fcalciner plant + (TCRPcarbonator + 2·TCRstorage·tmax)·fcarbonator + TCRcalciner·fcalciner | (6) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) plant, fcarbonator and fcalciner are the power fractions of the power plant, carbonator and calciner with respect to the total power input.
plant, fcarbonator and fcalciner are the power fractions of the power plant, carbonator and calciner with respect to the total power input.
In order to calculate the TCRTotal corresponding to the appropriate units of eqn (3) ($ per kWe), the net efficiency of the system depicted in Fig. 1 (ηplant) can be estimated by means of eqn (7):
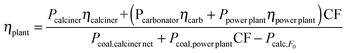 | (7) |
The thermal power associated with the calcination of the purge (Pcalc, F0) is discounted from the estimation of the efficiency to account for the credits associated with the utilization of the purge. According to this equation, efficiencies of 0.26 and 0.29 are calculated for the FlexiCaL system with Xave values of 0.52 and 0.26 respectively. The main cost parameters for the cases chosen as examples are summarized in Table 3. In order to focus the discussion on the specific capital requirements, the same fuel and fixed costs as those of the conventional CaL system have been assumed. The cost associated with the consumption of limestone has been included under variable costs (VOM).
| Parameter | Units | FlexiCaL/Xave = 0.52 | FlexiCaL/Xave = 0.26 |
|---|---|---|---|
| a Including the make-up limestone cost. | |||
| TCR | $ per kWth | 366 | 359 |
| ($ per kWe) | (1402) | (1222) | |
TCRpower![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) plant plant |
$ per kWth | 0 | 0 |
| TCRrefractory | $ per kWth | 125 | 125 |
| TCRCPU | $ per kWth | 80 | 80 |
| TCRPcarbonator | $ per kWth | 450 | 450 |
| TCRcalciner | $ per kWth | 1037 | 1037 |
| TCRstorage | $ per kWhth | 0.051 | 0.102 |
| t max | Days | 15 | 15 |
| TCRstorage | $ per kWth | 18.5 | 37 |
f
power![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) plant plant
|
0.61 | 0.61 | |
| f carbonator | 0.23 | 0.25 | |
| f calciner | 0.16 | 0.14 | |
| η plant | kWe kWth−1 | 0.261 | 0.294 |
| FOM | $ per kWe | 40 | 40 |
| FCF | year−1 | 0.1 | 0.1 |
| VOMa | $ per kWhe | 0.015 | 0.004 |
| Fuel cost | $ per GJ | 2 | 2 |
| Limestone cost | $ per ton | 10 | 10 |
| CO2 emission factor | kgCO2 MW he−1 | 67 | 58 |
Based on the assumptions summarized in Table 3 the COE calculated by means of eqn (3) for the FlexiCaL system are 0.150 and 0.126 $ per kWhe for Xave values of 0.52 and 0.26 respectively. The higher COE obtained for FlexiCaL/Xave = 0.52 is mainly due to the higher make-up flow of limestone and the slightly lower efficiency. The potential advantages of the system proposed in this work can be appreciated when compared with the COE calculated for a conventional CaL system retrofitted into a power plant operating with a CF value of 0.2. In this case, the system is heavily penalized by the higher capital requirements, with the COE increasing up to 0.192 $ per kWhe (see Table 2).
If the system in Fig. 1 is designed for capturing CO2 from a power plant operating with a high capacity factor, the contribution of the energy storage system to the whole process is reduced. As a result, the contribution of the costly oxy-fuel calciner is higher and the total capital requirements increase. In that case, the FlexiCaL system and the standard CaL would show similar COE values of around 0.064–0.082 $ per kWhe at the high CF values of the power plant. However, as the capacity factor decreases (see Fig. 4a), the COE of the FlexiCaL integrated with an energy storage system becomes much more favorable, with a reduction of 35% in the case of FlexiCaL/Xave = 0.26 when compared to a standard CaL system at CF = 0.2.
