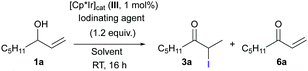Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
2,2-Diiododimedone: a mild electrophilic iodinating agent for the selective synthesis of α-iodoketones from allylic alcohols†
Samuel
Martinez-Erro‡
,
Antonio
Bermejo Gómez‡
 ,
Ana
Vázquez-Romero
,
Elis
Erbing
and
Belén
Martín-Matute
,
Ana
Vázquez-Romero
,
Elis
Erbing
and
Belén
Martín-Matute
 *
*
Department of Organic Chemistry, Arrhenius Laboratory, Stockholm University, 10691 Stockholm, Sweden. E-mail: belen.martin.matute@su.se
First published on 7th August 2017
Abstract
2,2-Diiodo-5,5-dimethylcyclohexane-1,3-dione is reported as a new electrophilic iodinating agent that selectively iodinates electron-rich aromatics. In contrast to other common electrophilic iodinating reagents, its mild nature allows it to be used for the selective synthesis of α-iodinated carbonyl compounds from allylic alcohols through a 1,3-hydrogen shift/iodination process catalyzed by iridium(III) complexes.
Halogenated organic compounds are ubiquitous, not only as natural products,1 but also as synthetic compounds, and they have applications in medicinal chemistry,2 agrochemistry,3 and materials science.4 The halogen functional group is very versatile. It can participate in many synthetic transformations, including nucleophilic substitutions, eliminations, dehydrohalogenations, and oxidative additions to metal centers. The nature of the halogen gives each carbon–halogen bond unique properties,5 resulting in numerous applications in synthetic organic chemistry.
Significant progress has been made in the development of synthetic methods for the construction of C–F bonds, via both electrophilic and nucleophilic approaches.6 There also exist many synthetic methods for the nucleophilic and electrophilic chlorination7 and bromination8 of organic molecules. When it comes to electrophilic methods for the construction of C–I bonds, reagents such as I2, NIS (N-iodosuccinimide), IPy2BF4 (Barluenga's reagent), and ICl have been widely used in organic synthesis.9 Also, methods based on the conversion of iodide into the corresponding iodonium species through oxidation have also been reported.10 These methods have some drawbacks, however, such as the high reactivity of the electrophilic iodinating agents, or the need for strong oxidizing agents, which result in low functional-group tolerance and low chemo- or regioselectivity. Thus, the development of mild and selective electrophilic iodination methods remains a challenging and desirable goal for synthetic chemists.
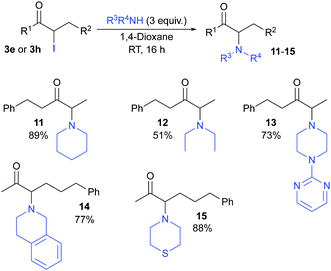
We have previously reported the use of iridium catalysts for the conversion of allylic alcohols (1) into α-functionalized carbonyl compounds.11,7c,8c,12 Carbon and halogen (i.e., F, Cl, and Br) functional groups could be introduced through a process involving a 1,3-hydrogen shift mediated by the metal complex. This process allows the selective formation of either the α or α′ constitutional isomer, as the new bond is constructed exclusively at the carbon that was originally part of the olefin. Attempts to introduce an iodide failed in our hands, mainly due to the high reactivity of the available electrophilic iodinating agents, but also due to the instability of the starting allylic alcohols and/or the final iodoketones under the reaction conditions. In this communication, we report the development of a new mild iodinating agent (Scheme 1, top) and its application in electrophilic iodination of aromatic compounds, and also in the synthesis of α-iodoketones (3) from allylic alcohols mediated by iridium catalysts (Scheme 1, bottom). This methodology allows for the first time the one-step synthesis of α-iodoketones as single constitutional isomers from allylic alcohols in a one-step procedure. The utility of α-iodoketones for the preparation of valuable building blocks is also presented.
