Open Access Article
Open Access ArticleColorimetric analysis of painting materials using polymer-supported polydiacetylene films†
Alexander
Trachtenberg
a,
Orit
Malka
a,
Kaviya Parambath
Kootery
a,
Stella
Beglaryan
b,
Danilo
Malferrari
b,
Paola
Galletti
b,
Silvia
Prati
*c,
Rocco
Mazzeo
c,
Emilio
Tagliavini
b and
Raz
Jelinek
*a
aDepartment of Chemistry, Ben Gurion University of the Negev, Beer Sheva 84105, Israel. E-mail: razj@bgu.ac.il
bUniversity of Bologna, Chemistry Department “G. Ciamician”, Green Chemistry Lab, Via S. Alberto 163, 48100 Ravenna, Italy. E-mail: paola.galletti@unibo.it
cUniversity of Bologna, Chemistry Department “G. Ciamician”, Microchemistry and Microscopy Art Diagnostic Laboratory (M2ADL), via Guaccimanni 42, 48100 Ravenna, Italy. E-mail: s.prati@unibo.it
First published on 12th September 2016
Abstract
Analysis of artworks and identification of their molecular components are of utmost importance for selecting proper conservation strategies and monitoring restoration. Accordingly, development of simple and readily applicable paint analysis assays is highly sought in conservation science and technology. We prepared transparent spin-coated films comprising polydiacetylene (PDA) derivatives upon poly(methyl methacrylate) (PMMA) substrates and employed the PDA/PMMA films for color analysis of organic constituents in painting materials. We show that distinct color transformations were recorded upon addition of paint compounds to different PDA films. Quantitative analysis revealed that the colorimetric transitions were dictated by the interactions between the diacetylene headgroups and the tested compounds. This study underscores the significance of the diacetylene headgroup for molecular discrimination and points to the possible uses of the PMMA-supported PDA films for colorimetric screening of organic components in painting materials.
Introduction
Proper restoration of old paintings and artworks often necessitates identification of painting materials and diagnosis of their state of conservation. Cleaning operations of paintings are often necessary to remove the aged and yellowed varnish layer applied on the painting. However, the selective removal of the varnish layer may be difficult due to its thickness (10–50 μm) and the affinity to the painting surface. Thus, identification of organic compounds present both in the painting and in the varnish layers is of the utmost importance to identify the most suitable cleaning method. Moreover, the materials used as binders in painting layers or as ingredients in the formulation of varnishes belong to different organic classes, such as triglycerides (i.e., siccative oil used both as painting binders and in varnishes), terpenes (i.e., natural resins used in varnishes), proteinaceous materials (i.e., animal glue, egg, milk or casein used as binders and sometimes in varnishes), and polysaccharides (i.e., natural gums, used as binders and sometimes in varnishes). This poses additional challenges to the development of accurate and effective on-site identification methods.Several analytical methods have been employed for paint analysis. Micro-destructive methods, such as gas chromatography-mass spectrometry (GC-MS) and high performance liquid chromatography (HPLC), enable specific identification of the organic compounds present in a paint sample.1 While these methods are very useful and informative, they cannot be applied during routine restoration work because they require technical expertise to carry out and analyse, and they could be expensive and time consuming. Portable Fourier transform infrared (FTIR) and Raman spectroscopies can be used to monitor the molecular compositions of the external painting layers.1 Development of simple on-site analytical systems has been lacking in conservation science. While such applications are technically challenging, introduction of such instruments would be of significant benefit in conservation and restoration work and maintaining cultural heritage.
