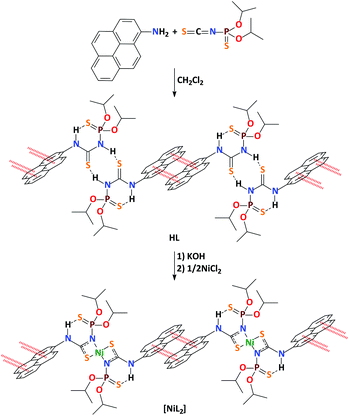
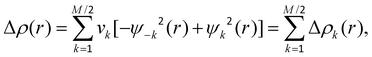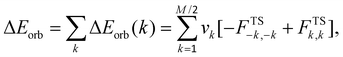An intermolecular pyrene excimer in the pyrene-labeled N-thiophosphorylated thiourea and its nickel(II) complex†
Damir A.
Safin
*a,
Maria G.
Babashkina
a,
Mariusz P.
Mitoraj
*b,
Piotr
Kubisiak
b,
Koen
Robeyns
a,
Michael
Bolte
c and
Yann
Garcia
*a
aInstitute of Condensed Matter and Nanosciences, Molecules, Solids and Reactivity (IMCN/MOST), Université catholique de Louvain, Place L. Pasteur 1, 1348 Louvain-la-Neuve, Belgium. E-mail: damir.a.safin@gmail.com; yann.garcia@uclouvain.be
bDepartment of Theoretical Chemistry, Faculty of Chemistry, Jagiellonian University, R. Ingardena 3, 30-060 Cracow, Poland. E-mail: mitoraj@chemia.uj.edu.pl
cInstitut für Anorganische Chemie J.-W.-Goethe-Universität, Frankfurt/Main, Germany
First published on 26th September 2016
Abstract
A new N-thiophosphorylated thiourea (1-pyrene)NHC(S)NHP(S)(OiPr)2 (HL) has been synthesized. The molecular structure of HL was elucidated by X-ray diffraction revealing a linear intramolecular hydrogen bond. Additionally, its crystal structure is stabilized by two intermolecular hydrogen bonds, which in turn leads to a centrosymmetric R22(8) dimer formation. These dimers are packed into polymeric chains through π⋯π stacking interactions between the pyrene rings. The reaction of the deprotonated HL with NiII leads to the NiII complex ([NiL2]). The crystal structure of [NiL2] exhibits a centrosymmetric homoleptic structure, where the NiII ion is coordinated in a square-planar fashion with the ligands arranged in a trans-N2S2 configuration. The pyreneNH protons in [NiL2] are involved in intramolecular hydrogen bonds of the pyreneN–H⋯S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P type. Molecules of [NiL2] form π⋯π stacking interactions between the pyrene rings, yielding 1D polymeric chains similar to those observed in the structure of HL. These π⋯π stacked 1D polymeric chains are linked to each other through C–H⋯S and anagostic C–H⋯Ni interactions, yielding 2D sheets. Hirshfeld surface analysis showed that the structures of both HL and [NiL2] are highly dominated by H⋯H, H⋯C, H⋯S and C⋯C contacts and also characterized by H⋯O and H⋯N contacts. The molecular surfaces of HL and [NiL2] also contain O⋯S and H⋯Ni contacts, respectively. Both HL and [NiL2] were found to be emissive in CH2Cl2 solution, which is due to the concentration dependent emission of the pyrene monomer and excimer. It was established that the latter fluorescence is due to the intermolecular excimer formation. The DFT calculations allowed us to confirm the aggregation ability of the synthesized species in solution through the numerous non-covalent interactions C–H⋯S, C–H⋯Ni and C–H⋯π, which, in turn, might be responsible for the concentration dependent photophysical properties.
P type. Molecules of [NiL2] form π⋯π stacking interactions between the pyrene rings, yielding 1D polymeric chains similar to those observed in the structure of HL. These π⋯π stacked 1D polymeric chains are linked to each other through C–H⋯S and anagostic C–H⋯Ni interactions, yielding 2D sheets. Hirshfeld surface analysis showed that the structures of both HL and [NiL2] are highly dominated by H⋯H, H⋯C, H⋯S and C⋯C contacts and also characterized by H⋯O and H⋯N contacts. The molecular surfaces of HL and [NiL2] also contain O⋯S and H⋯Ni contacts, respectively. Both HL and [NiL2] were found to be emissive in CH2Cl2 solution, which is due to the concentration dependent emission of the pyrene monomer and excimer. It was established that the latter fluorescence is due to the intermolecular excimer formation. The DFT calculations allowed us to confirm the aggregation ability of the synthesized species in solution through the numerous non-covalent interactions C–H⋯S, C–H⋯Ni and C–H⋯π, which, in turn, might be responsible for the concentration dependent photophysical properties.
Introduction
Since pyrene was first discovered in 1837 by Laurent,1 this tetracyclic aromatic hydrocarbon has continuously been in the focus of much research.2,3 Much later, in 1913, the first synthesis of pyrene was suggested by Weitzenböck.4One of the first industrial applications of pyrene was in the production of synthetic dyes.5 In the middle of 1950s, the first observation of intermolecular excimers (excited dimers) in a pyrene solution was reported.6 This efficient excimer formation, with exceptional distinction of the fluorescence bands for monomer and excimer, is a key feature of pyrene in comparison to other polyaromatic fluorophores.7 In particular, two informative parameters, namely the ratio of the excimer to monomer emission (IE/IM) and the wavelength corresponding to the maximum of excimer emission, are characteristic of the pyrene excimer. Pyrene exhibits a well-defined monomer emission at 370–430 nm along with an efficient excimer emission at about 480 nm.2,3 The ratio IE/IM, being highly sensitive to the conformational change of the pyrene-appended receptors, is useful for analyte sensing.8–14
A great number of NiII complexes with the imidodiphosphinates R2P(X)NHP(Y)R′2 (IDP) (X, Y = O, S, Se, Te),15–23 acylthioamides RC(S)NHC(X)R′ (AA) and aroylthioureas R2NC(S)NHC(X)R′ (ATU) (X = O, S)24–27 have been described. The chelate backbones of the IDP, AA and ATU ligands are coordinated towards the NiII cation through the donor X and Y atoms. This is, obviously, explained by the efficient delocalization of the negative charge in the symmetrically conjugated chelate backbone upon deprotonation. Contrarily, the coordination mode of N-(thio)phosphorylated thioamides and thioureas RC(S)NHP(X)(OR′)2 (X = O, S), which are IDP's, AA's and ATU's asymmetrical analogues, towards NiII still remains lesser studied. This is rather surprising since the negative charge is delocalized asymmetrically in these deprotonated ligands and, hence, they might show ambidental coordination properties through the donor atoms of the thiocarbonyl and (thio)phosphoryl groups, and the nitrogen atom of the phosphorylamide group. Furthermore, the nature of the R and R′ substituents might influence considerably the coordination mode both by electronic and steric factors. Additional donor functions in these substituents might also crucially affect complexation properties. In this context, we have studied the coordination properties of N-(thio)phosphorylated thioureas RR′NC(S)NHP(X)(OR′′)2 (X = O, S; R, R′ = H, alkyl, aryl; R′′ = OiPr, OPh) (NTTU) towards NiII and demonstrated that their square planar complexes exhibit either a 1,3-N,S- or 1,5-O,S- and 1,5-S,S′-coordination mode.28–47
In this contribution, the use of a fluorescent molecular probe approach48 to determine the conformation of the coordination core in the NiII complex [NiL2] with the N-thiophosphorylated thiourea in solution is presented for the first time. For this purpose, the thiourea (1-pyrene)NHC(S)NHP(S)(OiPr)2 (HL) was applied as a pyrene-labeled system. Moreover, to examine and discuss the contribution and influence of intermolecular interactions, responsible for the crystal packing, Hirshfeld surface analysis49 and associated 2D fingerprint plots,50 obtained using the CrystalExplorer 3.1 software,51 as well as the enrichment ratios,52 derived as the decomposition of the crystal contact surface between pairs of interacting chemical species, have been performed for HL and [NiL2].
Results and discussion
The N-thiophosphorylated thiourea HL was synthesized by reacting 1-aminopyrene with the isothiocyanate (iPrO)2P(S)NCS (Scheme 1). Complex [NiL2] was prepared by deprotonating the ligand in situ with KOH, followed by the reaction with NiCl2 (Scheme 1). Notably, we could not obtain the complexes of HL with other metals due to the instability of the parent ligand upon complex formation under the applied synthetic conditions.The IR spectrum of HL contains a band at 644 cm−1 for the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S group. The same band is only slightly shifted to low frequencies in the spectrum of [NiL2] and found at 631 cm−1. This observation testifies to the 1,3-N,S-coordination mode of the deprotonated ligand L in the crystal structure of [NiL2].28–47 In the IR spectra of HL there is a band at 1528 cm−1, corresponding to the S
S group. The same band is only slightly shifted to low frequencies in the spectrum of [NiL2] and found at 631 cm−1. This observation testifies to the 1,3-N,S-coordination mode of the deprotonated ligand L in the crystal structure of [NiL2].28–47 In the IR spectra of HL there is a band at 1528 cm−1, corresponding to the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N fragment, whereas the spectrum of [NiL2] contains a band at 1531 cm−1, which is the signature of the conjugated S–C–N fragment.53,54 In addition, there is a broad intense band arising from the POC group at 982 and 993 cm−1 in the spectra of HL and [NiL2], respectively. Besides these, the IR spectrum of HL contains two characteristic bands for the pyreneNH and PNH groups at 3081 and 3234 cm−1, whereas there is one broad band in the spectrum of [NiL2] at 3238 cm−1.
C–N fragment, whereas the spectrum of [NiL2] contains a band at 1531 cm−1, which is the signature of the conjugated S–C–N fragment.53,54 In addition, there is a broad intense band arising from the POC group at 982 and 993 cm−1 in the spectra of HL and [NiL2], respectively. Besides these, the IR spectrum of HL contains two characteristic bands for the pyreneNH and PNH groups at 3081 and 3234 cm−1, whereas there is one broad band in the spectrum of [NiL2] at 3238 cm−1.