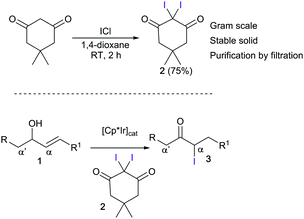 | ||
| Scheme 1 Synthesis of 2,2-diiododimedone (2, top) and of α-iodocarbonyl compounds from allylic alcohols. | ||
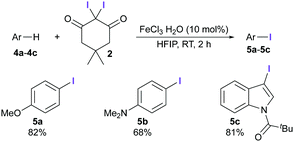
The synthesis of the new iodinating reagent, 2,2-diiododimedone (2), was easily accomplished by the treatment of dimedone with iodine monochloride in 1,4-dioxane at room temperature. The synthesis of 2 can be carried out on a gram scale, and purification by simple filtration gives the product in excellent yield. We initially tested 2 as an iodinating agent for aromatic compounds (Scheme 2). In optimization studies, the best results were obtained using a catalytic amount of FeCl3, and hexafluoroisopropanol (HFIP) as the solvent. The need to use these conditions reflects the mildness of 2, which is important for increasing the functional group tolerance in electrophilic halogenation reactions. After a short optimization (see ESI†), we found that anisole 4a could be iodinated under an air atmosphere in just 2 h. Two other substrates were also iodinated using these reaction conditions; both N,N-dimethylaniline (4b) and N-pivaloyl indole (4c) were converted into the corresponding iodinated analogues (5b and 5c) in very good yields after short reaction times and with excellent regioselectivity.
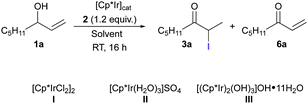
Next, we evaluated the performance of 2 in the iridium-catalyzed isomerization/α-iodination of allylic alcohols. For a successful outcome, the reaction must take place under mild conditions, and the starting alcohols (1) and final products (3) should not undergo undesired iodination. Oct-1-en-3-ol (1a) was chosen as a model substrate for the optimization studies, and several Ir(III) catalysts and solvents were evaluated (Table 1). When [Cp*IrCl2]2 (I, 2 mol% [Ir]) was used as the catalyst in a mixture of acetone and H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), full conversion was observed, but only 34% of the desired product 3a was formed, together with 64% of α,β-unsaturated ketone 6a (entry 1). This solvent mixture gave, on the contrary, excellent results in the bromination of allylic alcohols.8c The use of THF/H2O (1
1), full conversion was observed, but only 34% of the desired product 3a was formed, together with 64% of α,β-unsaturated ketone 6a (entry 1). This solvent mixture gave, on the contrary, excellent results in the bromination of allylic alcohols.8c The use of THF/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) resulted in a clear decrease in the formation of 6a (entry 2). This effect was even clearer when THF was replaced by 2-methyltetrahydrofuran (2-MeTHF). In this case, the desired α-iodinated compound (3a) was obtained in 85% yield and only 11% of 6a was formed (entry 3). The amount of 6a could be decreased further by using [Cp*Ir(H2O)3]SO4 (II) or [(Cp*Ir)2(OH)3]OH·11H2O (III) as the catalyst (entries 4 and 5). When the proportion of H2O in the solvent mixture was increased (i.e., 1
1) resulted in a clear decrease in the formation of 6a (entry 2). This effect was even clearer when THF was replaced by 2-methyltetrahydrofuran (2-MeTHF). In this case, the desired α-iodinated compound (3a) was obtained in 85% yield and only 11% of 6a was formed (entry 3). The amount of 6a could be decreased further by using [Cp*Ir(H2O)3]SO4 (II) or [(Cp*Ir)2(OH)3]OH·11H2O (III) as the catalyst (entries 4 and 5). When the proportion of H2O in the solvent mixture was increased (i.e., 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), higher amounts of the undesired 6a were formed (entries 6 and 7); when the proportion of 2-MeTHF was increased, the yield of 6a dropped to just 5–6% (entries 8 and 9), while a very good yield for 3a was maintained. Increasing the proportion of 2-MeTHF further did not prevent the formation of 6a, and also compromised the conversion (see the ESI† for a full description of solvent effects). When the catalyst loading was decreased to 1 mol% [Ir], catalyst III was better than II (entries 10 and 11). When 0.6 and 0.2 equiv. of 2 were used, yields of 70 and 27%, respectively, were observed (see ESI†). This result suggests that more than one iodo unit can be donated by the reagent. All reactions were carried out under an atmosphere of air.