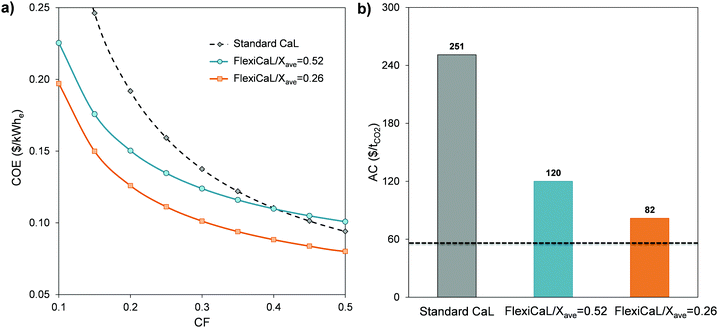 | ||
| Fig. 4 (a) Comparison of the cost of electricity (COE) of the FlexiCaL systems reported in Table 3 and the standard CaL as a function of the CF. (b) CO2 avoidance costs (AC) of the CaL and FlexiCaL systems when CF = 0.2. Dotted line corresponds to an AC of 56 $ per tCO2 corresponding to a standard CaL operating with a CF value of 0.9 assuming that it retrofits an amortized coal power plant (CAPEX = 0). | ||
To calculate the CO2 avoidance costs, an existing amortized power plant operating with the same capacity factor of 0.2 has been chosen as a reference. It must be noted here that, as it has been assumed that both the reference CaL and FlexiCaL systems retrofit an amortized power plant with a lower COE than a new power plant (see Table 2), the AC calculated by means of eqn (4) are increased when compared with systems coupled to new power plants (e.g. 56 $ per tCO2vs. 26 $ per tCO2 for a standard CaL system operated at base load). As can be seen in Fig. 4b in all the cases the AC are higher than those corresponding to a standard CaL system operating at base load (for a typical capacity factor of 0.9). However, if the standard CaL operates at CF = 0.2, the cost of CO2 avoided increases almost 5-fold up to a value of 251 $ per tCO2. This very high cost can make this kind of system economically unviable for such low capacity factors. In contrast, the increase in the CO2 avoidance costs for the FlexiCaL cases operating with capacity factors of 0.2 is relatively low for the examples presented in this work. For example, an increment of only 26 $ per tCO2 is obtained for the FlexiCaL/Xave = 0.26 configuration compared to the standard CaL system operating at base load. It should be noted however that it is an immense challenge to operate any other CO2 capture system at such low capacity factors as those required for power plants used for back-up purposes. The need to store very large quantities of the functional material (such as amines in post-combustion or cryogenic O2 in oxy-fuel systems) would be at the expense of substantial economic penalties due to the inherently higher costs of these materials. In contrast, the FlexiCaL system proposed in this work allows the storage of very large masses of sorbent at a very low cost, due to the low cost of the CaO precursor (limestone), the lack of environmental risks, and the low thermal conductivity of stagnant solids. Another important advantage of this concept is the possibility of retrofitting to existing coal power plants as there is no need for any modification to the existing power plant as with other technologies (e.g. the need to extract steam in the case of post-combustion amine-based systems, to desulfurize the flue gas, to modify the boiler in the case of oxy-combustion etc.). Most important of all, these plants do not have much chance of being incorporated into future energy systems where there is an increasing share of renewable power, other than as back-up systems for brief periods of time, unless a competitive method for CO2 capture during the brief periods of operation can be found.