The 31P{1H} NMR signal of HL in CDCl3 appears as a singlet at 52.8 ppm and in the area characteristic for the neutral NTTU (X = S).28–47 The 31P{1H} NMR spectrum of [NiL2] in the same solvent contains a unique signal at 53.6 ppm, which indicates the exclusive presence of diamagnetic 1,3-N,S-coordinated complex forms.28–471H NMR spectra of HL and [NiL2] in CDCl3 each reveals a single set of signals. The signals for the iPrO protons are observed as two doublets at 1.31–1.69 ppm of the diastereotopic CH3 protons and a doublet of septets or multiplet at 4.69–5.00 ppm, corresponding to the CHO protons. The signals for the pyrene protons are observed as a multiplet at 7.85–8.40 ppm. Besides, the 1H NMR spectrum of HL contains two singlets for the PNH and pyreneNH protons at 7.36 and 10.12 ppm, respectively, while only one singlet at 11.19 ppm, corresponding to the pyreneNH proton, is observed in the spectrum of [NiL2]. The singlets at 10.12 and 11.19 ppm in the 1H NMR spectra of HL and [NiL2] are considerably low-field shifted, indicating a strong intramolecular hydrogen bonding between the sulfur atom of the thiophosphoryl function and the hydrogen atom of the pyreneNH fragment (Scheme 1).
Compounds HL and [NiL2] crystallize in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and each contains one independent molecule in the asymmetric unit.
and each contains one independent molecule in the asymmetric unit.
The parameters of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S, C–N, P–N and P
S, C–N, P–N and P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bonds for HL are in the typical range for N-thiophosphorylated thiourea derivatives (Table 1).28–47 In particular, the C
S bonds for HL are in the typical range for N-thiophosphorylated thiourea derivatives (Table 1).28–47 In particular, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S and P
S and P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond lengths are about 1.66 and 1.92 Å, respectively. The P–N bond is about 1.66 Å and the C–N(P) bond length is ∼1.39 Å, and is slightly elongated compared to the C–N(C) one (∼1.33 Å). The S
S bond lengths are about 1.66 and 1.92 Å, respectively. The P–N bond is about 1.66 Å and the C–N(P) bond length is ∼1.39 Å, and is slightly elongated compared to the C–N(C) one (∼1.33 Å). The S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N–P backbone has an E conformation, while the S
C–N–P backbone has an E conformation, while the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P–N–C exhibits a Z conformation (Scheme 1 and Fig. 1). This is also echoed in the bond angles within the S
P–N–C exhibits a Z conformation (Scheme 1 and Fig. 1). This is also echoed in the bond angles within the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(N)–N–P
C(N)–N–P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S moiety. The S
S moiety. The S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N(C) and S
C–N(C) and S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N(P) angles are about 122°, while the S
C–N(P) angles are about 122°, while the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P–N and N–C–N ones are ∼116° (Table 1). Finally, the P–N–C angle is the largest one and about 127°. According to the S
P–N and N–C–N ones are ∼116° (Table 1). Finally, the P–N–C angle is the largest one and about 127°. According to the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N–P, S
C–N–P, S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N–C, S
C–N–C, S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P–N–C and N–C–N–P torsion angles (Table 1), the S
P–N–C and N–C–N–P torsion angles (Table 1), the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(N)–N–P
C(N)–N–P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S moiety is significantly deviated from planarity, which is caused by the deviation of the phosphorus atom from the least-squares plane formed by the S
S moiety is significantly deviated from planarity, which is caused by the deviation of the phosphorus atom from the least-squares plane formed by the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(N)–N–P fragment, which, in turn, is significantly flat.
C(N)–N–P fragment, which, in turn, is significantly flat.
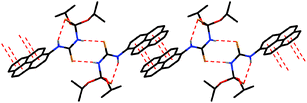 | ||
| Fig. 1 View of the hydrogen bonded dimers, linked through π⋯π stacking interactions in the structure of HL (hydrogen atoms not involved in H-bonding are omitted for clarity). | ||
| HL | [NiL2] | |
|---|---|---|
| Bond lengths | ||
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S S |
1.663(4) | 1.7157(17) |
P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S S |
1.9213(14) | 1.9350(5) |
| C–N(P) | 1.385(5) | 1.339(2) |
| C–N(C) | 1.325(5) | 1.333(2) |
| P–N | 1.661(3) | 1.6466(17) |
| Ni–N | — | 1.9068(13) |
| Ni–S | — | 2.2233(4) |
| Bond angles | ||
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P–N P–N |
115.64(13) | 117.01(6) |
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N(P) C–N(P) |
121.5(3) | 109.79(11) |
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N(C) C–N(C) |
122.8(3) | 124.84(15) |
| N–C–N | 115.7(4) | 125.37(16) |
| P–N–C | 126.8(3) | 130.31(12) |
| Ni–N–C | — | 98.64(11) |
| Ni–S–C | — | 77.20(6) |
| Ni–N–P | — | 129.30(9) |
| N–Ni–Sendocyclic | — | 74.36(5) |
| N–Ni–Sexocyclic | — | 105.64(5) |
| Torsion angles | ||
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N–P C–N–P |
146.8(3) | 165.15(10) |
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N–C C–N–C |
−3.4(6) | 1.6(3) |
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P–N–C P–N–C |
62.1(3) | 24.34(17) |
| N–C–N–P | −33.7(5) | −14.0(3) |
The crystal structure of HL contains an intramolecular hydrogen bond of the pyreneN–H⋯S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P type, which is formed between the pyreneNH hydrogen atom and the P
P type, which is formed between the pyreneNH hydrogen atom and the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S sulfur atom (Scheme 1, Fig. 1 and Table 2). Furthermore, the crystal structure of HL is additionally stabilized by intermolecular hydrogen bonds of the (P)N–H⋯S#1
S sulfur atom (Scheme 1, Fig. 1 and Table 2). Furthermore, the crystal structure of HL is additionally stabilized by intermolecular hydrogen bonds of the (P)N–H⋯S#1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C#1 type, which are formed between the phosphorylamide hydrogen atoms and the thiocarbonyl sulfur atoms of two neighboring molecules (Scheme 1, Fig. 1 and Table 2). As a result of such kinds of intermolecular interactions, centrosymmetric R22(8) dimers are formed. Additionally, the centrosymmetric dimers of HL form π⋯π stacking interactions between the pyrene rings (Scheme 1, Fig. 1 and Table 3). These interactions produce 1D polymeric chains.
C#1 type, which are formed between the phosphorylamide hydrogen atoms and the thiocarbonyl sulfur atoms of two neighboring molecules (Scheme 1, Fig. 1 and Table 2). As a result of such kinds of intermolecular interactions, centrosymmetric R22(8) dimers are formed. Additionally, the centrosymmetric dimers of HL form π⋯π stacking interactions between the pyrene rings (Scheme 1, Fig. 1 and Table 3). These interactions produce 1D polymeric chains.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Cg(I) | Cg(J) | d[Cg(I)–Cg(J)] | α | β | γ | |
|---|---|---|---|---|---|---|
| a Cg(I)–Cg(J): distance between ring centroids; α: dihedral angle between planes Cg(I) and Cg(J); β: angle Cg(I) → Cg(J) vector and normal to plane I; γ: angle Cg(I) → Cg(J) vector and normal to plane J. b Symmetry transformations used to generate equivalent atoms: #1 1 − x, 1 − y, 1 − z. Cg(1): C(2)–C(3)–C(17)–C(13)–C(14)–C(15); Cg(2): C(3)–C(4)–C(5)–C(6)–C(16)–C(17); Cg(3): C(6)–C(7)–C(8)–C(9)–C(10)–C(16), Cg(4): C(10)–C(11)–C(12)–C(13)–C(17)–C(16). c Symmetry transformations used to generate equivalent atoms: #1 2 − x, 1 − y, −z. Cg(7): C(51)–C(54)–C(57)–C(60)–C(52)–C(58); Cg(8): C(51)–C(58)–C(53)–C(64)–C(62)–C(63); Cg(9): C(52)–C(56)–C(55)–C(59)–C(53)–C(58); Cg(10): C(53)–C(59)–C(61)–C(66)–C(65)–C(64). | ||||||
| HL | Cg(1) | Cg(2)#1 | 3.938(2) | 0.92(19) | 27.2 | 26.7 |
| Cg(2) | Cg(1)#1 | 3.938(2) | 0.92(19) | 26.7 | 27.2 | |
| Cg(1) | Cg(3)#1 | 3.764(3) | 2.4(2) | 19.3 | 21.6 | |
| Cg(3) | Cg(1)#1 | 3.765(3) | 2.4(2) | 21.6 | 19.3 | |
| Cg(1) | Cg(4)#1 | 3.713(3) | 0.96(19) | 20.1 | 19.2 | |
| Cg(4) | Cg(1)#1 | 3.713(3) | 0.96(19) | 19.2 | 20.1 | |
| Cg(2) | Cg(4)#1 | 3.718(2) | 0.86(19) | 20.5 | 20.4 | |
| Cg(4) | Cg(2)#1 | 3.718(2) | 0.86(19) | 20.4 | 20.5 | |
| [NiL2] | Cg(7) | Cg(8)#1 | 3.9116(10) | 1.03(8) | 26.6 | 27.6 |
| Cg(8) | Cg(7)#1 | 3.9116(10) | 1.03(8) | 27.6 | 26.6 | |
| Cg(7) | Cg(9)#1 | 3.5989(11) | 1.21(8) | 15.5 | 15.5 | |
| Cg(9) | Cg(7)#1 | 3.5989(11) | 1.21(8) | 15.5 | 15.5 | |
| Cg(7) | Cg(10)#1 | 3.8109(10) | 1.37(8) | 25.4 | 24.4 | |
| Cg(10) | Cg(7)#1 | 3.8110(10) | 1.37(8) | 24.4 | 25.4 | |
| Cg(8) | Cg(9)#1 | 3.8358(10) | 0.44(8) | 24.9 | 24.5 | |
| Cg(9) | Cg(8)#1 | 3.8359(10) | 0.44(8) | 24.5 | 24.9 | |
Complex [NiL2] crystallizes in the centrosymmetric structure with the Ni atom positioned on the inversion center. The NiII ion is coordinated in a square-planar fashion with the ligands arranged in a trans-N2S2 configuration (Fig. 2). The four-membered Ni–S–C–N metallocycles are planar. The endocyclic N–Ni–S and Ni–S–C angles fall in the range of 74–77°, while the exocyclic N–Ni–S and Ni–N–P angles are about 106 and 129°, respectively (Table 1). The Ni–N–C angle is ∼99°. While the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P–N, S
P–N, S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N(C) and P–N–C angles in the structure of [NiL2] are almost the same as those in the structure of parent HL, the S
C–N(C) and P–N–C angles in the structure of [NiL2] are almost the same as those in the structure of parent HL, the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N(P) angles are about 12° smaller and the N–C–N ones are about 10° bigger than those in HL (Table 1). The Ni–N and Ni–S distances are about 1.91 and 2.22 Å, respectively. Inspection of bond lengths within the S
C–N(P) angles are about 12° smaller and the N–C–N ones are about 10° bigger than those in HL (Table 1). The Ni–N and Ni–S distances are about 1.91 and 2.22 Å, respectively. Inspection of bond lengths within the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(N)–N–P
C(N)–N–P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S moieties of [NiL2] indicates that the P
S moieties of [NiL2] indicates that the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S, C–N(C) and P–N distances are almost the same as those in the structure of HL, while the C
S, C–N(C) and P–N distances are almost the same as those in the structure of HL, while the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond is elongated and the C–N(P) bond shortened up to about 0.05 Å each (Table 1).