2), higher amounts of the undesired 6a were formed (entries 6 and 7); when the proportion of 2-MeTHF was increased, the yield of 6a dropped to just 5–6% (entries 8 and 9), while a very good yield for 3a was maintained. Increasing the proportion of 2-MeTHF further did not prevent the formation of 6a, and also compromised the conversion (see the ESI† for a full description of solvent effects). When the catalyst loading was decreased to 1 mol% [Ir], catalyst III was better than II (entries 10 and 11). When 0.6 and 0.2 equiv. of 2 were used, yields of 70 and 27%, respectively, were observed (see ESI†). This result suggests that more than one iodo unit can be donated by the reagent. All reactions were carried out under an atmosphere of air.
| Entry | Catalyst | Solvent (v/v) | Conv.b [%] | 3a/6ab,c [%] |
|---|---|---|---|---|
| a Unless otherwise noted, 1a (0.5 mmol, 0.1 M) and 2 mol% of [Ir] were used. b Determined by 1H NMR spectroscopy. c Octan-3-one was not formed. d 1 mol% of [Ir]. | ||||
| 1 | I | Acetone/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
>99 | 36/64 |
| 2 | I | THF/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
86 | 58/28 |
| 3 | I | 2-MeTHF/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
96 | 85/11 |
| 4 | II | 2-MeTHF/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
99 | 93/6 |
| 5 | III | 2-MeTHF/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
>99 | 92/8 |
| 6 | II | 2-MeTHF/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
>99 | 91/9 |
| 7 | III | 2-MeTHF/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
>99 | 90/10 |
| 8 | II | 2-MeTHF/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
98 | 93/5 |
| 9 | III | 2-MeTHF/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
>99 | 94/6 |
| 10d | II | 2-MeTHF/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
86 | 79/7 |
| 11d | III | 2-MeTHF/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
92 | 88/4 |
When compared to other electrophilic iodinating reagents, the superiority of 2 was clearly seen (Table 2). None of the other reagents tested were able to give good yields of 3a, mainly due to the decomposition of the starting allylic alcohol 1a (entries 1–5). This is not surprising, since it is well known that double bonds promptly react with these reagents to give cyclic iodonium ions. This undesired reaction was not observed when 2,2-diiododimedone (2) was used. A series of control experiments was also carried out (Table 2, entries 6–11). Catalyst III alone was not able to react with the allylic alcohol in the absence of 2 (entry 6). We recently reported that the presence of a halogen ligand on Ir is important for the activity of [Cp*Ir] complexes of this type, and described the in situ formation of such an Ir–halogen bond through the reaction of the Ir precatalyst with the halogenating agent.13 In another control experiment, we observed that 2 does not react with allylic alcohol 1a in the absence of the iridium catalyst after 16 h (entry 7). It can therefore be concluded that the mildness of the iodinating agent 2 is key to the success of this tandem isomerization/halogenation reaction. Mechanistic investigations were carried out with deuterium-labelled alcohols, and the results suggest that an intramolecular 1,3-H shift is operating under these reaction conditions (see ESI†).