Conclusions
A flexible calcium looping process integrated within an energy storage system has been analyzed in this work. This FlexiCaL system uses piles of CaO/CaCO3 for storing chemical energy and the decoupling of the calciner from the carbonator reactors. It has been shown that the calciner in such systems can have a highly reduced environmental footprint and be operated at base load irrespective of the flue gas load fed to the carbonator. To capture CO2 from a typical 500 MWth coal power plant operated with low capacity factors, it has been shown that the thermal load to the calciner can be as low as 110–130 MWth. An analysis of the effect of the activity of the material stored in the solids piles has confirmed that temperatures of around 500–600 °C in the carbonator are compatible with the storage of solids at low temperature (<250 °C), which facilitates the use of large solid piles intended for daily or even inter-seasonal storage solids. A basic economic analysis of the system proposed indicates that the cost of the electricity could be around 0.13–0.15 $ per kWhe when capturing CO2 from an amortized coal power plant operated with a capacity factor as low as 0.2. This translates into CO2 avoidance costs of 80–120 $ per tCO2 for a system with a capacity factor of just 0.2. This represents only a 44% increase in the CO2 avoidance costs with respect to the equivalent standard CaL system operating in base load mode (i.e. CF of 0.9), which would have a CO2 avoidance cost of 251 $ per tCO2 if requested to operate under the same low capacity factor (i.e. CF of 0.2). This sharp reduction in capture cost is the result of moderating the capital cost requirements by reducing the calciner footprint and by the low cost of the very large inventory of Ca-derived solids in the solid storage system. Thus, the flexible CaL process is presented in this work as a highly advantageous option for capturing CO2 from existing back-up power plants when compared with conventional capture systems.Conflict of interest
There are no conflicts of interest to declare.Acknowledgements
The authors acknowledge the financial support provided by the European Union under the Research Fund for Coal and Steel (RFCS) Program (FlexiCaL Project, No. 709629). Y. A. Criado thanks the Government of the Principality of Asturias for a PhD fellowship (Severo Ochoa Program).References
- IPCC, Climate change 2014: Synthesis report. Contribution of Working Group I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, International Panel on Climate Change, Geneva, Switzerland, 2014.
- IEA, World energy outlook special report: Energy and climate change, International Energy Agency, Paris, France, 2015.
- IEA, Technology roadmap: Energy storage, International Energy Agency, Paris, France, 2014.
- IEA, Harnessing variable renewables: A guide to the balancing challenge, International Energy Agency, Paris, France, 2011.
- N. Kumar, P. Besuner, S. Lefton and D. Agan, Power plant cycling costs, National Renevable Laboratory, 2012 Search PubMed.
- P. Keatley, A. Shibli and N. J. Hewitt, Appl. Energy, 2013, 111, 550–557 CrossRef.
- R. M. Montañés, M. Korpås, L. O. Nord and S. Jaehnert, Energy Procedia, 2016, 86, 22–31 CrossRef.
- J. Davison, Energy Procedia, 2011, 4, 2548–2555 CrossRef.
- ZEP, The cost of CO2 capture: post-demostration CCS in the EU, European technology platform for zero emissions fossil fuel power plants, 2011.
- IPCC, Special report on carbon dioxide capture and storage, Intergovernmental Panel on Climate Change, New York, USA, 2005.
- R. Domenichini, L. Mancuso, N. Ferrari and J. Davison, Energy Procedia, 2013, 37, 2727–2737 CrossRef CAS.
- H. Chalmers, J. Gibbins and M. Leach, Mitig. Adapt. Strategies Glob. Chang., 2012, 17, 621–649 CrossRef.
- IEAGHG, Operating flexibility of power plants with CCS, International Energy Agency Greenhouse Gas R&D Programme, 2012.
- N. Mac Dowell and I. Staffell, Int. J. Greenhouse Gas Control, 2016, 48(Part 2), 327–344 CrossRef.
- ZEP, Future CCS technologies, European technology platform for zero emissions fossil fuel power plants, 2017.
- E. Sanchez Fernandez, M. Sanchez del Rio, H. Chalmers, P. Khakharia, E. L. V. Goetheer, J. Gibbins and M. Lucquiaud, Int. J. Greenhouse Gas Control, 2016, 48(part 2), 275–289 CrossRef CAS.