Herein, we present application of spin-coated polydiacetylene (PDA) films for in situ colorimetric sensing of a selection of organic materials present in paintings. PDAs are π-conjugated polymers displaying unique structural and chromatic properties.2 These polymers, first synthesized in the late 1960s, have attracted considerable interest both scientifically and as promising sensing platforms, primarily due to their visible colour transformations (generally from blue to red), induced by varied external stimuli such as heat,3 ionic strength,4 mechanical pressures, and interactions with biological and chemical molecules.5 Recently, we demonstrated that PDA films spin-coated on poly(methyl methacrylate) (PMMA), a widely used transparent polymer, constituted a powerful colorimetric sensing platform for varied organic substances.6
In this study, we constructed PMMA-coated PDA films using diacetylene monomers displaying varied headgroups exhibiting different polarities and functional moieties, designed to tune the interactions with the tested analytes, i.e. paint constituents. The premise of this work is that the interactions of the organic substances with the different headgroups would generate distinct colorimetric transformations within the PMMA-supported PDA films, aiding in distinguishing among the tested compounds. In particular, different PMMA-supported PDA films were tested on not-aged painting materials in order to make a feasibility study and a screening of the most performing tools for the on-site analysis of paint materials.
Experimental
Materials
All solvents were purchased from Sigma-Aldrich Chemicals. The diacetylene monomer, 10,12-tricosadiynoic acid (TRCDA), was purchased from Aldrich (98% purity) and eventually purified to remove the polymerized part (if present) before use. For natural gums study, arabic, ghatti, tragacanth, guar and karaya gums were purchased from Sigma Aldrich. 1H,1H,2H,2H,perfluoro-1-decanol was purchased from Fluka. Analytical thin-layer chromatography (TLC) was conducted on Sigma-Aldrich silica gel aluminum plates (60 Å, with fluorescent indicator) or silica gel 60 F-254 with a 0.2 mm layer thickness. For flash chromatography, 60 Å silica gel (Merck, 230–400 mesh) was employed. 1H, 13C and 19F NMR spectra were obtained using a Varian Mercury 400 spectrometer with a 5 mm probe.Synthesis of diacetylene monomer derivatives
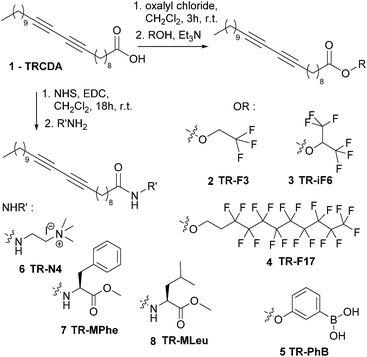
Synthesis routes developed for synthesizing the TRCDA derivatives 2–8 are summarized in Scheme 1. All synthesis routes started from 10,12-tricosadiynoic acid (TRCDA) 1. Ester derivatives were prepared by treating 1 with oxalylchloride and the suitable fluorinated alcohol to obtain 2–4 or phenylboronic acid to obtain 5. Amide derivatives 6–8 were prepared by activating 1via its hydroxysuccinamide derivative, in the presence of EDC, and then coupling with the corresponding amine or amino-acid methyl ester. All diacetylene derivatives 2–8 were characterized by standard analytical and spectroscopic techniques and proved very stable when stored under nitrogen (or at −20 °C) in the dark. Details on synthesis and spectroscopic data are reported in the supporting material.Film preparation
40 mg of TRCDA and TR derivatives (mole ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were dissolved in a solvent mixture comprising 500 μL CH2Cl2 and 500 μL THF. 40 μL of this solution was placed upon a PMMA disk (0.5 cm diameter). After 30 seconds, spin coating was carried out at 1500 rpm for 30 seconds. The films were subsequently UV irradiated (254 nm) for 0.3 minutes in a UV oven, yielding blue-phase PDA.
1) were dissolved in a solvent mixture comprising 500 μL CH2Cl2 and 500 μL THF. 40 μL of this solution was placed upon a PMMA disk (0.5 cm diameter). After 30 seconds, spin coating was carried out at 1500 rpm for 30 seconds. The films were subsequently UV irradiated (254 nm) for 0.3 minutes in a UV oven, yielding blue-phase PDA.