| Entry | Reagent | Conv.b [%] | 3a/6ab [%] |
|---|---|---|---|
| a Unless otherwise noted, 1a (0.5 mmol, 0.1 M). b Determined by 1H NMR spectroscopy. c 28% (entry 2) and 21% (entry 3) of octan-3-one were formed. d Without [Ir] catalyst. e A mixture of unknown by-products was observed. | |||
| 1 | 2 | >99 | 94/6 |
| 2 | I2 | 66 | —/3c |
| 3 | ICl | 66 | —/1c |
| 4 | NIS | >99 | 17/— |
| 5 | IPy2BF4 | >99 | —/— |
| 6 | — | <5 | — |
| 7 | 2 | 4 | — |
| 8 | I2d | 25 | —e |
| 9 | ICld | 95 | —e |
| 10 | NISd | 90 | —e |
| 11 | IPy2BF4d | 76 | —e |
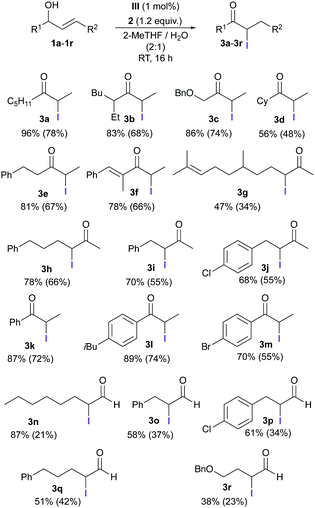
Having established these optimized reaction conditions, we went on to study the scope of the reaction (Table 3). Alcohols 1a–1f and 1k–1m bearing a terminal double bond gave the corresponding α-iodocarbonyl compounds (3a–3f and 3k–3m) in high yields within 16 h. Allylic alcohols with internal double bonds (1g–1j) were also converted into the corresponding α-iodinated products in good yields. Additional double bonds in the molecule were unaffected (1f and 1g); only the allylic alcohol functionality reacted. The reaction is not restricted to secondary alcohols; primary alcohols gave the corresponding α-iodoaldehydes (3n–3r) in moderate to good yields. The stability of the products during the purification process accounts for the difference between the yields estimated against an internal standard and the isolated yields.
| a Reactions carried out on a 1 mmol scale (0.1 M). Yield determined by 1H NMR spectroscopy. Isolated yields in parentheses. |
|---|
|
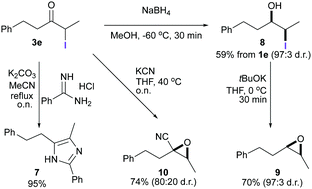
The utility of α-iodoketones as versatile intermediates for organic synthesis was demonstrated in a series of transformations (Schemes 3 and 4). First, the synthesis of imidazole 7 was easily accomplished by allowing an amidine to react with α-iodoketone 3e. In a one-pot procedure starting from 1e, iodohydrin 8 was synthesized in 59% yield with excellent diastereoselectivity (93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7). Subsequent basic treatment gave the corresponding epoxide 9 with excellent diastereoselectivity. α-Cyanoepoxide 10 was also prepared in good yield and with a good diastereomeric ratio in one step by the reaction of 3e with KCN.
7). Subsequent basic treatment gave the corresponding epoxide 9 with excellent diastereoselectivity. α-Cyanoepoxide 10 was also prepared in good yield and with a good diastereomeric ratio in one step by the reaction of 3e with KCN.
The synthetic utility of α-iodocarbonyl compounds was also demonstrated via the preparation of valuable α-aminoketones. This structural motif is found in many compounds with important biological activities.14 Although there are several procedures for the synthesis of compounds containing this motif, their regioselective preparation when two enolizable positions are present remains a challenge.14b–d We found that secondary amines react with α-iodocarbonyl derivatives – prepared from allylic alcohols as described in this communication – to give α-amino ketones as single constitutional isomers (Scheme 4).
In conclusion, we present a new mild and selective electrophilic iodinating reagent, 2,2-diiododimedone (2). Using this reagent, we have been able to carry out the first selective synthesis of α-iodocarbonyl compounds as single constitutional isomers. The method relies on an iridium-catalyzed 1,3-hydrogen shift mediated by iridium(III) complexes. The utility of α-iodocarbonyl compounds has been demonstrated by their conversion into other important organic structures for which selective synthetic procedures have not previously been available.
This project was supported by the Swedish Research Council (VR), the Swedish Research Council Formas, and the Knut and Alice Wallenberg Foundation. We also thank the Swedish Governmental Agency for Innovation Systems (VINNOVA) for a VINNMER grant to B. M.-M.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) M. M. Haeggblom and I. D. Bossert, Dehalogenation, Kluwer Academic Publishers, New York, 2003, pp. 6–29 Search PubMed; (b) G. W. Gribble, Naturally occurring organohalogen compounds – A comprehensive update, Springer, Wien, 2010 Search PubMed; (c) G. W. Gribble, Prog. Heterocycl. Chem., 2003, 15, 58 CAS.