- M. R. Haines and J. E. Davison, Energy Procedia, 2009, 1, 1457–1464 CrossRef CAS.
- N. Perrin, R. Dubettier, F. Lockwood, J.-P. Tranier, C. Bourhy-Weber and P. Terrien, Appl. Therm. Eng., 2015, 74, 75–82 CrossRef CAS.
- D. P. Hanak, D. Powell and V. Manovic, Appl. Energy, 2017, 191, 193–203 CrossRef CAS.
- A. Ozarslan, Int. J. Hydrogen Energy, 2012, 37, 14265–14277 CrossRef CAS.
- N. Böttcher, U.-J. Görke, O. Kolditz and T. Nagel, Environ. Earth Sci., 2017, 76, 98 CrossRef.
- A. Brown, presented in part at the Workshop on Operating Flexibility of Power Plants with CCS, Imperial College, London, November, 2009.
- J. Davison, S. Arienti, P. Cotone and L. Mancuso, Energy Procedia, 2009, 1, 4063–4070 CrossRef CAS.
- A.-M. Cormos, C. Dinca and C.-C. Cormos, Appl. Therm. Eng., 2015, 74, 20–27 CrossRef CAS.
- W. Hausz, B. J. Berkowitz and R. C. Hare, Conceptual design of thermal energy storage systems for near term electric utility applications: screening of concepts, Report DOE/NASA/0012-78/1, 1978.
- M. K. Drost, S. Somasundaram, D. R. Brown and Z. I. Antionak, presented in part at the Fossil-fuel plant cycling conference, Washington D.C., December, 1990.
- B. Arias, Y. A. Criado, A. Sanchez-Biezma and J. C. Abanades, Appl. Energy, 2014, 132, 127–136 CrossRef CAS.
- B. Arias, Int. J. Greenhouse Gas Control, 2016, 45, 172–180 CrossRef CAS.
- B. Arias, M. E. Diego, J. C. Abanades, M. Lorenzo, L. Diaz, D. Martínez, J. Alvarez and A. Sánchez-Biezma, Int. J. Greenhouse Gas Control, 2013, 18, 237–245 CrossRef CAS.
- A. Galloy, J. Ströhle and B. Epple, VGB PowerTech, 2011, 91, 64–68 CAS.
- H. Dieter, A. R. Bidwe, G. Varela-Duelli, A. Charitos, C. Hawthorne and G. Scheffknecht, Fuel, 2014, 127, 23–37 CrossRef CAS.
- M. Alonso, M. E. Diego, C. Pérez, J. R. Chamberlain and J. C. Abanades, Int. J. Greenhouse Gas Control, 2014, 29, 142–152 CrossRef CAS.
- M. H. Chang, W. C. Chen, C. M. Huang, W. H. Liu, Y. C. Chou, W. C. Chang, W. Chen, J. Y. Cheng, K. E. Huang and H. W. Hsu, Energy Procedia, 2014, 63, 2100–2108 CrossRef CAS.
- M. E. Diego and M. Alonso, Fuel, 2016, 181, 325–329 CrossRef CAS.
- A. Sánchez-Biezma, J. Paniagua, L. Diaz, M. Lorenzo, J. Alvarez, D. Martínez, B. Arias, M. E. Diego and J. C. Abanades, Energy Procedia, 2013, 37, 1–8 CrossRef.
- J. C. Abanades, B. Arias, A. Lyngfelt, T. Mattisson, D. E. Wiley, H. Li, M. T. Ho, E. Mangano and S. Brandani, Int. J. Greenhouse Gas Control, 2015, 40, 126–166 CrossRef CAS.
- I. Martínez, G. Grasa, J. Parkkinen, T. Tynjälä, T. Hyppänen, R. Murillo and M. C. Romano, Int. J. Greenhouse Gas Control, 2016, 50, 271–304 CrossRef.