Colorimetric measurements and analysis
The organic painting material samples were solubilized in different solvents (outlined in the Results section) at a concentration of 10 mg mL−1 and placed upon the spin-coated PDA films and incubated for 90 minutes. Ultraviolet (UV)-vis spectroscopy measurements were subsequently carried out at 25 °C on a Varioskan (Thermo, Finland) in a wavelength range of 400–800 nm.Percentage colorimetric response (%CR) was calculated after baseline correction of the UV-vis absorbance spectrum, according to the equation:
| %CR = [(PB0 − PBi)/PB0] × 100 |
Analytical techniques
Results
Fig. 1 outlines the structural features of the diacetylene monomer derivatives used in this study (prepared according to Scheme 1). In particular, we designed and synthetized different diacetylene headgroups in order to examine both the distinct molecular interactions with the compounds tested, as well as modulate the overall structural organization and corresponding colorimetric properties of the sensor films. In particular, the diacetylene monomers examined displayed carboxylic acid (e.g., the commercial product TRCDA, compound 1), fluorine-containing headgroups with different fluorine chain lengths designed to enhance non polar interaction (compounds 2, 3 and 4), phenyl boronic acid (compound 5)7 designed to establish polar/ionic interactions and eventually form boronic esters with diols-containing materials with the aim of exhibiting affinity to carbohydrates,8 ammonium residue (compound 6)9 aimed to promote ionic interactions with negative compounds, and aminoacid ester derivatives (compounds 7 and 8) for attaining protein-like hydrogen bonding with polar targets.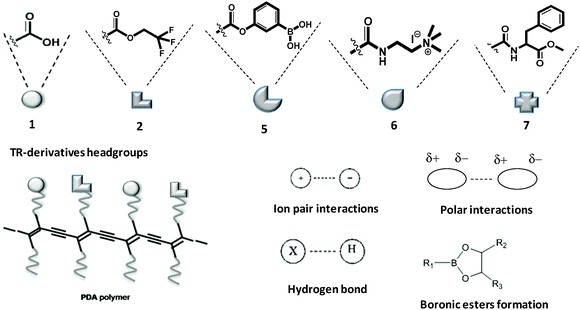 | ||
| Fig. 1 Structures of TR-derivatives and PDA polymers and possible intermolecular interactions between PDA and painting material components. | ||
The colour sensor films were assembled through spin-coating mixtures comprising the starting monomer 10,12-tricosadiynoic acid (TRCDA, 1) and the different functionalized-headgroup derivatives at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ratio (TRCDA
1 mole ratio (TRCDA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) derivative) upon PMMA disks.6 Notably, we found that films comprising pure diacetylene derivatives exhibit lower stability and/or were too sensitive to buffer/solvents, thereby rendering very high background signals. Following deposition and drying of the organic solvent, the films were subjected to UV (254 nm) irradiation to generate the blue polymerized polydiacetylene (PDA) phase.
derivative) upon PMMA disks.6 Notably, we found that films comprising pure diacetylene derivatives exhibit lower stability and/or were too sensitive to buffer/solvents, thereby rendering very high background signals. Following deposition and drying of the organic solvent, the films were subjected to UV (254 nm) irradiation to generate the blue polymerized polydiacetylene (PDA) phase.
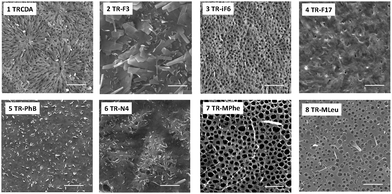
Fig. 2 presents the scanning electron microscopy (SEM) images of the PMMA-coated PDA films. The SEM experiments demonstrate significantly different surface morphologies among the PDA derivatives, and attest the pronounced effects of diacetylene headgroups upon film organization. In particular, the cooperative film properties, determined to a large extent by the distinct headgroups, might have generated different morphologies during the dewetting accompanying the spin coating process. Previous reports also underscored the significant effect of headgroup interactions in determining macro-scale organization of PDA assemblies.10 While the SEM results in Fig. 2 reveal distinct macro-scale organizations of the spin-coated films, representative UV-vis spectra and Raman scattering data in Fig. 3 indicate that the PDA backbones within the different films exhibit the same chromatic properties, specifically the presence of the well-known blue and red PDA phases.11Fig. 3A depicts the UV-vis spectra of a spin-coated PDA film in the blue phase (after polymerization), and after transformation to the red phase induced by placing the PMMA-supported film at 50 °C.