- (a) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320 RSC; (b) M. Z. Hernandes, S. M. T. Cavalcanti, D. R. M. Moreira, W. F. de Azeved Jr. and A. C. L. Leite, Curr. Drug Targets, 2010, 11, 303 CrossRef CAS PubMed; (c) F. Adasme-Carreno, C. Muñoz-Gutierrez and J. H. Alzate-Morales, RSC Adv., 2016, 6, 61837 RSC; (d) S. Jiang, L. Zhang, D. Cui, Z. Yao, B. Gao, J. Lin and D. Wei, Sci. Rep., 2016, 6, 34750 CrossRef CAS PubMed.
-
P. Jeschke, W. Kra
![[m with combining umlaut]](https://www.rsc.org/images/entities/char_006d_0308.gif) er, U. Schirmer and M. Witschel, Modern Methods in Crop Protection Research, Wiley-VCH, Weinheim, 2013, ch. 4, pp. 73–119 Search PubMed.
er, U. Schirmer and M. Witschel, Modern Methods in Crop Protection Research, Wiley-VCH, Weinheim, 2013, ch. 4, pp. 73–119 Search PubMed. - (a) M. L. Tang and Z. Bao, Chem. Mater., 2011, 23, 446 CrossRef CAS; (b) F. Yang, C. Li, W. Lai, A. Zhang, H. Huang and W. Li, Mater. Chem. Front., 2017, 1, 1389–1395 RSC.
- G. Cavallo, P. Metrengolo, R. Milani, T. Pilati, A. Priimagi, G. Resnati and G. Terraneo, Chem. Rev., 2016, 116, 2478 CrossRef CAS PubMed.
- (a) C. Hollingworth and V. Gouverneur, Chem. Commun., 2012, 48, 2929 RSC; (b) T. Umemoto, J. Fluorine Chem., 2014, 167, 3 CrossRef CAS; (c) M. G. Campbell and T. Ritter, Chem. Rev., 2015, 115, 612 CrossRef CAS PubMed; (d) J.-D. Yang, Y. Wang, X.-S. Xue and J.-P. Cheng, J. Org. Chem., 2017, 82, 4129 CrossRef CAS PubMed.
- (a) I. Lengyel, V. Cesare and R. Stephani, Synth. Commun., 1998, 28, 1891 CrossRef CAS; (b) X. Wan, Z. Ma, B. Li, K. Zhang, S. Cao, S. Zhang and Z. Shi, J. Am. Chem. Soc., 2006, 128, 7416 CrossRef CAS PubMed; (c) N. Ahlsten, A. Bermejo Gómez and B. Martín-Matute, Angew. Chem., Int. Ed., 2013, 52, 6273 CrossRef CAS PubMed; (d) R. A. Rodriguez, C.-M. Pan, Y. Yabe, Y. Kawamata, M. D. Eastgate and P. S. Baran, J. Am. Chem. Soc., 2014, 136, 6908 CrossRef CAS PubMed; (e) S. M. Maddox, C. J. Nalbandian, D. E. Smith and J. L. Gustafson, Org. Lett., 2015, 17, 1042 CrossRef CAS PubMed; (f) M. Wang, Y. Zhang, T. Wang, C. Wang, D. Xue and J. Xiao, Org. Lett., 2016, 18, 1976 CrossRef CAS PubMed.
- (a) O. A. Attanasi, S. Beretta, G. Favi, P. Filippone, G. Mele, G. Moscatelli and R. Saladino, Org. Lett., 2006, 8, 4291 CrossRef CAS PubMed; (b) F. Mo, J. M. Yan, D. Qiu, F. Li, Y. Zhang and J. Wang, Angew. Chem., Int. Ed., 2010, 49, 2028 CrossRef CAS PubMed; (c) A. Bermejo Gómez, E. Erbing, M. Batuecas, A. Vázquez-Romero and B. Martín-Matute, Chem. – Eur. J., 2014, 20, 10703 CrossRef PubMed; (d) I. Saikia, A. J. Borah and P. Phukan, Chem. Rev., 2016, 116, 6837 CrossRef CAS PubMed; (e) M. H. Shinde and U. A. Kshirsagar, Green Chem., 2016, 18, 1455 RSC.