- R. Barker, J. Appl. Chem. Biotechnol., 1973, 23, 733–742 CAS.
- K. Kyaw, H. Matsuda and M. Hasatani, presented in part at the Japan Atomic Energy Research Institute Conference, Japan, 1996.
- R. Chacartegui, A. Alovisio, C. Ortiz, J. M. Valverde, V. Verda and J. A. Becerra, Appl. Energy, 2016, 173, 589–605 CrossRef CAS.
- S. E. B. Edwards and V. Materić, Sol. Energy, 2012, 86, 2494–2503 CrossRef CAS.
- B. Müller, W. Arlt and P. Wasserscheid, Energy Environ. Sci., 2011, 4, 4322–4331 Search PubMed.
- A. Vandersickel, R. P. Field, W. Chen, N. D. Mancini and A. Mitsos, Ind. Eng. Chem. Res., 2014, 53, 12032–12043 CrossRef CAS.
- J. C. Abanades, B. Arias and Y. A. Criado, EP2762781 B1, 2013.
- D. P. Hanak, C. Biliyok and V. Manovic, Energy Environ. Sci., 2016, 9, 971–983 CAS.
- T. Shimizu, T. Hirama, H. Hosoda, K. Kitano, M. Inagaki and K. Tejima, Chem. Eng. Res. Des., 1999, 77, 62–68 CrossRef CAS.
- K. Schwaiger, M. Haider, F. Holzleithner and R. Eisl, presented in part at the 21st International Conference on Fluidized Bed Combustion, Naples, Italy, 2012.
- N. Rodríguez, M. Alonso and J. C. Abanades, Chem. Eng. J., 2010, 156, 388–394 CrossRef.
- L. Zhen-shan, C. Ning-sheng and E. Croiset, AIChE J., 2008, 54, 1912–1925 CrossRef.
- G. S. Grasa, J. C. Abanades, M. Alonso and B. González, Chem. Eng. J., 2008, 137, 561–567 CrossRef CAS.
- G. Grasa, R. Murillo, M. Alonso and J. C. Abanades, AIChE J., 2009, 55, 1246–1255 CrossRef CAS.
- P. Sun, J. R. Grace, C. J. Lim and E. J. Anthony, Chem. Eng. Sci., 2008, 63, 47–56 CrossRef CAS.
- Z.-S. Li, F. Fang, X.-Y. Tang and N.-S. Cai, Energy Fuels, 2012, 26, 2473–2482 CrossRef CAS.
- S. K. Bhatia and D. D. Perlmutter, AIChE J., 1983, 29, 79–86 CrossRef CAS.
- A. Darde, R. Prabhakar, J.-P. Tranier and N. Perrin, Energy Procedia, 2009, 1, 527–534 CrossRef CAS.
- L. M. Romeo, J. C. Abanades, J. M. Escosa, J. Paño, A. Giménez, A. Sánchez-Biezma and J. C. Ballesteros, Energy Convers. Manage., 2008, 49, 2809–2814 CrossRef CAS.
- T. Hirsch and A. Khenissi, Energy Procedia, 2014, 49, 1165–1176 CrossRef.
- J. Spelling, A. Gallo, M. Romero and J. González-Aguilar, Energy Procedia, 2015, 69, 1160–1170 CrossRef CAS.
- DOE/NETL, Cost and performance baseline for fossil energy plants volume 1a: Bituminous coal (PC) and natural gas to electricity. Revision 3., Report DOE/NETL-2015/1723, US Department of Energy, National Energy Technology Laboratory, 2015.
- IEAGHG, CO2 capture in the cement industry, Report 2008/3, International Energy Agency Greenhouse Gas R&D Programme, 2008.
- DOE/NETL, Cost and perfomance for low rank pulverized coal oxycombustion energy plants, Report DOE/NETL-401/093010 US Department of Energy, National Energy Technology Laboratory, 2010.
| This journal is © The Royal Society of Chemistry 2017 |