S bond is elongated and the C–N(P) bond shortened up to about 0.05 Å each (Table 1).
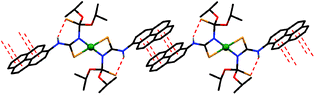 | ||
| Fig. 2 View of the molecules, linked through π⋯π stacking interactions in the structure of [NiL2] (hydrogen atoms not involved in H-bonding are omitted for clarity). | ||
The pyreneNH protons in [NiL2], as in HL, are involved in intramolecular hydrogen bonds of the pyreneN–H⋯S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P type (Scheme 1, Fig. 2 and Table 2). Due to their formation, a flat syn,syn-conformation of the N–C–N–P–S unit is favored (Fig. 2). Molecules of [NiL2] form π⋯π stacking interactions between the pyrene rings (Scheme 1, Fig. 1 and Table 3). These interactions produce 1D polymeric chains similar to those observed in the structure of HL (Scheme 1 and Fig. 1). Notably, the π⋯π stacked 1D polymeric chains are linked to each other through C–H⋯S interactions, formed by the 7-H hydrogen atom of pyrene and the sulfur atom of the thiocarbonyl fragment, and, interestingly, C–H⋯Ni interactions, formed by the 8-H hydrogen atom of pyrene (Fig. 3), yielding 2D sheets. The latter interaction is characterized by the following parameters: d(Ni⋯H) = 2.88 Å and ∠(C–H⋯Ni) = 140°. Three forms of C–H⋯M interactions, namely: hydrogen bonds, agostic and anagostic interactions were reported.55–63 Hydrogen bonds are 3-centre-4-electron interactions with an almost linear geometry. Agostic interactions are 3-centre-2-electron interactions and characterized by the short M⋯H distance (1.8–2.2 Å) and C–H⋯M bond angles (90–130°). Anagostic interactions are largely electrostatic in nature and characterized by the long M⋯H distance (2.3–2.9 Å) and the large C–H⋯M bond angles (110–170°). The observed C–H⋯Ni parameters in the structure of [NiL2] nicely fit those for the anagostic interactions.
P type (Scheme 1, Fig. 2 and Table 2). Due to their formation, a flat syn,syn-conformation of the N–C–N–P–S unit is favored (Fig. 2). Molecules of [NiL2] form π⋯π stacking interactions between the pyrene rings (Scheme 1, Fig. 1 and Table 3). These interactions produce 1D polymeric chains similar to those observed in the structure of HL (Scheme 1 and Fig. 1). Notably, the π⋯π stacked 1D polymeric chains are linked to each other through C–H⋯S interactions, formed by the 7-H hydrogen atom of pyrene and the sulfur atom of the thiocarbonyl fragment, and, interestingly, C–H⋯Ni interactions, formed by the 8-H hydrogen atom of pyrene (Fig. 3), yielding 2D sheets. The latter interaction is characterized by the following parameters: d(Ni⋯H) = 2.88 Å and ∠(C–H⋯Ni) = 140°. Three forms of C–H⋯M interactions, namely: hydrogen bonds, agostic and anagostic interactions were reported.55–63 Hydrogen bonds are 3-centre-4-electron interactions with an almost linear geometry. Agostic interactions are 3-centre-2-electron interactions and characterized by the short M⋯H distance (1.8–2.2 Å) and C–H⋯M bond angles (90–130°). Anagostic interactions are largely electrostatic in nature and characterized by the long M⋯H distance (2.3–2.9 Å) and the large C–H⋯M bond angles (110–170°). The observed C–H⋯Ni parameters in the structure of [NiL2] nicely fit those for the anagostic interactions.
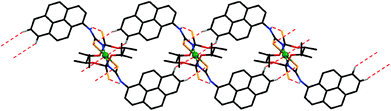 | ||
| Fig. 3 View of the molecules, linked through C–H⋯Ni and C–H⋯S interactions in the structure of [NiL2] (hydrogen atoms not involved in H-bonding and weak interactions are omitted for clarity). | ||
The bulk samples of HL and [NiL2] were studied by means of X-ray powder diffraction analysis (Fig. 4). Experimental X-ray powder patterns are in agreement with the calculated powder patterns obtained from the single crystal X-ray analysis showing that the bulk materials HL and [NiL2] are free from phase impurities.
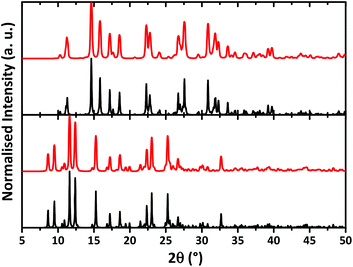 | ||
| Fig. 4 Calculated (black) and experimental (red) X-ray powder diffraction patterns of HL (top) and [NiL2] (bottom). | ||
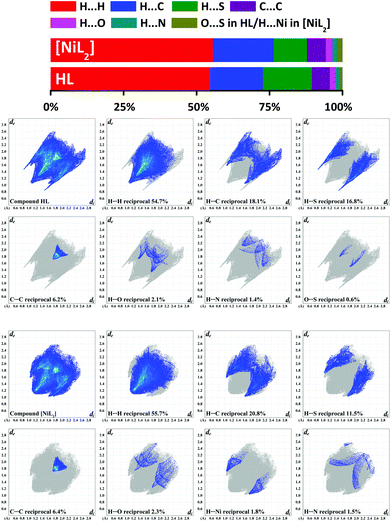
In order to examine the interactions in the crystal structures of HL and [NiL2], the Hirshfeld surface analysis49 and the 2D fingerprint plots50 were obtained using CrystalExplorer 3.1.51
According to the Hirshfeld surface analysis, for both HL and [NiL2], the intermolecular H⋯H contacts, comprising 54.7% and 55.7% (Fig. 5) of the total number of contacts are highly dominant contributors to the crystal packing. The shortest H⋯H contacts are shown in the fingerprint plots as characteristic spikes at de + di ≈ 2.2–2.3 Å (Fig. 5). A subtle feature is evident in the fingerprint plot of HL. There is a distinct splitting of the short H⋯H fingerprint. This splitting occurs when the shortest contact is between three atoms, rather than for a direct two-atom contact.50 Both structures are also dominated by H⋯C contacts, comprising 18.1% and 20.8% for HL and [NiL2], respectively (Fig. 5) of the total Hirshfeld surface areas. These contacts in the corresponding fingerprint plots are shown with the shortest de + di ≈ 2.6–2.7 Å (Fig. 5). It is worth mentioning that the fingerprint plot of HL exhibits a number of points at large de and di, shown as tails at the top right of the plots (Fig. 5). These points, similar to those observed in the fingerprint plot of benzene50 and phenyl-containing compounds,64,65 correspond to regions on the Hirshfeld surface without any close contacts to nuclei in adjacent molecules. The structures are further characterized by a significant proportion of H⋯S contacts, comprising 16.8% in HL and, comparably less, 11.5% in [NiL2] (Fig. 5). These contacts in the fingerprint plot of HL are shown as a pair of sharp spikes with the shortest de + di ≈ 2.4 Å (Fig. 5) and correspond to the (P)N–H⋯S#1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C#1 hydrogen bonds (Fig. 1). The same contacts in the fingerprint plot of [NiL2] are shown as a pair of “horns” with the shortest de + di ≈ 2.8 Å (Fig. 5) and attributed to the intermolecular C–H⋯S interactions, formed by the 7-H hydrogen atom of pyrene and the sulphur atom of the thiocarbonyl fragment (Fig. 3). Also worth mentioning is fact that the contribution to the total Hirshfeld surface area of all molecules of HL and [NiL2] arises from the C⋯C contacts being 6.3% for both structures (Fig. 5). They are shown on the fingerprint plots as blue/pale blue areas on the diagonal at de = di ≈ 1.7–2.0 Å (Fig. 5). These contacts correspond to the presence of the abovementioned π⋯π stacking interactions. Close inspection of other intermolecular contacts also revealed a small proportion of H⋯O (2.1–2.3%) and H⋯N (1.4–1.5%) contacts in HL and [NiL2] (Fig. 5). The structure of HL is also characterized by a negligible proportion of O⋯S (0.6%). Interestingly, the molecular surface of [NiL2] also contains H⋯Ni (1.8%) intermolecular contacts, which in the corresponding fingerprint plot are shown as a pair of broad “horns” with the shortest de + di ≈ 2.8 Å (Fig. 5) and are attributed to the abovementioned intermolecular anagostic bond (Fig. 3).
C#1 hydrogen bonds (Fig. 1). The same contacts in the fingerprint plot of [NiL2] are shown as a pair of “horns” with the shortest de + di ≈ 2.8 Å (Fig. 5) and attributed to the intermolecular C–H⋯S interactions, formed by the 7-H hydrogen atom of pyrene and the sulphur atom of the thiocarbonyl fragment (Fig. 3). Also worth mentioning is fact that the contribution to the total Hirshfeld surface area of all molecules of HL and [NiL2] arises from the C⋯C contacts being 6.3% for both structures (Fig. 5). They are shown on the fingerprint plots as blue/pale blue areas on the diagonal at de = di ≈ 1.7–2.0 Å (Fig. 5). These contacts correspond to the presence of the abovementioned π⋯π stacking interactions. Close inspection of other intermolecular contacts also revealed a small proportion of H⋯O (2.1–2.3%) and H⋯N (1.4–1.5%) contacts in HL and [NiL2] (Fig. 5). The structure of HL is also characterized by a negligible proportion of O⋯S (0.6%). Interestingly, the molecular surface of [NiL2] also contains H⋯Ni (1.8%) intermolecular contacts, which in the corresponding fingerprint plot are shown as a pair of broad “horns” with the shortest de + di ≈ 2.8 Å (Fig. 5) and are attributed to the abovementioned intermolecular anagostic bond (Fig. 3).
