Accurate structure determination of a borosilicate zeolite EMM-26 with two-dimensional 10 × 10 ring channels using rotation electron diffraction†
Peng
Guo
a,
Karl
Strohmaier
b,
Hilda
Vroman
b,
Mobae
Afeworki
b,
Peter I.
Ravikovitch
b,
Charanjit S.
Paur
b,
Junliang
Sun
a,
Allen
Burton
*b and
Xiaodong
Zou
*a
aBerzelii Center EXSELENT on Porous Materials, Department of Materials and Environmental Chemistry, Stockholm University, SE-106 91 Stockholm, Sweden. E-mail: xzou@mmk.su.se; Tel: (+) 46-8-162389
bCorporate Strategic Research, ExxonMobil Research & Engineering Co. Inc, 1545 Route 22 East, Annandale, New Jersey 08801, USA. E-mail: allen.w.burton@exxonmobil.com
First published on 19th September 2016
Abstract
A new borosilicate zeolite |N2H36C16|[Si22B2O48]·H2O, denoted as EMM-26, has been synthesized by employing a linear dicationic organic structure directing agent 1,6-bis(N-methylpyrrolidinium)hexane (OSDA). EMM-26 has a novel zeolite framework and contains two-dimensional (2D) intersecting 10 × 10-ring channels. Its structure was solved from sub-micrometer sized crystals using rotation electron diffraction (RED) and refined against both the RED and synchrotron powder diffraction data. We have shown for the first time that RED data alone can be used to accurately determine zeolite structures. The OSDAs can be removed from the framework generating permanent pores. EMM-26 shows good CO2 uptake and CO2/CH4 selectivity.
Zeolites, a class of crystalline microporous materials with well-defined channels or cavities of molecular dimensions within their structures, are attracting increasing attention in both academia and industry, due to their applications in ion exchange, gas separation and storage, and organic catalysis.1,2 Although big efforts have been made to synthesize zeolites with new pore structures, there are currently 231 different zeolite framework types in the Zeolite Structure Database of the International Zeolite Association (IZA).3 Based on the size of pore opening, zeolites can be categorized by the number of TO4 tetrahedra (T = Si, Al, B, P, Ge, etc.) that define the pores, such as small (8 T-atoms), medium (10 T-atoms), large (12 T-atoms) and extra-large (larger than 12 T-atoms) pore zeolites. The discovery of new medium pore zeolites has attracted great attention. Zeolite ZSM-5 belongs to the medium pore zeolites with three-dimensional (3D) 10 × 10 × 10-ring channels. It is one of the most important catalysts with excellent shape selectivity in organic transformations. Two empirical approaches have been developed for the synthesis of novel zeolite frameworks as recently reviewed by Corma and co-workers.4 One of them is the utilization of linear dication organic structure directing agents (OSDAs). Based on this approach, a series of zeolites with small and medium pores or medium pores solely such as ZSM-57 (framework type code: MFS),5,6 TNU-10 (STI),7 ITQ-13 (ITH),8 ITQ-34 (ITR),9 TNU-9 (TUN),10,11 ITQ-52 (IFW),12 IM-5 (IMF),13 SSZ-74 (-SVR)14 and Nu-87 (NES),15 have been discovered. Another approach is topotactic transformation of layered precursors into 3D zeolite frameworks. Zeolites RUB-41 (RRO),16 IPC-4 (PCR)17 and CDS-1(CDO)18 have been obtained via this approach.
Zeolites are often synthesized as poly-crystallites, which are too small to be investigated by single crystal X-ray diffraction. Powder X-ray diffraction (PXRD) has been used for structure determination of such crystals. However, the structure determination by PXRD becomes very challenging for complex structures with severe peak overlapping, for samples with poor crystallinity, and for samples containing impurities or stacking disorders. Electron crystallography based on electron diffraction and high resolution transmission electron microscopy (HRTEM) imaging can overcome the aforementioned problems. For example, a complicated zeolite IM-5 was solved using HRTEM images along three main zone axes.13 HRTEM has also been applied for structural investigations of zeolites containing stacking disorders such as ITQ-38,19 ITQ-39,20 and SU-78.21 However, because of the electron beam damage on zeolites, it is very challenging to obtain high quality HRTEM images, even for TEM experts.