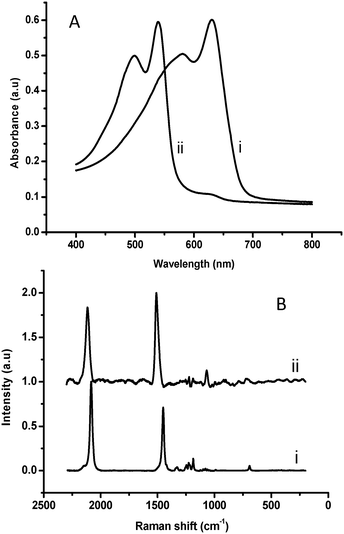 | ||
| Fig. 3 Spectroscopic characterization of PMMA-supported PDA films. (A) UV-vis spectra of blue (i) and red (ii) phase; (B) Raman scattering spectra of the blue (i) and red (ii) PDA film. | ||
Importantly, similar spectral features corresponding to the blue-red transitions were recorded for the other PDA derivatives, indicating that the chromatic properties of the conjugated polymer backbone were retained in all films. Indeed, the Raman scattering data in Fig. 3B corroborate this interpretation. The Raman spectral region corresponding to the conjugated alkyne–alkene groups initially featured the typical blue-phase signals at around 1450 cm−1 and 2090 cm−1 (Fig. 3B, i). Distinct peaks corresponding to red-phase PDA appeared at approximately 1520 cm−1 and 2125 cm−1 following heating (Fig. 3B, ii). Similar to the UV-vis results in Fig. 3A, Raman signals presented in Fig. 3B were recorded for all PDA derivative films prepared, confirming the formation of the conjugated PDA networks. Table 1 outlines the paint materials tested in this study. The selected substances belong to different classes of molecules employed as painting binders or in varnish formulation. In particular, the screened substances include polymerized triglycerides (linseed oil), proteinaceous compounds (fish glue, rabbit glue, and ovalbumine), terpenic compounds (mastic, dammar, colophony, and sandarac), and polysaccharide gums (arabic gum, gum karaya, ghatti gum, guar gum and gum tragacanth). Detailed compositions of the materials are provided in Table S1 of the ESI.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12
| Paint material | Family | Main composition |
| Linseed oil | Triglycerides | Polymerized tryglicerides, low chain carboxylic fatty acids |
| Ovalbumin | Proteinaceous | |
| Fish glue, rabbit glue | Proteinaceous | Denaturated collagen |
| Sandarac | Terpenes | Diterpenic compounds, with a polymerized component |
| Mastic, dammar | Terpenes | Triterpenic compounds |
| Colophony | Terpenes | Diterpenic compounds |
| Arabic, karaya, ghatti, guar, tragacanth gums | Polysaccharides | Carbohydrates, monosaccharides % in ESI |
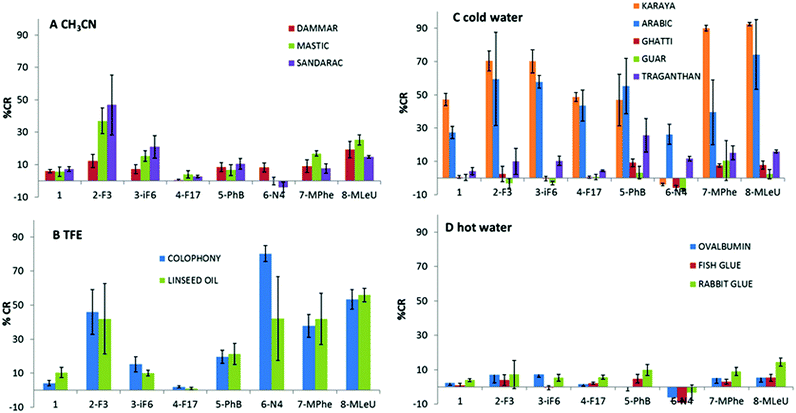
Fig. 4 presents the colorimetric responses of the PDA films comprising the different headgroup derivatives upon incubation with the compounds outlined in Table 1.
In particular, the bar diagrams in Fig. 4A–D depict the percentage colorimetric response (%CR)13 after subtraction of the buffer recorded upon incubation of the painting materials with the PDA films, calculated through comparison of the relative intensities of the UV-vis peaks at 500 nm (i.e., “red” peak in the UV-vis spectrum) and 640 nm (“blue” peak), see Experimental section.14 Essentially, the %CR values reflect the degrees of blue-red transformations with respect to the reference solution (here a tri buffer pH 8.0). In particular, high %CRs correspond to more pronounced red appearance, whereas lower %CR values reflect less significant blue-red transformations (e.g., more blue PDA films).15 Negative %CR values are occasionally recorded, likely reflecting the surface interactions of target analytes, which stabilize the PDA headgroups in comparison with the effects of buffer/solvents. Notably, the colorimetric data in Fig. 4 were grouped according to the solvent used, making sure the comparison of %CR is reliable in terms of compounds dissolution.
Fig. 4 demonstrates significant variations both among the colorimetric responses of the PDA films to the different painting materials, as well as among the diacetylene derivatives used in the PMMA-supported films. Among the painting materials dissolved in acetonitrile (Fig. 4A, terpenic compounds), films comprising PDA units displaying fluorinated residues (i.e., monomers 2 and 3) successfully distinguished between dammar, mastic, and sandarac resins. Derivative 2, in particular, gave rise to the most significant blue-red transformations (%CR of 15, 40, and 50 induced by dammar, mastic, and sandarac, respectively, Fig. 4A). This specificity is likely ascribed to the terpenic apolar backbone interacting with the apolar terminus of fluorinated derivatives. The dammar and mastic are triterpenic resins with very similar composition, whereas sandarac is partially polymerized diterpenic materials.16 Among the diacetylene derivatives, monomer 2 exhibited the most versatile interactions, both polar through the ester moiety, as well as apolar affinity via the fluorinated group. Indeed, the significance of the fluorine-containing PDA headgroups is supported by the observation of small %CR and no distinction among the terpenic compounds apparent in the films comprising the parent polymerized TRCDA (monomer 1, Fig. 4A). Similarly, polar or charged residues (i.e., derivatives 5 and 6) exhibited small %CR, which was similar to the parent diacetylene 1, reflecting insignificant interactions with terpenes.
Fig. 4B depicts the colorimetric data recorded for colophony and linseed oil, substances that were only dissolved in trifluoroethanol (TFE). The bar diagram in Fig. 4B indicates that apolar fluorinated derivatives 3–4 produced low %CR, whereas polar derivatives generated more pronounced %CR. Notably, only films comprising ionic positively-charged headgroup, PDA derivative 6, could distinguish between the two substances, possibly reflecting interactions of the amine residues with carboxylic acid functional groups in colophony.
Fig. 4C depicts the colorimetric transformations induced by paint substances solubilized in cold water, specifically gum derivatives with different saccharide compositions. Among these compounds, gum arabic and gum karaya induced the most pronounced blue-red transformations. Interestingly, Fig. 4C reveals that both the gums did not induce a colour change in the films comprising diacetylene 6, which exhibits an ionic headgroup. Moreover, a clear distinction between the two gums was accomplished by films containing the parent diacetylene monomer 1 and derivatives 7–8. Compound 8, in fact, appears the most promising for screening other gums (Fig. 4C). This result points to hydrogen bonding and interactions between the polar headgroup of 7 (Scheme 1) and hydroxyl units in the carbohydrate gums, rather than boronate ester formation, as the most prominent factors affecting the colorimetric transformations.
The colorimetric results recorded upon interactions of the proteinaceous substances dissolved in hot water with the PDA/PMMA films (Fig. 4D) underscore low %CR values, likely reflecting weak interactions between the tested compounds and the PDA headgroups. Films comprising PDA derivatives 2, 7, and 8 generated %CR values that were somewhat higher than from the background, although the %CR signals could not be used for distinguishing among the tested substances. Nevertheless, identification of generic proteinaceous substances rather than the precise protein type may be sufficient for selecting a suitable paint restoration treatment.
Discussion
This study explores the use of PMMA-supported PDA films as colorimetric sensors for common constituents in artwork paint. In particular, we synthesized novel diacetylene derivatives displaying headgroups, exhibiting different molecular properties and functionalities, and prepared spin-coated films comprising the polymerized diacetylene derivatives. In particular, different PMMA-supported PDA films were tested on not-aged painting materials in order to evaluate the PDA platform for paint screening and analysis. The PMMA-supported PDA films featured distinct morphologies and particularly important, different colorimetric response upon interaction with paint materials.The experimental data demonstrate that the extent of blue-red transformations of the PDA films was closely related to the affinity between the headgroup and tested analytes. In particular, the results point to the significance of polar, non-polar, and electrostatic interactions between the PDA headgroup and paint constituents as fundamental parameters affecting the colour transitions. As such, this study points to the potential of the PDA/PMMA system as a viable tool for distinguishing among paint substances. Further studies will be performed to evaluate the response on aged and real paintings.
Polar interactions affecting enhanced colour transformations are manifested, for example, in the more pronounced transformations involving the fluorine-displaying headgroups, particularly derivatives 2 and 3. These films gave rise to stronger blue-red transformations upon addition of different paint material families (e.g.Fig. 4A–C). The three fluorine-based PDA derivatives will be further tested to monitor the conservation state of terpenic varnishes considering that during ageing, they change their polarity and solubility. Thus, we expect a change in their response to the three derivatives at different level of apolarity compared with the non-aged materials.
While polar or apolar interactions (as well as combined polar/apolar effects involving different parts of the PDA framework) contributed to the colorimetric transformations and aided in distinguishing among paint substances, our results additionally illuminate other chemical effects modulating the colour response of the PDA films. Specifically, Fig. 4 revealed that hydrogen bonding and ionic interactions played significant roles in certain instances. These observations point to the possibility to employ different diacetylene derivatives in a comprehensive “colorimetric toolbox” for distinguishing paint analytes.
It should be emphasized that the experimental results presented in Fig. 4 suggest that ionic PDA films may be suitable to monitor the polarity changes of oil painting constituents. Indeed, siccative oils adopt three-dimensional networks upon drying, in which low molecular weight compounds formed during the polymerization are entrapped. In particular, dicarboxylic fatty acids are produced during the photo-polymerization of siccative oils and tend to increase in concentration due to ageing.16 In addition, degradation processes are responsible for the formation of free fatty acids and metal carboxylates, which further contribute to overall polarity changes in paintings.
PDA-derivatives capable to distinguish different types of gums may find applications not only in conservation science, but also in other disciplines, such as food production, in which gum formulation may be required.17 The colorimetric results obtained for the proteinaceous substances, for example, provided %CR values that were sufficiently different from the background colorimetric signals, even if it is not possible yet to clearly distinguish among the different materials. However, the PDA films might be applied to develop readily applicable tools for in situ identification of generic proteinaceous substances. Indeed, exact determination of the type of protein may not be necessary to define a suitable restoration treatment. Further studies will be performed to evaluate the ageing effect on the painting materials and to verify the applicability of the PDA-based sensors to other proteinaceous materials used in conservation. One example of application may be related to the evaluation of residues after the bio-cleaning procedures. Biotechnological researches have proposed the use of enzymes and bacteria as a cleaning method for the selective removal of specific class of compounds.18 When applied, conservators have to verify that any residue of the biological treatments were removed from the surface to avoid any interaction with the clean surface.
Conclusions
In conclusion, this study shows that poly(methyl methacrylate)-supported polydiacetylene films comprising different diacetylene monomer derivatives could distinguish among paint constituents. The film assembly might be employed as a simple, on-site platform for monitoring degradation processes and planning restoration intervention. Importantly, the colour transformations can be readily quantified using simple spectrophotometer instrumentation. Application of the colorimetric polydiacetylene film assay is simple and analysis can be readily carried out by the use of visible spectrophotometry. This study shows that the polydiacetylene technology might open new analytical avenues in molecular analysis, in general, painting restoration and conversation science, in particular.Acknowledgements
We acknowledge the University of Bologna (RFO Ricerca Fondamentale Orientata) and the Regione Emilia Romagna (POR-FESR) for funding.Notes and references
- Scientific Examination for the Investigation of Paintings: A Handbook for Conservators and Restorers, ed. D. Pinna; M. Galeotti and R. Mazzeo, Centro Di, Firenze, 2010, ISBN: 88703845 Search PubMed.
- X. Sun, T. Chen, S. Huang, L. Li and H. Peng, Chem. Soc. Rev., 2010, 39, 4244–4257 RSC.
- J. Lee, H. J. Kim and J. Kim, J. Am. Chem. Soc., 2008, 130, 5010–5011 CrossRef CAS PubMed.
- C. H. Park, J. P. Kim, S. W. Lee, N. L. Jeon, P. J. Yoo and S. J. Sim, Adv. Funct. Mater., 2009, 19, 3703–3710 CrossRef CAS.
- N. Gal, D. Malferarri, S. Kolusheva, P. Galletti, E. Tagliavini and R. Jelinek, Biochim. Biophys. Acta, Biomembr., 2012, 1818, 2967–2974 CrossRef CAS PubMed.
- K. Parambath Kootery, H. Jiang, S. Kolusheva, T. P. Vinod, M. Ritenberg, L. Zeiri, R. Volinsky, D. Malferrari, P. Galletti, E. Tagliavini and R. Jelinek, ACS Appl. Mater. Interfaces, 2014, 6, 8613–8620 CAS.
- J. Lee, O. Yarimaga, C. H. Lee, Y. K. yu Choi and J. M. Kim, Adv. Funct. Mater., 2011, 21, 1032–1039 CrossRef CAS.
- T. D. James, K. R. A. S. Sandanayake and S. Shinkai, Angew. Chem., 1996, 35, 1910–1922 Search PubMed; G. Springsteen and B. Wang, Tetrahedron, 2002, 58, 5291–5300 CrossRef CAS.
- H. Jeon, S. Lee, Y. Li, S. Park and J. Yoon, J. Mater. Chem., 2012, 22, 3795–3799 RSC.
- B. Yoon, S. Lee and J. M. Kim, Chem. Soc. Rev., 2009, 38, 1958–1968 RSC; H. Jiang, G. Hershtig, S. Richter and R. Jelinek, J. Phys. Chem. Lett., 2016, 7, 1628–1631 CrossRef CAS PubMed.
- X. Q. Chen, G. D. Zhou, X. J. Peng and J. Yoon, Chem. Soc. Rev., 2012, 41, 4610–4630 RSC.
- C. Torri, E. Soragni, S. Prati and D. Fabbri, Microchem. J., 2013, 110, 719–725 CrossRef CAS.
- X. Chen, S. Kang, M. Kim, J. Kim, Y. Kim, H. Kim, B. Chi, S.-J. Kim, Y. Lee and J. Yoon, Angew. Chem., Int. Ed., 2010, 49, 1422 CrossRef CAS PubMed.
- Y. Demikhovsky, S. Kolusheva, M. Geyzer and R. Jelinek, J. Colloid Interface Sci., 2011, 364, 428–434 CrossRef CAS PubMed.
- S. Okada, S. Peng, W. Spevak and D. Charych, Acc. Chem. Res., 1998, 31, 229–239 CrossRef CAS.
- The Organic Chemistry of Museum Objects, ed. J. S. Mills and R. White, Butterworths, London, 1987 Search PubMed.
- P. Vanloot, N. Dupuy, M. Guiliano and J. Artaud, Food Chem., 2012, 135, 2554 CrossRef CAS PubMed.
- P. Bosch-Roig and G. Ranalli, Front. Microbiol., 2014, 5, 155 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed synthetic routes, 1H NMR, 13C NMR and 19F NMR spectra of TR-derivatives. See DOI: 10.1039/c6nj02092e |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 |