We have also determined the enrichment ratios (E)52 of the intermolecular contacts for HL and [NiL2] to study the propensity of two chemical species to be in contact. E, derived from the Hirshfeld surface analysis,49,51 is defined as the ratio between the proportion of actual contacts and the theoretical proportion of random contacts. E is larger than unity for a pair of elements with a higher propensity to form contacts, while pairs which tend to avoid contacts yield an E value lower than unity.
The H⋯H contacts in both structures show enrichment ratios EHH equal to unity, which is commonly the case for compounds with a high hydrogen content on the surface and generate an overwhelming majority (∼55.0%) of the interaction surface (Table 4). For the polar contacts H⋯N, H⋯O and H⋯S, an increased propensity to form is observed (EHN = 1.25–1.40, EHO = 1.05–1.28, EHS = 1.30–1.32). This is due to the comparably lower values of random contacts RHN, RHO and RHS compared to their corresponding proportions on the total Hirshfeld surface area (Table 4). Notably, the C⋯C contacts in both structures are remarkably enriched (ECC = 2.29–2.70), which is also due to a relatively high amount of aromatic, or more generally Csp2 carbons on the total Hirshfeld surface (Table 4). Contrarily, the H⋯C contacts are less favored in the structures of HL and [NiL2] (EHC = 0.80–0.83), showing an increased tendency of the aromatic rings to be engaged in π⋯π stacking rather than C–H⋯π interactions. Remarkably, the molecular surface of [NiL2] is also characterized by highly favored H⋯Ni contacts (EHNi = 1.38), which is caused by both a negligible amount of random contacts RHNi as well as a significantly high value of the SH proportion (Table 4).
| HL | [NiL2] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H | C | N | O | S | H | C | N | O | S | Ni | |
| a Values are obtained from CrystalExplorer 3.1.51 b E were not computed when the “random contacts” were lower than 0.9%, as they are not meaningful.52 | |||||||||||
| Contactsa (C, %) | |||||||||||
| H | 54.7 | — | — | — | — | 55.7 | — | — | — | — | — |
| C | 18.1 | 6.2 | — | — | — | 20.8 | 6.4 | — | — | — | — |
| N | 1.4 | 0.0 | 0.0 | — | — | 1.5 | 0.0 | 0.0 | — | — | — |
| O | 2.1 | 0.0 | 0.0 | 0.0 | — | 2.3 | 0.0 | 0.0 | 0.0 | — | — |
| S | 16.8 | 0.0 | 0.0 | 0.6 | 0.0 | 11.5 | 0.0 | 0.0 | 0.0 | 0.0 | — |
| Ni | — | — | — | — | — | 1.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Surface (S, %) | |||||||||||
| 73.9 | 15.3 | 0.7 | 1.35 | 8.7 | 74.7 | 16.8 | 0.8 | 1.2 | 5.8 | 0.9 | |
| Random contacts (R, %) | |||||||||||
| H | 54.6 | — | — | — | — | 55.8 | — | — | — | — | — |
| C | 22.5 | 2.3 | — | — | — | 25.1 | 2.8 | — | — | — | — |
| N | 1.0 | 0.2 | 0.0 | — | — | 1.2 | 0.3 | 0.0 | — | — | — |
| O | 2.0 | 0.4 | 0.0 | 0.0 | — | 1.8 | 0.4 | 0.0 | 0.0 | — | — |
| S | 12.9 | 2.7 | 0.1 | 0.2 | 0.8 | 8.7 | 1.9 | 0.1 | 0.1 | 0.3 | — |
| Ni | — | — | — | — | — | 1.3 | 0.3 | 0.0 | 0.0 | 0.1 | 0.0 |
| Enrichment (E)b | |||||||||||
| H | 1.00 | — | — | — | — | 1.00 | — | — | — | — | — |
| C | 0.80 | 2.70 | — | — | — | 0.83 | 2.29 | — | — | — | — |
| N | 1.40 | — | — | — | — | 1.25 | — | — | — | — | — |
| O | 1.05 | — | — | — | — | 1.28 | — | — | — | — | — |
| S | 1.30 | 0.0 | — | — | — | 1.32 | 0.0 | — | — | — | — |
| Ni | — | — | — | — | — | 1.38 | — | — | — | — | — |
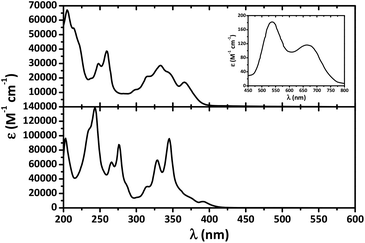
Pyrene is known to display three major highly intense absorption bands with maxima at about 240, 275 and 350 nm,66 while absorption bands for the free NTTUs (X = O, S) are rather high in energy (λabsmax < 250 nm) and have been assigned to intraligand transitions.67–69 The UV-Vis spectrum of HL in CH2Cl2 exhibits intense absorption bands exclusively in the UV region and mainly characteristic of pyrene (Fig. 6).
Complex [NiL2] was obtained as greenish-blue microcrystals and dissolving these crystals in CH2Cl2 leads to greenish-blue solutions. In the UV-Vis absorption spectrum of [NiL2] in CH2Cl2 a number of intense bands in the UV region are discernible (Fig. 6). In the visible region essentially two absorption bands were observed at 538 and 665 nm. From the rather low intensities (183 and 117 M−1 cm−1, respectively) of the two long-wavelength bands they are assigned to ligand field (d–d) transitions. For a square planar 3d8 system three bands [1A1g → 1B1g (dx2−y2 → dxy), 1A1g → 1B3g (dxz → dxy), and 1A1g → 1B1g (dz2 → dxy)], assuming the orbital ordering: [dxy > dx2−y2 > dyz > dxz > dz2], are expected.28–47 However comparison with the spectra of related complexes suggests that the third band is hidden under the tail of the intense intraligand bands.
Emission spectra of both HL and [NiL2] at a concentration of 10−3 M revealed two bands centered at about 400 and 500 nm, corresponding to the pyrene monomer and excimer, respectively (Fig. 7).
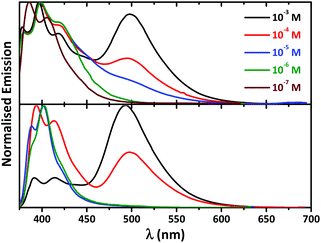 | ||
| Fig. 7 Normalized concentration dependent fluorescence spectra (λexc = 350 nm) of HL (bottom) and [NiL2] (top) in CH2Cl2. | ||
Interestingly, 10-fold dilution resulted in an immediate and dramatic effect, which is more pronounced in the spectrum of [NiL2], on the emission of both compounds. As a result of this dilution the intensity of the excimer bands decreases with the simultaneous increase of the monomer band (Fig. 7). Further 10-fold dilution revealed only well resolved fluorescence of the pyrene monomer and the complete disappearance of the broad band at about 500 nm in the spectrum of HL (Fig. 7). A similar effect was observed in the emission spectrum of [NiL2] at a concentration of 10−5 M, where only traces of the initial excimer band were observed. This band completely disappeared upon further 10-fold dilution (Fig. 7). Spectra of both HL and [NiL2] obtained at even lower concentrations, as low as 10−6 and 10−7 M, respectively, showed almost no further variation (Fig. 7). The disappearance of the broad emission band centered at about 500 nm upon gradual dilution allowed the assignment of this band to intermolecular excimer formation.
It should be noted that the 31P{1H} and 1H NMR spectra of [NiL2] in CDCl3 at concentrations of 10−3 and 10−5 M show the presence of a single set of signals exclusively corresponding to the complex. This testifies to the absence of dissociation of the complex and means that the excimer is formed thanks to an intermolecular aggregation of molecules of [NiL2].
In order to shed some light on the mechanism of [NiL2] aggregation in CH2Cl2 solution, we have performed theoretical studies at the DFT/BLYP-D3/TZP level of theory as implemented in the Amsterdam Density Functional (ADF) package.70,71 At the first stage we have determined the lowest free energy conformation of the [NiL2] dimer in CH2Cl2 solution, approximated by the continuum solvation model COSMO.72 The lowest free energy adduct 1-[NiL2]2 contains the monomers connected through the numerous intermolecular non-covalent interactions: Ni⋯H–C, S⋯H–C and π⋯H–C (Fig. 8). This isomer appeared to be significantly more stable, by ca. 10.4 kcal mol−1, than the corresponding conformation 2-[NiL2]2, where only weak π⋯π stacking is noted (Fig. 8). This result is of particular interest as in many pyrene based systems, π⋯π stacking interactions are of a vital importance in the ground state and excimer formation.2,3 It should be noted, however, that in order to draw detailed conclusions on the fluorescence mechanism it would be necessary to optimize the excited state geometry of a very large system 1-[NiL2]2. Nowadays it is not possible, considering current ab initio, accurate and reliable quantum chemical methods.
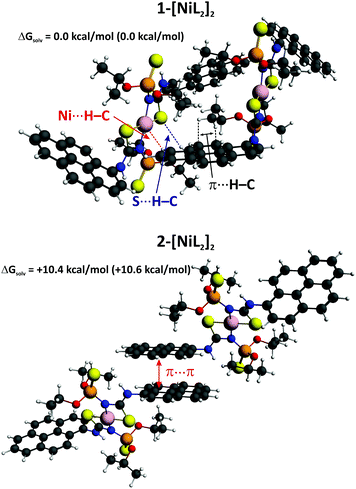 | ||
| Fig. 8 The lowest free energy conformations of the dimer [NiL2]2 in CH2Cl2 solution based on the ADF/BLYP-D3/TZP/COSMO calculations. The relative electronic energies are given in parenthesis. | ||
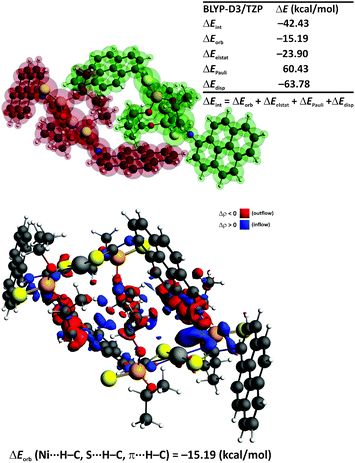
We have further determined the magnitude and the nature of interactions in the preferred aggregate 1-[NiL2]2 by the charge and energy decomposition scheme ETS–NOCV (Fig. 9).73–75 It is found that the overall interaction energy between the monomers is extremely low (ΔEint = −42.43 kcal mol−1). Decomposition of the interaction energy ΔEint into the ETS–NOCV contributions demonstrate that the most crucial stabilizing factor is the dispersion (ΔEdisp = −63.78 kcal mol−1) followed by the electrostatic (ΔEelstat = −23.90 kcal mol−1) and charge transfer (ΔEorb = −15.19 kcal mol−1) components (Fig. 9). An incorporation of the geometry reorganization leads to the overall dimerization energy (ΔEtotal = −39.2 kcal mol−1). This falls in the regime of exceptionally strong non-covalent interactions or even typical dative bonds.76–80 Further inclusion of solvent effects, entropic contribution and internal energy leads to the free energy change ΔGsolv. = −11.6 kcal mol−1, which demonstrates a possibility for the spontaneous aggregation of the [NiL2] units in solution through non-covalent Ni⋯H–C, S⋯H–C and π⋯H–C interactions. This, in turn, might lead to the excimer formation and, consequently, to the fluorescence activity. The reduced density gradient also demonstrates that a significant stability of 1-[NiL2]2 might result from the existence of numerous non-covalent interactions (Fig. S1 in the ESI†).
The overall deformation density, due to the dimerization of the [NiL2] species, reveals the outflow of electron density from the lone electron pair of sulphur onto the empty σ*(C–H) as well as from the occupied σ(C–H) of the pyrene ring onto the nickel center (Fig. 9). In addition, the charge transfer between the π-system of the pyrene fragment and the closely situated C–H bonds is seen. Notably, the engagement of the pyrene C–H bonds as well as the methyl C–H bonds of the iPrO units in the intermolecular interaction between the [NiL2] units in 1-[NiL2]2 is also visible from the calculated 1H NMR spectra based on the GIAO approach as implemented in the ADF program.70,71 Namely, the chemical shifts (with respect to the TMS reference) for these protons are at 10–11 ppm, whereas for the remaining protons (not involved in the intermolecular interaction) the chemical shifts are close to 8 ppm – the average calculated 1H NMR shift for the hydrogen atoms of the pyrene units is 8.6 ppm, which agrees well with the experimental results (δexp 7.85–8.40 ppm). The non-bonded protons of the iPrO groups exhibit a calculated shift of about 1.26 ppm, whereas lower chemical shifts of ca. ∼0.1 ppm are found for the hydrogen atoms involved in the π⋯H–C interactions. As far as the remaining hydrogen atoms are concerned we have also obtained good agreement with the experiment. Particularly, the hydrogen atom involved in the intramolecular S⋯H–N interaction exhibits the averaged shift at 12.5 ppm (δexp 11.19 ppm), whereas the value of 4.93 ppm is noted for the CHO hydrogen atoms (δexp 4.69–5.00 ppm).
We have also performed the ETS–NOCV calculations for the 2-[NiL2]2 isomer and have found that the interaction energy, ΔEint, is only −27.27 kcal mol−1 (Fig. S2 in the ESI†). Hence, the lower stability of 2-[NiL2]2 originates from the presence of relatively weak π⋯π stacking between the pyrene rings as compared with the numerous non-covalent Ni⋯H–C, S⋯H–C and π⋯H–C interactions identified in the dimeric adduct 1-[NiL2]2 (Fig. 9 and S2 in the ESI†).
In order to gain some knowledge on the electronic transitions in [NiL2], we have performed the TD-DFT based study for the aggregate 1-[NiL2]2 by means of the Gaussian 09 rev. E01 package.84 We have tested various exchange correlation functionals (XC). It appears that only long-range corrected XC correctly identify the main absorption region of 200–400 nm (Fig. S3 in the ESI†). According to the PCM/LC-BLYP results, the two most intense absorption peaks are located at 224 and 313 nm (Fig. 10). The molecular orbitals demonstrate that the transitions occur mostly within the pyrene rings, π → π* (Fig. 10). Notably, this UV region also exhibits a number of other less intense bands (Table S1 in the ESI†), which constitute the overall shape of the absorption spectrum (Fig. 10). Furthermore, we have also located the two transitions in the visible region at 540 and 545 nm, however, their oscillator strengths appeared to be almost zero. The presence of numerous intense bands in the UV region is in qualitative agreement with the experimental spectrum (Fig. 6).
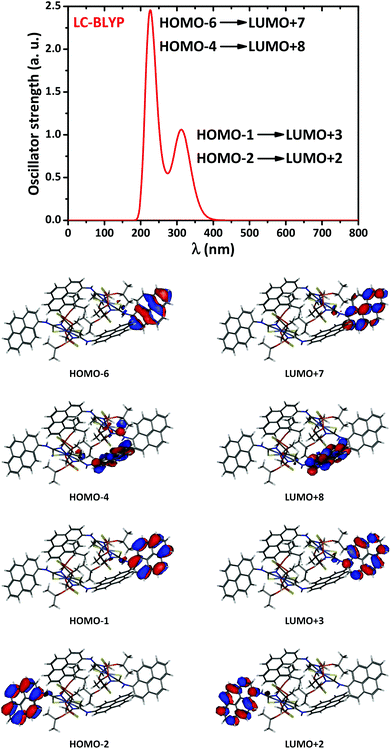 | ||
| Fig. 10 The simulated TD-DFT/LC/BLYP absorption spectrum of 1-[NiL2]2 in CH2Cl2 solution (top), and the contours of molecular orbitals (0.03 a.u.) involved in the dominant transitions (bottom). | ||
Conclusions
In summary, we have synthesized a N-thiophosphorylated thiourea (1-pyrene)NHC(S)NHP(S)(OiPr)2 (HL) and its homoleptic NiII complex ([NiL2]). Single crystal X-ray diffraction studies showed that the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N–P backbone has an E conformation, while the S
C–N–P backbone has an E conformation, while the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P–N–C exhibits a Z conformation in the structure of HL. The crystal structure of HL contains a linear intramolecular hydrogen bond of the pyreneN–H⋯S
P–N–C exhibits a Z conformation in the structure of HL. The crystal structure of HL contains a linear intramolecular hydrogen bond of the pyreneN–H⋯S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P type, and is additionally stabilized by intermolecular hydrogen bonds of the (P)N–H⋯S#1
P type, and is additionally stabilized by intermolecular hydrogen bonds of the (P)N–H⋯S#1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C#1 type. As a result of this kind of intermolecular interaction, centrosymmetric R22(8) dimers are formed. Additionally, the centrosymmetric dimers of HL form π⋯π stacking interactions between the pyrene rings, yielding 1D polymeric chains. According to single crystal X-ray diffraction, complex [NiL2] exhibits a centrosymmetric homoleptic structure, where a NiII ion is coordinated in a square-planar fashion with the ligands arranged in a trans-N2S2 configuration. The pyreneNH protons in [NiL2] are involved in intramolecular hydrogen bonds of the pyreneN–H⋯S
C#1 type. As a result of this kind of intermolecular interaction, centrosymmetric R22(8) dimers are formed. Additionally, the centrosymmetric dimers of HL form π⋯π stacking interactions between the pyrene rings, yielding 1D polymeric chains. According to single crystal X-ray diffraction, complex [NiL2] exhibits a centrosymmetric homoleptic structure, where a NiII ion is coordinated in a square-planar fashion with the ligands arranged in a trans-N2S2 configuration. The pyreneNH protons in [NiL2] are involved in intramolecular hydrogen bonds of the pyreneN–H⋯S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P type. Molecules of [NiL2] form π⋯π stacking interactions between the pyrene rings, yielding 1D polymeric chains similar to those observed in the structure of HL. Furthermore, these π⋯π stacked 1D polymeric chains are linked to each other through C–H⋯S and anagostic C–H⋯Ni interactions, yielding 2D sheets.
P type. Molecules of [NiL2] form π⋯π stacking interactions between the pyrene rings, yielding 1D polymeric chains similar to those observed in the structure of HL. Furthermore, these π⋯π stacked 1D polymeric chains are linked to each other through C–H⋯S and anagostic C–H⋯Ni interactions, yielding 2D sheets.
Hirshfeld surface analysis showed that the structures of both HL and [NiL2] are highly dominated by H⋯H, H⋯C, H⋯S and C⋯C contacts and also characterized by H⋯O and H⋯N contacts. The molecular surfaces of HL and [NiL2] also contain O⋯S and H⋯Ni contacts, respectively.
Both HL and [NiL2] were found to be emissive in CH2Cl2 solution, which is due to the concentration dependent emission of the pyrene monomer and excimer. It was established that the latter fluorescence is due to the intermolecular excimer formation. DFT calculations have demonstrated, in line with experimental findings that the monomers of [NiL2] can spontaneously aggregate in solution through the numerous, dispersion dominated, intermolecular non-covalent interactions, i.e. Ni⋯H–C, S⋯H–C and π⋯H–C. Therefore, such interactions might be crucial for the concentration dependent fluorescence activity of the reported systems. TD-DFT simulations allowed us to conclude that the photophysical activity occurs predominantly within the molecular orbitals located at the pyrene rings (π → π*).
Experimental
Physical measurements
Infrared spectra were recorded with a Thermo Nicolet 380 FT-IR spectrometer in the range 400–3600 cm−1. NMR spectra in CDCl3 were obtained on a Bruker Avance 300 MHz spectrometer at 25 °C. 1H and 31P{1H} NMR spectra were recorded at 299.948, and 121.420 MHz, respectively. Chemical shifts are reported with reference to SiMe4 (1H) and 85% H3PO4 (31P{1H}). UV-Vis and fluorescence spectra in CH2Cl2 were measured on a Lambda-35 spectrometer and a Varian Cary Eclipse fluorescence spectrophotometer, respectively. Elemental analyses were performed on a Thermoquest Flash EA 1112 Analyzer from CE Instruments.Hirshfeld surface analysis
The Hirshfeld molecular surfaces49 and their associated 2D fingerprint plots50 were generated using the CrystalExplorer 3.1 software51 on the basis of crystal structures. The dnorm (normalized contact distance) surface and the breakdown of the 2D fingerprint plots were used for decoding and quantifying the intermolecular interactions in the crystal lattice. The dnorm is a symmetric function of distances to the surface from the nuclei inside (di) and outside (de) the Hirshfeld surface, relative to their respective van der Waals radii. 2D fingerprint plots were generated using di and de in the translated 0.4–3.0 Å range and including reciprocal contacts as a pair of coordinates in 2D histograms. A color gradient in the fingerprint plots ranging from blue to red is used to visualize the proportional contribution of contact pairs in the global surface.Enrichment ratio
The enrichment ratio (E)52 of a pair of elements (X,Y) is the ratio between the proportion of actual contacts in the crystal and the theoretical proportion of random contacts. E is larger than unity for pairs of elements which have a high propensity to form contacts in crystals, while pairs which tend to avoid contacts with each other yield an E value lower than unity. E values are calculated from the percentage of contacts, which, in turn, are given by the CrystalExplorer 3.1 software,51 between one type or two types of chemical elements in a crystal packing.DFT calculations
We have performed the DFT calculations based on the BLYP-D3/TZP as implemented in the ADF package70,71 as well as by the Gaussian 09 rev. E01 program.84 The COSMO solvent model,72 as implemented in the ADF program, was applied for conformational analyses. In addition, the TD-DFT calculations have been done by means of Gaussian 09 rev. E01 using long-range corrected functionals (i.e. CAM-B3LYP, wB97XD, LC-BLYP, LC-BP, LC-PBE, LC-wPBE) in combination with the cc-pVTZ basis set for nickel and cc-pVDZ for the remaining atoms. The solvation effects within the TD-DFT calculations are accounted for via the polarizable continuum model.85ETS–NOCV bonding analysis
Natural Orbitals for Chemical Valence (NOCV) are the eigenvectors that diagonalize the deformation density matrix.73–75 It was shown that the natural orbitals for chemical valence pairs (ψ−k,ψk) decompose the differential density Δρ into NOCV-contributions (Δρk):where νk and M stand for the NOCV eigenvalues and the number of basis functions, respectively. Visual inspection of deformation density plots (Δρk) helps to attribute the symmetry and direction of the charge flow. In addition, these pictures are enriched by providing the energetic estimations, ΔEorb(k), for each Δρk within the ETS–NOCV scheme.73–75 The exact formula, which links the ETS and NOCV methods, will be given in the next paragraph, after we briefly present the basic concept of the ETS scheme. In this method the total bonding energy ΔEint between interacting fragments, exhibiting geometry as in the combined complex, is divided into three components: ΔEint = ΔEelstat + ΔEPauli + ΔEorb. The first term, ΔEelstat, corresponds to the classical electrostatic interaction between the promoted fragments as they are brought to their positions in the final complex. The second term, ΔEPauli, accounts for the repulsive Pauli interaction between occupied orbitals on the two fragments in the combined molecule. Finally, the last stabilizing term, ΔEorb, represents interactions between the occupied molecular orbitals of one fragment with the unoccupied molecular orbitals of the other fragment as well as the mixing of occupied and virtual orbitals within the same fragment (inner-fragment polarization). Importantly, an inclusion of the geometry distortion term (ΔEdist) provides the overall bonding energy according to the formula: ΔEtotal = ΔEint + ΔEdist (−ΔEtotal is the bond dissociation energy). The orbital interaction contribution may be further linked to the electronic bonding effect arising from the formation of a chemical bond. In the combined ETS–NOCV scheme73–75 the orbital interaction term, ΔEorb, is expressed in terms of NOCV's eigenvalues (vk) as:
where
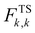 are diagonal Kohn–Sham matrix elements defined over NOCV with respect to the transition state (TS) density at the midpoint between the density of the molecule and the sum of fragment densities. The above components ΔEorb(k) provide the energetic estimation of Δρk that may be related to the importance of a particular electron flow channel for the bonding between the considered molecular fragments. ETS–NOCV analysis was performed based on the Amsterdam Density Functional (ADF) package,70,71 in which this scheme was implemented. We have applied predominantly the BLYP-D3/TZP computational details as it has been shown that they provide accurate results on non-covalent interactions.81–83
are diagonal Kohn–Sham matrix elements defined over NOCV with respect to the transition state (TS) density at the midpoint between the density of the molecule and the sum of fragment densities. The above components ΔEorb(k) provide the energetic estimation of Δρk that may be related to the importance of a particular electron flow channel for the bonding between the considered molecular fragments. ETS–NOCV analysis was performed based on the Amsterdam Density Functional (ADF) package,70,71 in which this scheme was implemented. We have applied predominantly the BLYP-D3/TZP computational details as it has been shown that they provide accurate results on non-covalent interactions.81–83
Non-covalent index (NCI) technique
It has been shown that the reduced density gradient,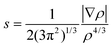 , appeared to be a useful quantity86,87 for the description of non-covalent covalent interactions. In order to obtain information about the type of bonding, plots of the reduced density gradient s vs. molecular density ρ are examined. When a weak inter- or intramolecular interaction is present, a characteristic spike, lying at low values of both density ρ and reduced density gradient s, exists. To distinguish between attractive and repulsive interactions, the eigenvalues (λi) of the second derivative of density (Hessian, ∇2ρ) are used, ∇2ρ = λ1 + λ2 + λ3. Namely, bonding interactions are characterized by λ2 < 0, whereas λ2 > 0 indicates that atoms are in a non-bonded contact. Therefore, within the NCI technique, one can draw information about non-covalent interactions from the plots of sign(λ2)ρ vs. s. In such plots the low gradient spike, an indicator of the stabilizing interaction, is located within the region of negative values of density. In contrast, the repulsive interaction is characterized by positive values of sign(λ2)ρ. The contour of s, colored by the value of sign(λ2)ρ, can also be plotted. This gives a pictorial representation of non-covalent interactions.
, appeared to be a useful quantity86,87 for the description of non-covalent covalent interactions. In order to obtain information about the type of bonding, plots of the reduced density gradient s vs. molecular density ρ are examined. When a weak inter- or intramolecular interaction is present, a characteristic spike, lying at low values of both density ρ and reduced density gradient s, exists. To distinguish between attractive and repulsive interactions, the eigenvalues (λi) of the second derivative of density (Hessian, ∇2ρ) are used, ∇2ρ = λ1 + λ2 + λ3. Namely, bonding interactions are characterized by λ2 < 0, whereas λ2 > 0 indicates that atoms are in a non-bonded contact. Therefore, within the NCI technique, one can draw information about non-covalent interactions from the plots of sign(λ2)ρ vs. s. In such plots the low gradient spike, an indicator of the stabilizing interaction, is located within the region of negative values of density. In contrast, the repulsive interaction is characterized by positive values of sign(λ2)ρ. The contour of s, colored by the value of sign(λ2)ρ, can also be plotted. This gives a pictorial representation of non-covalent interactions.
Synthesis of HL
A solution of 1-aminopyrene (0.217 g, 1 mmol) in CH2Cl2 (15 mL) was treated under vigorous stirring with a solution of (iPrO)2P(S)NCS (0.263 g, 1.1 mmol) in the same solvent. The mixture was stirred for 1 h. The solvent was removed under vacuum, and the product was purified by recrystallisation from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (v/v) mixture of CH2Cl2 and n-hexane. Yield: 0.415 g (91%). IR, ν: 644 (P
5 (v/v) mixture of CH2Cl2 and n-hexane. Yield: 0.415 g (91%). IR, ν: 644 (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S), 982 (POC), 1528 (SCN), 3081 (PNH), 3234 (pyreneNH) cm−1. 1H NMR, δ: 1.46 (d, 3JH,H = 6.2 Hz, 6H, CH3), 1.52 (d, 3JH,H = 6.2 Hz, 6H, CH3), 5.00 (d sept, 3JPOCH = 10.3 Hz, 3JH,H = 6.2 Hz, 2H, OCH), 7.36 (br. s, 1H, PNH), 7.96–8.40 (m, 9H, pyrene), 10.12 (s, 1H, pyreneNH) ppm. 31P{1H} NMR, δ: 52.8 ppm. Anal. Calc. for C23H25N2O2PS2 (456.56): C 60.51, H 5.52, N 6.14. Found: C 60.44, H 5.49, N 6.17%.
S), 982 (POC), 1528 (SCN), 3081 (PNH), 3234 (pyreneNH) cm−1. 1H NMR, δ: 1.46 (d, 3JH,H = 6.2 Hz, 6H, CH3), 1.52 (d, 3JH,H = 6.2 Hz, 6H, CH3), 5.00 (d sept, 3JPOCH = 10.3 Hz, 3JH,H = 6.2 Hz, 2H, OCH), 7.36 (br. s, 1H, PNH), 7.96–8.40 (m, 9H, pyrene), 10.12 (s, 1H, pyreneNH) ppm. 31P{1H} NMR, δ: 52.8 ppm. Anal. Calc. for C23H25N2O2PS2 (456.56): C 60.51, H 5.52, N 6.14. Found: C 60.44, H 5.49, N 6.17%.
Synthesis of [NiL2]
A suspension of HL (0.228 g, 0.5 mmol) in MeOH (10 mL) was mixed with a MeOH (10 mL) solution of KOH (0.034 g, 0.6 mmol). An aqueous (10 mL) solution of NiCl2 (0.039 g, 0.3 mmol) was added dropwise under vigorous stirring to the resulting potassium salt. The mixture was stirred at room temperature for a further 2 h and left overnight. The resulting complex was extracted with CH2Cl2, washed with water and dried with anhydrous MgSO4. The solvent was removed under vacuum, and the product was purified by recrystallisation from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (v/v) mixture of CH2Cl2 and n-hexane. Yield: 0.201 g (83%). IR, ν: 631 (P
3 (v/v) mixture of CH2Cl2 and n-hexane. Yield: 0.201 g (83%). IR, ν: 631 (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S), 993 (POC), 1531 (SCN), 3238 (pyreneNH) cm−1. 1H NMR, δ: 1.31 (d, 3JH,H = 6.0 Hz, 12H, CH3), 1.69 (d, 3JH,H = 6.0 Hz, 12H, CH3), 4.69–4.88 (m, 4H, OCH), 7.85–8.32 (m, 18H, pyrene), 11.19 (s, 1H, pyreneNH) ppm. 31P{1H} NMR, δ: 53.6 ppm. Anal. Calc. for C46H48N4NiO4P2S4 (969.79): C 56.97, H 4.99, N 5.78. Found: C 57.08, H 4.92, N 5.74%.
S), 993 (POC), 1531 (SCN), 3238 (pyreneNH) cm−1. 1H NMR, δ: 1.31 (d, 3JH,H = 6.0 Hz, 12H, CH3), 1.69 (d, 3JH,H = 6.0 Hz, 12H, CH3), 4.69–4.88 (m, 4H, OCH), 7.85–8.32 (m, 18H, pyrene), 11.19 (s, 1H, pyreneNH) ppm. 31P{1H} NMR, δ: 53.6 ppm. Anal. Calc. for C46H48N4NiO4P2S4 (969.79): C 56.97, H 4.99, N 5.78. Found: C 57.08, H 4.92, N 5.74%.
X-Ray powder diffraction
X-Ray powder diffraction for bulk samples was carried out on a MAR345 diffractometer equipped with a rotating anode (Mo Kα radiation) and a XENOCS focusing mirror.Single-crystal X-ray diffraction study
The X-ray data for HL were collected at 173(2) K on a STOE IPDS-II diffractometer with graphite-monochromatised Mo Kα radiation generated by a fine-focus X-ray tube operated at 50 kV and 40 mA. The reflections of the images were indexed, integrated and scaled using the X-Area data reduction package.88 Data were corrected for absorption using the PLATON program.89 The structure was solved by direct methods using the SHELXS-97 program90 and refined first isotropically and then anisotropically using SHELXL-97.90 Hydrogen atoms were revealed from Δρ maps and those bonded to C were refined using appropriate riding models. H atoms bonded to N were freely refined.The X-ray data for [NiL2] were collected at 100(2) K on the Swiss-Norwegian beamline BM1A at the European Synchrotron Radiation Facility (Grenoble, France) with a PILATUS 2M pixel detector and λ = 0.68239 Å. The data were integrated with the CrysAlis(Pro) software.22 The implemented empirical absorption correction was applied. The structures of 1 and 2 were solved by SHELXS-9790 and refined by full-matrix least squares on |F2|, using SHELXL-97.90 Non-hydrogen atoms were anisotropically refined and the hydrogen atoms were placed on the calculated positions in riding mode with temperature factors fixed at 1.2 times Ueq. of the parent atoms. Figures were generated using the program Mercury.91
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 10.244(2), b = 10.4376(19), c = 11.148(2) Å, α = 104.961(15), β = 90.250(16), γ = 103.255(15)°, V = 1118.3(4) Å3, Z = 2, ρ = 1.356 g cm−3, μ(Mo Kα) = 0.332 mm−1, reflections: 10
, a = 10.244(2), b = 10.4376(19), c = 11.148(2) Å, α = 104.961(15), β = 90.250(16), γ = 103.255(15)°, V = 1118.3(4) Å3, Z = 2, ρ = 1.356 g cm−3, μ(Mo Kα) = 0.332 mm−1, reflections: 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 227 collected, 3924 unique, Rint = 0.079, R1(all) = 0.1057, wR2(all) = 0.1223.
227 collected, 3924 unique, Rint = 0.079, R1(all) = 0.1057, wR2(all) = 0.1223.
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 10.2046(6), b = 10.7201(7), c = 10.8463(5) Å, α = 90.022(5), β = 108.105(5), γ = 105.302(5)°, V = 1083.39(12) Å3, Z = 1, ρ = 1.486 g cm−3, μ(λ = 0.68239) = 0.678 mm−1, reflections: 9801 collected, 5086 unique, Rint = 0.016, R1(all) = 0.0361, wR2(all) = 0.0840.
, a = 10.2046(6), b = 10.7201(7), c = 10.8463(5) Å, α = 90.022(5), β = 108.105(5), γ = 105.302(5)°, V = 1083.39(12) Å3, Z = 1, ρ = 1.486 g cm−3, μ(λ = 0.68239) = 0.678 mm−1, reflections: 9801 collected, 5086 unique, Rint = 0.016, R1(all) = 0.0361, wR2(all) = 0.0840.
Acknowledgements
D. A. Safin and M. G. Babashkina thank WBI (Belgium) for post-doctoral positions. We acknowledge the Swiss-Norwegian beamline for the beamtime allocation at the European Synchrotron Radiation Facility (Grenoble, France). DFT calculations were performed using the PL-Grid Infrastructure and resources provided by the ACC Cyfronet AGH (Cracow, Poland).References
- A. Laurent, Ann. Chim. Phys., 1837, 66, 136 Search PubMed.
- F. M. Winnik, Chem. Rev., 1993, 93, 587 CrossRef CAS.
- T. M. Figueira-Duarte and K. Müllen, Chem. Rev., 2011, 111, 7260 CrossRef CAS PubMed.
- R. Weitzenböck, Mh. Chem., 1913, 34, 193 Search PubMed.
- R. D. Welham, J. Soc. Dyers Color, 1963, 79, 98 CrossRef CAS.
- T. Förster and K. Kasper, Z. Elektrochem., 1955, 59, 976 Search PubMed.
- J. B. Birks, Photophysics of Aromatic Molecules, Wiley-Interscience, London, 1970 Search PubMed.
- C. Broan, Chem. Commun., 1996, 699 RSC.
- F. D. Lewis, Y. Zhang and R. L. Letsinger, J. Am. Chem. Soc., 1997, 119, 5451 CrossRef CAS.
- J. Lou, T. A. Hatton and P. E. Laibinis, Anal. Chem., 1997, 69, 1262 CrossRef CAS.
- A. T. Reis e Sousa, E. M. S. Castanheira, A. Fedorov and J. M. G. Martinho, J. Phys. Chem. A, 1998, 102, 6406 CrossRef.
- Y. Suzuki, T. Morozumi, H. Nakamura, M. Shimomura, T. Hayashita and R. A. Bartsch, J. Phys. Chem. B, 1998, 102, 7910 CrossRef CAS.
- S. Das, A. Sahana, A. Banerjee, S. Lohar, D. A. Safin, M. G. Babashkina, M. Bolte, Y. Garcia, I. Hauli, S. K. Mukhopadhyay and D. Das, Dalton Trans., 2013, 42, 4757 RSC.
- S. Lohar, D. A. Safin, A. Sengupta, A. Chattopadhyay, J. S. Matalobos, M. G. Babashkina, K. Robeyns, M. P. Mitoraj, P. Kubisiak, Y. Garcia and D. Das, Chem. Commun., 2015, 51, 8536 RSC.
- R. Rösler, C. Silvestru, G. Espinosa-Perez, I. Haiduc and R. Cea-Olivares, Inorg. Chim. Acta, 1996, 241, 47 CrossRef.
- A. Silvestru, D. Bilc, R. Rosler, J. E. Drake and I. Haiduc, Inorg. Chim. Acta, 2000, 305, 106 CrossRef CAS.
- E. V. Garcia-Baez, M. J. Rozales-Hoz, H. Nöth, I. Haiduc and C. Silvestru, Inorg. Chem. Commun., 2000, 3, 173 CrossRef CAS.
- S. D. Robertson, T. Chivers, J. Akhtar, M. Afzaal and P. O'Brien, Dalton Trans., 2008, 7004 RSC.
- I. Haiduc, J. Organomet. Chem., 2001, 623, 29 CrossRef CAS.
- A. Panneerselvam, M. A. Malik, M. Afzaal, P. O'Brien and M. Helliwell, J. Am. Chem. Soc., 2008, 130, 2420 CrossRef CAS PubMed.
- N. Levesanos, S. D. Robertson, D. Maganas, C. P. Raptopoulou, A. Terzis, P. Kyritsis and T. Chivers, Inorg. Chem., 2008, 47, 2949 CrossRef CAS PubMed.
- D. Maganas, A. Grigoropoulos, S. S. Staniland, S. D. Chatziefthimiou, A. Harrison, N. Robertson, P. Kyritsis and F. Neese, Inorg. Chem., 2010, 49, 5079 CrossRef CAS PubMed.
- E. Ferentinos, D. Maganas, C. P. Raptopoulou, A. Terzis, V. Psycharis, N. Robertson and P. Kyritsis, Dalton Trans., 2011, 40, 169 RSC.
- K. R. Koch, O. Hallale, S. A. Bourne, J. Miller and J. Bacsa, J. Mol. Struct., 2001, 561, 185 CrossRef CAS.
- S. A. Bourne, O. Hallale and K. R. Koch, Cryst. Growth Des., 2005, 5, 307 CAS.
- O. Hallale, S. A. Bourne and K. R. Koch, New J. Chem., 2005, 29, 1416 RSC.
- O. Hallale, S. A. Bourne and K. R. Koch, CrystEngComm, 2005, 7, 161 RSC.
- F. D. Sokolov, S. V. Baranov, D. A. Safin, F. E. Hahn, M. Kubiak, T. Pape, M. G. Babashkina, N. G. Zabirov, J. Galezowska, H. Kozlowski and R. A. Cherkasov, New J. Chem., 2007, 31, 1661 RSC.
- F. D. Sokolov, V. V. Brusko, D. A. Safin, R. A. Cherkasov and N. G. Zabirov, Coordination Diversity of N-Phosphorylated Amides and Ureas Towards VIIIB Group Cations, in Transition Metal Chemistry: New Research, ed. B. Varga and L. Kis, Nova Science Publishers Inc., Hauppauge NY, USA, 2008, p. 101 Search PubMed.
- D. A. Safin, F. D. Sokolov, Ł. Szyrwiel, M. G. Babashkina, T. R. Gimadiev, F. E. Hahn, H. Kozlowski, D. B. Krivolapov and I. A. Litvinov, Polyhedron, 2008, 27, 2271 CrossRef CAS.
- D. A. Safin, F. D. Sokolov, T. R. Gimadiev, V. V. Brusko, M. G. Babashkina, D. R. Chubukaeva, D. B. Krivolapov and I. A. Litvinov, Z. Anorg. Allg. Chem., 2008, 634, 967 CrossRef CAS.
- D. A. Safin, M. G. Babashkina, A. Klein, F. D. Sokolov, S. V. Baranov, T. Pape, F. E. Hahn and D. B. Krivolapov, New J. Chem., 2009, 33, 2443 RSC.
- M. G. Babashkina, D. A. Safin, M. Bolte and A. Klein, Inorg. Chem. Commun., 2009, 12, 678 CrossRef CAS.
- D. A. Safin, M. Bolte, M. G. Babashkina and H. Kozlowski, Polyhedron, 2010, 29, 488 CrossRef CAS.
- D. A. Safin, M. G. Babashkina, M. Bolte and A. Klein, Inorg. Chim. Acta, 2011, 365, 32 CrossRef CAS.
- M. G. Babashkina, D. A. Safin, M. Bolte, M. Srebro, M. Mitoraj, A. Uthe, A. Klein and M. Köckerling, Dalton Trans., 2011, 40, 3142 RSC.
- D. A. Safin, M. G. Babashkina, M. Bolte and F. E. Hahn, Dalton Trans., 2011, 40, 4806 RSC.
- M. G. Babashkina, D. A. Safin, M. Srebro, P. Kubisiak, M. P. Mitoraj, M. Bolte and Y. Garcia, CrystEngComm, 2011, 13, 5321 RSC.
- M. G. Babashkina, D. A. Safin, K. Robeyns and Y. Garcia, Dalton Trans., 2012, 41, 1451 RSC.
- M. G. Babashkina, D. A. Safin and Y. Garcia, Dalton Trans., 2012, 41, 2234 RSC.
- M. G. Babashkina, D. A. Safin, M. Srebro, P. Kubisiak, M. P. Mitoraj, M. Bolte and Y. Garcia, CrystEngComm, 2012, 14, 370 RSC.
- M. G. Babashkina, D. A. Safin, M. Srebro, P. Kubisiak, M. P. Mitoraj, M. Bolte and Y. Garcia, Eur. J. Inorg. Chem., 2013, 545 CrossRef CAS.
- D. A. Safin, M. G. Babashkina, P. Kubisiak, M. P. Mitoraj, K. Robeyns, E. Goovaerts and Y. Garcia, Dalton Trans., 2013, 42, 5252 RSC.
- D. A. Safin, M. G. Babashkina, K. Robeyns, M. P. Mitoraj, P. Kubisiak, M. Brela and Y. Garcia, CrystEngComm, 2013, 15, 7845 RSC.
- D. A. Safin, M. G. Babashkina, K. Robeyns, M. Rouzières, R. Clérac and Y. Garcia, Dalton Trans., 2013, 42, 16470 RSC.
- M. G. Babashkina, D. A. Safin, K. Robeyns and Y. Garcia, Eur. J. Inorg. Chem., 2015, 1160 CrossRef CAS.
- D. A. Safin, M. G. Babashkina, K. Robeyns, M. P. Mitoraj, P. Kubisiak and Y. Garcia, Chem. – Eur. J., 2015, 21, 16679 CrossRef CAS PubMed.
- D. M. Kolpashchikov, Chem. Rev., 2010, 110, 4709 CrossRef CAS PubMed.
- M. A. Spackman and D. Jayatilaka, CrystEngComm, 2009, 11, 19 RSC.
- M. A. Spackman and J. J. McKinnon, CrystEngComm, 2002, 4, 378 RSC.
- S. K. Wolff, D. J. Grimwood, J. J. McKinnon, M. J. Turner, D. Jayatilaka and M. A. Spackman, CrystalExplorer 3.1, University of Western Australia, 2012 Search PubMed.
- C. Jelsch, K. Ejsmont and L. Huder, IUCrJ, 2014, 1, 119 CAS.
- E. G. Yarkova, N. R. Safiullina, I. G. Chistyakova, N. G. Zabirov, F. M. Shamsevaleev and R. A. Cherkasov, Zh. Obshch. Khim., 1990, 60, 1790 Search PubMed.
- N. G. Zabirov, V. N. Soloviev, F. M. Shamsevaleev, R. A. Cherkasov, A. N. Chechlov, A. G. Tsyfarkin and I. V. Martynov, Russ. J. Gen. Chem., 1991, 61, 597 Search PubMed.
- W. Yao, O. Eisenstein and R. H. Crabtree, Inorg. Chim. Acta, 1997, 254, 105 CrossRef CAS.
- Y. Zhang, J. C. Lewis, R. G. Bergman, J. A. Ellman and E. Oldfield, Organometallics, 2006, 25, 3515 CrossRef CAS.
- M. Brookhart, M. L. H. Green and G. Parkin, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 6908 CrossRef CAS PubMed.
- H. V. Huynh, L. R. Wong and P. S. Ng, Organometallics, 2008, 27, 2231 CrossRef CAS.
- J. Saßmannshausen, Dalton Trans., 2012, 41, 1919 RSC.
- K. A. Siddiqui and E. R. T. Tiekink, Chem. Commun., 2013, 49, 8501 RSC.
- S. Schöler, M. H. Wahl, N. I. C. Wurster, A. Puls, C. Hättig and G. Dyker, Chem. Commun., 2014, 50, 5909 RSC.
- M. G. D. Holaday, G. Tarafdar, A. Kumar, M. L. P. Reddy and A. Srinivasan, Dalton Trans., 2014, 43, 7699 RSC.
- W. Scherer, A. C. Dunbar, J. E. Barquera-Lozada, D. Schmitz, G. Eickerling, D. Kratzert, D. Stalke, A. Lanza, P. Macchi, N. P. M. Casati, J. Ebad-Allah and C. Kuntscher, Angew. Chem., Int. Ed., 2015, 54, 2505 CrossRef CAS PubMed.
- D. A. Safin, M. P. Mitoraj, K. Robeyns, Y. Filinchuk and C. M. L. Vande Velde, Dalton Trans., 2015, 44, 16824 RSC.
- M. G. Babashkina, K. Robeyns, Y. Filinchuk and D. A. Safin, New J. Chem., 2016, 40, 1230 RSC.
- D. A. van Dyke, B. A. Pryor, P. G. Smith and M. R. Topp, J. Chem. Educ., 1998, 75, 615 CrossRef CAS.
- D. A. Safin, M. G. Babashkina, M. Bolte, T. Pape, F. E. Hahn, M. L. Verizhnikov, A. R. Bashirov and A. Klein, Dalton Trans., 2010, 39, 11577 RSC.
- D. A. Safin, F. D. Sokolov, H. Nöth, M. G. Babashkina, T. R. Gimadiev, J. Galezowska and H. Kozlowski, Polyhedron, 2008, 27, 2022 CrossRef CAS.
- D. A. Safin, A. Klein, M. G. Babashkina, H. Nöth, D. B. Krivolapov, I. A. Litvinov and H. Kozlowski, Polyhedron, 2009, 28, 1504 CrossRef CAS.
- G. te Velde, F. M. Bickelhaupt, E. J. Baerends, C. Fonseca Guerra, S. J. A. van Gisbergen, J. G. Snijders and T. Ziegler, J. Comput. Chem., 2001, 22, 931 CrossRef CAS and references therein.
- E. J. Baerends, J. Autschbach, D. Bashford, A. Bérces, F. M. Bickelhaupt, C. Bo, P. M. Boerrigter, L. Cavallo, D. P. Chong, L. Deng, R. M. Dickson, D. E. Ellis, M. van Faassen, L. Fan, T. H. Fischer, C. Fonseca Guerra, A. Ghysels, A. Giammona, S. J. A. van Gisbergen, A. W. Götz, J. A. Groeneveld, O. V. Gritsenko, M. Grüning, F. E. Harris, P. van den Hoek, C. R. Jacob, H. Jacobsen, L. Jensen, G. van Kessel, F. Kootstra, M. V. Krykunov, E. van Lenthe, D. A. McCormack, A. Michalak, M. Mitoraj, J. Neugebauer, V. P. Nicu, L. Noodleman, V. P. Osinga, S. Patchkovskii, P. H. T. Philipsen, D. Post, C. C. Pye, W. Ravenek, J. I. Rodríguez, P. Ros, P. R. T. Schipper, G. Schreckenbach, M. Seth, J. G. Snijders, M. Solà, M. Swart, D. Swerhone, G. te Velde, P. Vernooijs, L. Versluis, L. Visscher, O. Visser, F. Wang, T. A. Wesolowski, E. M. van Wezenbeek, G. Wiesenekker, S. K. Wolff, T. K. Woo, A. L. Yakovlev and T. Ziegler, ADF2012.01, Theoretical Chemistry, Vrije Universiteit, Amsterdam Search PubMed.
- A. Klamt and G. Schüürmann, J. Chem. Soc., Perkin Trans. 2, 1993, 799 RSC.
- M. Mitoraj and A. Michalak, J. Mol. Model., 2007, 13, 347 CrossRef CAS PubMed.
- A. Michalak, M. Mitoraj and T. Ziegler, J. Phys. Chem. A, 2008, 112, 1933 CrossRef CAS PubMed.
- M. Mitoraj, A. Michalak and T. Ziegler, J. Chem. Theory Comput., 2009, 5, 962 CrossRef CAS PubMed.
- G. Frenking and N. Fröhlich, Chem. Rev., 2000, 100, 717 CrossRef CAS PubMed.
- S. J. Grabowski, Chem. Rev., 2011, 111, 2597 CrossRef CAS PubMed.
- The Chemical Bond. Fundamental Aspects of Chemical Bonding, ed. G. Frenking and S. Shaik, Wiley-VCH, Weinheim, 2014 Search PubMed.
- The Chemical Bond. Chemical Bonding Across the Periodic Table, ed. G. Frenking and S. Shaik, Wiley-VCH, Weinheim, 2014 Search PubMed.
- P. A. Hunt, C. R. Ashworth and R. P. Matthews, Chem. Soc. Rev., 2015, 44, 1257 RSC.
- T. van der Wijst, C. Fonseca Guerra, M. Swart, F. M. Bickelhaupt and B. Lippert, Angew. Chem., Int. Ed., 2009, 48, 3285 CrossRef CAS PubMed.
- C. Fonseca Guerra, T. van der Wijst, J. Poater, M. Swart and F. Matthias Bickelhaupt, Theor. Chem. Acc., 2010, 125, 245 CrossRef.
- W. Gao, H. Feng, X. Xuan and L. Chen, J. Mol. Model., 2012, 18, 4577 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Gaussian Inc., Wallingford CT, 2009 Search PubMed.
- S. Miertus, E. Scrocco and J. Tomasi, Chem. Phys., 1981, 55, 117 CrossRef CAS.
- E. R. Johnson, S. Keinan, P. Mori-Sánchez, J. Contreras-García, A. J. Cohen and W. Yang, J. Am. Chem. Soc., 2010, 132, 6498 CrossRef CAS PubMed.
- J. Contreras-García, E. R. Johnson, S. Keinan, R. Chaudret, J. P. Piquemal, D. Beratan and W. Yang, J. Chem. Theory Comput., 2011, 7, 625 CrossRef PubMed.
- Stoe & Cie, X-AREA. Area-Detector Control and Integration Software, Stoe & Cie, Darmstadt, Germany, 2001 Search PubMed.
- A. L. Spek, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2009, 65, 148 CrossRef CAS PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
- I. J. Bruno, J. C. Cole, P. R. Edgington, M. Kessler, C. F. Macrae, P. McCabe, J. Pearson and R. Taylor, Acta Crystallogr., Sect. B: Struct. Sci., 2002, B58, 389 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S3, Table S1 and cif files of HL and [NiL2]. CCDC 1453155 and 1453156. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qi00298f |
| This journal is © the Partner Organisations 2016 |