Recently, Kolb's group and our group have independently developed new methods for automated collection and processing of 3D electron diffraction (ED) data: automated diffraction tomography (ADT)22,23 and rotation electron diffraction (RED).24,25 The data collection is performed on a conventional TEM via control by software without the need for any additional accessories. The procedure is very similar to that for single crystal X-ray diffraction, but can be used for studying crystals too small to be studied by single crystal X-ray diffraction, down to nanometers in size. The unit cell parameters, reflection conditions, and possible space groups can be deduced from the 3D ED data. Furthermore, diffraction intensities extracted from the 3D ED data can be used for structure solution by utilizing the methods and software developed for SXRD data, such as direct methods (SHELX26 and SIR27) and charge flipping (Superflip28 and Jana29). To date, a number of novel zeolite structures have been solved from 3D ED data.30–34 However, in all cases, the 3D ED data could only provide an initial structural model, which needs to be further refined by Rietveld refinement using PXRD data. This brought up a serious limitation of the structure determination, because it is not possible to obtain high quality PXRD data needed for a good Rietveld refinement when the sample has poor crystallinity or contains impurities. It is thus desirable to be able to obtain accurate structural models from 3D ED data alone without the need for Rietveld refinement from PXRD data.
In this communication, we present a new medium pore zeolite EMM-26, a borosilicate synthesized by employing a linear dicationic organic structure directing agent (OSDA). Its structure was solved using RED data, from crystals of less than 0.5 μm in size. The structure was further refined using both the RED and PXRD data. We show for the first time that the framework structure of zeolites obtained from RED data is as accurate as that obtained from PXRD data, and even the preferential boron positions can be located.
EMM-26 was prepared by hydrothermal synthesis in fluoride media from boric acid and tetramethylorthosilicate (TMOS) using 1,6-bis(N-methylpyrrolidinium)hexane as the OSDA. The molar ratio of the final reaction gel was H3BO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMOS
TMOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SDA(OH)2
SDA(OH)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HF
HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1
H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 84. Polycrystalline EMM-26 crystals were obtained after being heated at 175 °C for 11 days in a Teflon-lined autoclave tumbled at ∼35–40 rpm. EMM-26 could also be synthesized in fluoride-free media. More details are described in section S1, ESI.†
84. Polycrystalline EMM-26 crystals were obtained after being heated at 175 °C for 11 days in a Teflon-lined autoclave tumbled at ∼35–40 rpm. EMM-26 could also be synthesized in fluoride-free media. More details are described in section S1, ESI.†
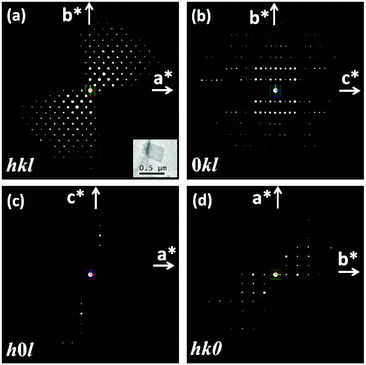
The RED data of the as-made EMM-26 were collected on a JEOL JEM2100 LaB6 using the RED data collection software.25 The data contain 484 ED frames covering a tilt range of 88.90° (more details in section S2 and Table S1, ESI†). 1444 reflections (of which 524 are unique) were obtained from this RED dataset, from which the 3D reciprocal lattice of EMM-26 was reconstructed using RED data processing software.25 The reconstructed 3D reciprocal lattice indicated that EMM-26 is C-centred with an orthorhombic unit cell a = 19.4 Å, b = 15.8 Å, and c = 17.9 Å (Fig. 1a). The reflection conditions were obtained from the 3D RED data (Fig. 1), which suggest two possible space groups: C2ce (no. 41) and Cmce (no. 64). At the start of the structure determination, we chose the highest centrosymmetric space group Cmce and assumed all T-atoms to be silicon. The structure of EMM-26 could be solved by direct methods using the program SHELXS97.26 All seven T atoms and 13 O atoms in the asymmetric unit were found. Five of the T atoms (T1, T3, T5, T6, and T7) are at general positions and the rest are at the special positions 8d (T2) and 8e (T4). Further structure refinement using the mixed occupancies of silicon and boron by the program SHELXL9726 revealed that boron atoms preferentially occupied the T4, T6 and T7 positions, with the occupancy of 12%, 5%, and 39%, respectively (see section S2, ESI† for details). This is the first time that the preferential boron positions could be located from electron diffraction data. The results also agree with those later obtained by Rietveld refinement against the PXRD data. The preferred boron positions were also reported in other borosilicate zeolites such as SSZ-82,14 MCM-70,35 and SSZ-87.36 From the catalytic point of view, it is important to know the location of boron in zeolites because its substitution for silicon creates exchange sites.