- (a) J. Barluenga, Pure Appl. Chem., 1999, 71, 431 CAS and references therein; (b) J. Barluenga, J. M. González, M. A. G. Martín, P. J. Campos and G. Asensio, J. Org. Chem., 1993, 58, 2058 CrossRef CAS; (c) R. Johnsson, A. Meijer and U. Ellervik, Tetrahedron, 2005, 61, 11657 CrossRef CAS; (d) X.-C. Wang, Y. Hu, S. Bonacorsi, Y. Hong, R. Burrell and J.-Q. Yu, J. Am. Chem. Soc., 2013, 135, 10326 CrossRef CAS PubMed; (e) J. He, J. Duan, H. Shi, J. Huang, J. Huang, L. Yu, M. Zeller, A. D. Hunter and Z. Xu, J. Inorg. Chem., 2014, 53, 6837 CrossRef CAS PubMed; (f) D. T. Racys, S. A. I. Sharif, S. L. Pimlott and A. Sutherland, J. Org. Chem., 2016, 81, 772 CrossRef CAS PubMed; (g) Y. Yang, L. Zhang, G.-J. Deng and H. Gong, Sci. Rep., 2017, 7, 40430 CrossRef PubMed.
- (a) J. Barluenga, M. Marco-Arias, F. González-Bobes, A. Balleteros and J. M. González, Chem. Commun., 2004, 2616 RSC; (b) S. Stavber, M. Jereb and M. Zupan, Synthesis, 2008, 1487 CrossRef CAS; (c) R. Singha, S. Dhara and J. K. Ray, RSC Adv., 2013, 3, 23989 RSC; (d) S. Song, X. Sun, X. Li, Y. Yuan and N. Jiao, Org. Lett., 2015, 17, 2886 CrossRef CAS PubMed; (e) J. Iskra and S. S. Murphree, Tetrahedron Lett., 2017, 58, 645 CrossRef CAS.
- (a) N. Ahlsten and B. Martín-Matute, Chem. Commun., 2011, 47, 8331 RSC; (b) A. Bartoszewicz, M. Livendahl and B. Martín-Matute, Chem. – Eur. J., 2008, 14, 10547 CrossRef CAS PubMed; (c) N. Ahlsten and B. Martín-Matute, Adv. Synth. Catal., 2009, 351, 2657 CrossRef CAS.
- Related metal-free isomerization example; S. Martinez-Erro, A. Sanz-Marco, A. Bermejo Gómez, A. Vázquez-Romero, M. S. G. Ahlquist and B. Martín-Matute, J. Am. Chem. Soc., 2016, 138, 13408 CrossRef CAS PubMed.
- E. Erbing, A. Vázquez-Romero, A. Bermejo Gómez, A. E. Platero-Prats, F. Carson, X. Zou, P. Tolstoy and B. Martín-Matute, Chem. – Eur. J., 2016, 22, 15659 CrossRef CAS PubMed.
- (a) P. C. Meltzer, D. Butler, J. R. Deschamps and B. K. Madras, J. Med. Chem., 2006, 49, 1420 CrossRef CAS PubMed; (b) R. W. Evans, J. R. Zbieg, S. Wu, W. Li and D. W. C. MacMillan, J. Am. Chem. Soc., 2013, 135, 16074 CrossRef CAS PubMed; (c) S. Guha, V. Rajeshkumar, S. S. Kotha and G. Sekar, Org. Lett., 2015, 17, 406 CrossRef CAS PubMed; (d) S. Liang, C.-C. Zeng, H.-Y. Tian, B.-G. Sun, X.-G. Luo and F.-Z. Ren, J. Org. Chem., 2016, 81, 11565 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed synthetic procedures, characterization, NMR spectra, optimization tables and deuterium labelling studies. See DOI: 10.1039/c7cc04823h |
| ‡ These authors contribute equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |