Synthesis, structure and properties of thiophene-fused BODIPYs and azaBODIPYs as near-infrared agents†
Abstract
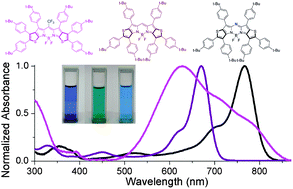
The synthesis, structure and photophysical properties of a series of thiophene-fused BODIPYs and azaBODIPYs from readily available thieno[2,3-b]pyrroles and thieno[3,2-b]pyrroles have been reported. These thiophene-fused (aza)BODIPYs all showed near infrared absorptions/emissions and excellent photostability. Among them, thieno[3,2-b]pyrrole derived azaBODIPYs 1 show the longest absorption (up to 788 nm) and emission maxima (up to 816 nm) in chloroform, while thieno[3,2-b]pyrrole derived BODIPYs 2 have the highest fluorescence quantum yields (up to 0.85 in chloroform). In contrast, thieno[2,3-b]pyrrole derived BODIPYs 3 have very broad absorption bands from 500 to 880 nm with an intense absorption λmax of around 628 nm and broad shoulder bands with a tail up to 880 nm.
- This article is part of the themed collection: Nitrogen Ligands

 Please wait while we load your content...
Please wait while we load your content...