The structural model of EMM-26 obtained from the RED data was further refined against the synchrotron PXRD data collected at the beamline ID22 in ESRF, Grenoble. Soft restraints were applied to all the B–O and Si–O bond distances and rigid body restraints were applied to OSDAs. The OSDAs were placed in the cavity according to the residual density in the difference electron density map (Fig. S1, ESI†). The refinement of EMM-26 converged to Rp = 0.051 and Rwp = 0.066, Rbrgg = 0.029 and GOF = 3.636 (Fig. S2 and Table S2, ESI†). The refinement result also showed that boron preferentially occupied sites at the T4 (14%), T6 (8%), and T7 (35%) positions (see section S3, ESI† for details). These results match with those of the refinement against the RED data with 12%, 5%, and 39% boron at T4, T6, and T7 positions, respectively.
The final R1 value of the structure refinement using the RED data was relatively high, 0.2185 for all 524 unique reflections. In order to find out the accuracy of the structure refinement from the RED data, we compared the atomic positions of the framework atoms refined against the RED data with those against the PXRD data, see Table S3, ESI.† The deviation was 0.036 Å on average and 0.051 Å at the largest for Si, and 0.070 Å on average and 0.139 Å at the largest for O. This indicates that the atomic positions obtained from the RED data are accurate. The T atom positions obtained from the RED data are more than twice as accurate as those of O atoms. The high R1 value is mainly due to the dynamical effects that make the RED intensities deviate from the kinematical intensities.
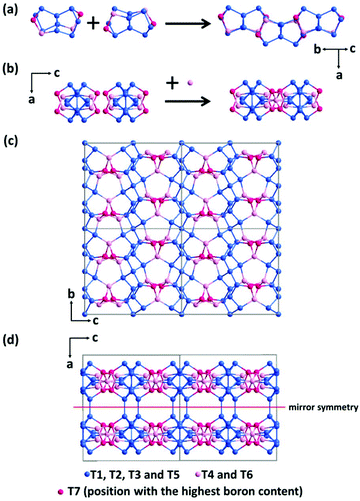
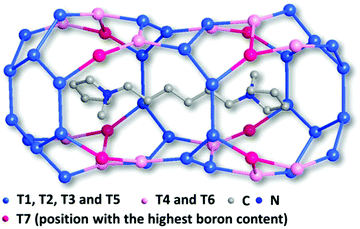
The framework of EMM-26 contains a 2D intersecting 10-ring channel system, and is built from the zeolite composite building unit (CBU) fer (Fig. 2a and S3, ESI†). The fer CBUs are connected by sharing the edge (two TO4 tetrahedra) in an up-down configuration to form a 1D chain along the b-axis as shown in Fig. 2a. Adjacent 1D chains are connected along the c-axis to form a 2D dense layer, through vertex-sharing together with one extra TO4 unit, as depicted in Fig. 2b. Adjacent dense layers (Fig. 2c), related by a mirror symmetry, are further linked to form a 3D zeolite framework, see Fig. 2d. The EMM-26 framework has elliptical zig-zag 10-ring channels along the b- and c-axes, with an effective pore diameter of ca. 6.58 Å × 3.58 Å (Fig. S3 and S4, ESI†). The structure contains a large cavity elongated along the c-axis, inside which the linear 1,6-bis(N-methylpyrrolidinium)hexane OSDA was occluded (Fig. 3). The T-site with the highest boron content (T7) is part of the 10-ring. A guest water molecule is found at the 10-ring window of the cavity. High resolution transmission electron microscopy (HRTEM) images along the b-axis also confirm our structural model (Fig. 4).
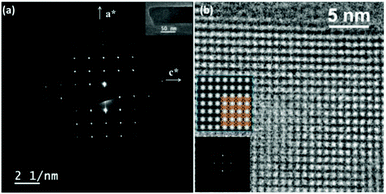 | ||
| Fig. 4 (a) Selected area electron diffraction pattern taken along the b-axis with a TEM image of the crystal inserted. (b) HRTEM image along the b-axis after compensation for the contrast transfer function (CTF) using the software QFocus.37 The insets are the corresponding Fourier transform and an averaged image with pmm symmetry imposed and the structural model overlaid. | ||
The OSDAs could be removed either by calcination or by ozone treatment, giving permanent pores in EMM-26. After the calcination, the peaks in the PXRD pattern are severely broadened presumably due to the deboronation. The d-spacing of the (200) plane decreases from 9.69 Å to 9.33 Å after the calcination in air. This shortening of the inter-layer distance is related either to the removal of a large portion of the framework boron or to a change in the coordination of the framework boron from tetrahedral to trigonal, as shown by 11B NMR (Fig. S5, ESI†). On the other hand, removal of the OSDAs by ozone treatment preserved nearly half (47%) of the BO4 tetrahedra. No significant peak shifts were observed for 0kl reflections after the calcination (Fig. S6, ESI†), indicating that the intra-layer d-spacings remain unchanged in the calcined EMM-26.
The final refinement result reveals that although the as-made EMM-26 has medium 10-ring pores, the effective pore size is approximately 6.58 Å × 3.58 Å due to its elliptical shape, which is close to those of small pores (Fig. S4, ESI†). We hypothesized that EMM-26 might be utilized for gas separation of CO2/CH4 because only smaller CO2 molecules (kinetic diameter 3.3 Å) would pass through this elliptical ring while the larger CH4 molecules (kinetic diameter 3.8 Å) would not. Although we do not have a structural model of the calcined zeolite, we speculate that the shortening of the a-axis may further decrease the 3.58 Å pore dimension that is parallel to the a-axis. So we measured CO2 and CH4 isotherms on a calcined EMM-26 sample after it was outgassed at 673 K for about 16 hours under dynamic vacuum (∼1 × 10−4 torr). At a pressure of 800 torr, the selectivity of CO2/CH4 is 4 (Fig. S7, ESI†).
In summary, we have successfully synthesized a new medium-pore borosilicate zeolite EMM-26 with small pore structural features by using a linear 1,6-bis(N-methylpyrrolidinium)hexane as the OSDA. EMM-26 is built from dense layers that are connected to form 2D 10 × 10-ring channels in parallel to the layers. We have shown that the structure of EMM-26 could be determined from the RED data to an accuracy of on average better than 0.04 Å for Si/B and 0.07 Å for oxygen. The locations of boron atoms and their occupancies could also be accurately determined from the RED data. We demonstrate that accurate zeolite structures can be obtained from RED data alone.
The authors gratefully acknowledge financial support from the Swedish Research Council (VR), the Swedish Governmental Agency for Innovation Systems (VINNOVA), the Röntgen-Ångström Cluster through the project grant MATsynCELL and the Knut & Alice Wallenberg Foundation through a grant for purchasing the TEMs and the project grant 3DEM-NATUR. We also acknowledge the ESRF, Grenoble (ID22, C. Drathen) for synchrotron X-ray beam time. We thank Gene Terefenko, Rich McEvoy, Bob Lemon, Khizera Tariq, and Maria Martinez for assistance in the preparation and early characterization of the zeolites.
Notes and references
- M. E. Davis, Nature, 2002, 417, 813–821 CrossRef CAS PubMed.
- M. E. Davis and R. F. Lobo, Chem. Mater., 1992, 4, 756–768 CrossRef CAS.
- Ch. Baerlocher and L. B. McCusker, Database of Zeolite Structures, http://www.iza-structure.org/databases/ Search PubMed.
- M. Moliner, C. Martínez and A. Corma, Angew. Chem., Int. Ed., 2015, 54, 3560–3579 CrossRef CAS PubMed.
- S. Ernst and J. Weitkamp, in Studies in Surface Science and Catalysis, ed. G. Öhlmann, H. Pfeifer and R. Fricke, Elsevier, 1991, vol. 65, pp. 645–652 Search PubMed.
- J. L. Schlenker, J. B. Higgins and E. W. Valyocsik, Zeolites, 1990, 10, 293–296 CrossRef CAS.
- S. B. Hong, E. G. Lear, P. A. Wright, W. Zhou, P. A. Cox, C.-H. Shin, J.-H. Park and I.-S. Nam, J. Am. Chem. Soc., 2004, 126, 5817–5826 CrossRef CAS PubMed.
- A. Corma, M. Puche, F. Rey, G. Sankar and S. J. Teat, Angew. Chem., Int. Ed., 2003, 42, 1156–1159 CrossRef CAS PubMed.
- A. Corma, M. J. Diaz-Cabanas, J. L. Jorda, F. Rey, G. Sastre and K. G. Strohmaier, J. Am. Chem. Soc., 2008, 130, 16482–16483 CrossRef CAS PubMed.
- S. B. Hong, H.-K. Min, C.-H. Shin, P. A. Cox, S. J. Warrender and P. A. Wright, J. Am. Chem. Soc., 2007, 129, 10870–10885 CrossRef CAS PubMed.
- F. Gramm, C. Baerlocher, L. B. McCusker, S. J. Warrender, P. A. Wright, B. Han, S. B. Hong, Z. Liu, T. Ohsuna and O. Terasaki, Nature, 2006, 444, 79–81 CrossRef CAS PubMed.
- R. Simancas, J. L. Jordá, F. Rey, A. Corma, A. Cantín, I. Peral and C. Popescu, J. Am. Chem. Soc., 2014, 136, 3342–3345 CrossRef CAS PubMed.
- C. Baerlocher, F. Gramm, L. Massüger, L. B. McCusker, Z. He, S. Hovmöller and X. Zou, Science, 2007, 315, 1113–1116 CrossRef CAS PubMed.
- C. Baerlocher, D. Xie, L. B. McCusker, S. J. Hwang, I. Y. Chan, K. Ong, A. W. Burton and S. I. Zones, Nat. Mater., 2008, 7, 631–635 CrossRef CAS PubMed.
- M. D. Shannon, J. L. Casci, P. A. Cox and S. J. Andrews, Nature, 1991, 353, 417–420 CrossRef CAS.
- Y. X. Wang, H. Gies, B. Marler and U. Müller, Chem. Mater., 2005, 17, 43–49 CrossRef CAS.
- W. J. Roth, P. Nachtigall, R. E. Morris, P. S. Wheatley, V. R. Seymour, S. E. Ashbrook, P. Chlubná, L. Grajciar, M. Položij, A. Zukal, O. Shvets and J. Čejka, Nat. Chem., 2013, 5, 628–633 CrossRef CAS PubMed.
- T. Ikeda, Y. Akiyama, Y. Oumi, A. Kawai and F. Mizukami, Angew. Chem., Int. Ed., 2004, 43, 4892–4896 CrossRef CAS PubMed.
- M. Moliner, T. Willhammar, W. Wan, J. González, F. Rey, J. L. Jorda, X. Zou and A. Corma, J. Am. Chem. Soc., 2012, 134, 6473–6478 CrossRef CAS PubMed.
- T. Willhammar, J. Sun, W. Wan, P. Oleynikov, D. Zhang, X. Zou, M. Moliner, J. Gonzalez, C. Martínez, F. Rey and A. Corma, Nat. Chem., 2012, 4, 188–194 CrossRef CAS PubMed.
- Z.-B. Yu, Y. Han, L. Zhao, S. Huang, Q.-Y. Zheng, S. Lin, A. Córdova, X. Zou and J. Sun, Chem. Mater., 2012, 24, 3701–3706 CrossRef CAS.
- U. Kolb, T. Gorelik, C. Kübel, M. T. Otten and D. Hubert, Ultramicroscopy, 2007, 107, 507–513 CrossRef CAS PubMed.
- U. Kolb, T. Gorelik and M. T. Otten, Ultramicroscopy, 2008, 108, 763–772 CrossRef CAS PubMed.
- D. Zhang, P. Oleynikov, S. Hovmöller and X. Zou, Z. Kristallogr., 2010, 225, 94–102 CrossRef CAS.
- W. Wan, J. Sun, J. Su, S. Hovmöller and X. Zou, J. Appl. Crystallogr., 2013, 46, 1863–1873 CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- M. C. Burla, R. Caliandro, M. Camalli, B. Carrozzini, G. L. Cascarano, C. Giacovazzo, M. Mallamo, A. Mazzone, G. Polidori and R. Spagna, J. Appl. Crystallogr., 2012, 45, 357–361 CrossRef CAS.
- L. Palatinus and G. Chapuis, J. Appl. Crystallogr., 2007, 40, 786–790 CrossRef CAS.
- V. Petříček, M. Dušek and L. Palatinus, Z. Kristallogr., 2014, 229, 345–352 Search PubMed.
- T. Willhammar, A. W. Burton, Y. Yun, J. Sun, M. Afeworki, K. G. Strohmaier, H. Vroman and X. Zou, J. Am. Chem. Soc., 2014, 136, 13570–13573 CrossRef CAS PubMed.
- J. Jiang, Y. Yun, X. Zou, J. L. Jorda and A. Corma, Chem. Sci., 2014, 6, 480–485 RSC.
- Y. Yun, M. Hernández, W. Wan, X. Zou, J. L. Jordá, A. Cantín, F. Rey and A. Corma, Chem. Commun., 2015, 51, 7602–7605 RSC.
- R. Martínez-Franco, M. Moliner, Y. Yun, J. Sun, W. Wan, X. Zou and A. Corma, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 3749–3754 CrossRef PubMed.
- J. Jiang, J. L. Jorda, J. Yu, L. A. Baumes, E. Mugnaioli, M. J. Diaz-Cabanas, U. Kolb and A. Corma, Science, 2011, 333, 1131 CrossRef CAS PubMed.
- D. Xie, L. B. McCusker, C. Baerlocher, L. Gibson, A. W. Burton and S.-J. Hwang, J. Phys. Chem. C, 2009, 113, 9845–9850 CAS.
- S. Smeets, L. B. McCusker, C. Baerlocher, D. Xie, C.-Y. Chen and S. I. Zones, J. Am. Chem. Soc., 2015, 137, 2015–2020 CrossRef CAS PubMed.
- W. Wan, S. Hovmöller and X. Zou, Ultramicroscopy, 2012, 115, 50–60 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and RED data collection and Rietveld refinement, X-ray and electron crystallography data (CIF), and additional tables and figures. CCDC 1471627 and 1471628. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qi00262e |
| This journal is © the Partner Organisations 2016 |