Open Access Article
Open Access ArticleFate of microplastics and other small anthropogenic litter (SAL) in wastewater treatment plants depends on unit processes employed†
Marlies R.
Michielssen
ab,
Elien R.
Michielssen
ab,
Jonathan
Ni
ac and
Melissa B.
Duhaime
*d
aDepartment of Civil and Environmental Engineering, University of Michigan, 2350 Hayward Rd., Ann Arbor, MI 48109, USA
bWashtenaw International High School, 510 Emerick St., Ypsilianti, MI 48198, USA
cDetroit Country Day School, 22400 Hilview Ln., Beverly Hills, MI 48025, USA
dDepartment of Ecology and Evolutionary Biology, University of Michigan, 830 North University Ave, Ann Arbor, MI 48109, USA. E-mail: duhaimem@umich.edu
First published on 14th October 2016
Abstract
The accumulation of microplastics (plastic particles less than 5 mm) and similarly sized small anthropogenic litter (SAL; e.g., cellulosic products manufactured from natural material) in aquatic ecosystems is a growing concern. These particles can serve as vectors of chemical toxins and microbial pathogens and thus, as organisms consume them, may lead to biomagnification of these contaminants. As collection points in managed water systems, wastewater treatment plants (WWTPs) provide an opportunity to develop and implement novel technologies to manage SAL pollution. Here, we assessed the efficiency of different unit processes at three WWTPs in removing SAL. Samples were collected from WWTPs that employ either secondary treatment (activated sludge) or tertiary treatment (granular sand filtration) as a final step, as well as a pilot membrane bioreactor system that finishes treatment with microfiltration. SAL from 20 μm to 4.75 mm was quantified and categorized by shape. The WWTP with secondary treatment removed 95.6% of SAL, discharging 5.9 SAL per L in the final effluent; the plant with tertiary treatment removed 97.2% of SAL, discharging 2.6 SAL per L; the membrane bioreactor plant removed 99.4% of SAL, discharging 0.5 SAL per L. The majority of SAL in effluent from all plants was comprised of thin fibers (e.g., textile fibers). While the WWTP with tertiary granular sand filtration and the membrane bioreactor exhibited greater overall removal of SAL, fibers represented a larger percentage of SAL in effluent from these plants (79 and 83%, respectively) than the plant with activated sludge as a final step (44% fibers). This study suggests that retrofitting existing secondary WWTPs with granular sand filtration or membrane filtration would result in the highest possible removal of SAL—though treatment facilities would continue to serve as pathways of SAL pollution to the environment. Further, the fate of the 95–99% of SAL that is retained or leaves WWTPs through means other than effluent (e.g., sludge) must be resolved to effectively address this problem.
Water impactConsidering their central role in urban and storm water infrastructure, wastewater treatment plants could serve as centralized points of mitigation to address the growing concern of microplastic contamination in nature. Our comparison of microplastic removal efficiency along three contrasting wastewater treatment plants informs recommendations regarding which systems and future innovations would optimally reduce loads of microplastics entering the aquatic environment. |
Introduction
Plastics have transformed our lives by providing numerous societal benefits and enabling technological and medical advancement.1 But recent evidence has indicated that our so-called “plastic age” brings with it ecological risk.2 Plastic accumulates in the environment as what is known as plastic debris pollution.3 Microplastics (MP), which are generally defined as plastic particles with a size smaller than 5 mm, are of concern because they can be harmful to aquatic and terrestrial life.4 MP have been detected in every major ocean and many freshwater lakes and rivers.5,6 While up to 80% of ocean litter—much of which is plastic—is estimated to be delivered by river systems from inland sources,3,7 less data are available depicting freshwater pathways of litter and MP. Runoff from urban, agricultural, and recreational activities, indiscriminate disposal, industrial release (including fisheries), atmospheric fallout, and wastewater treatment plant effluent discharge are among the pathways MP follow to environmental reservoirs.3–5,8,9 Organismal studies have confirmed that fauna across a range of feeding guilds ingest MP.4 These studies have raised concerns about the detrimental effects of MP in marine and freshwater ecosystems, as MP are ingested throughout the food chain more readily than larger plastic particles. MP are especially concerning because their bioaccumulation potential increases with decreasing size.3 Plastics contain chemical additives, adsorb organic contaminants from the surrounding area, and can serve as attachment media for bacterial pathogens.1,4,10 Since MP are ingested by organisms, they can serve as vectors for these chemical and microbial contaminants.1,10Wastewater treatment plants (WWTPs) are critical components of urban and inland water systems and further characterization of MP in these pathways has been called for.3 A number of studies of WWTP discharge have reported the total number of putative plastic particles visually detected,8,11–14 while others have differentiated labile particles from recalcitrant synthetic plastic by disintegrating the non-plastic particles with a wet peroxide oxidation (WPO) step before counting.15,16 The former studies that omit WPO processing capture particles of (i) plastic, which is manufactured from oil-based petroleum (e.g., polyethylene, polypropylene, polyester, nylon), as well as (ii) cellulosic particles manufactured from natural material (e.g., rayon, lyocell, modal derived from wood). To encompass this broader class, we introduce the term small anthropogenic litter (SAL), as an extension of the introduced term, anthropogenic litter.10 While it has not been confirmed that such cellulosic particles explicitly pose ecosystem threats through the same mechanisms as MP, they are ingested by aquatic organisms9,17 and the possible breakdown products of their dyes are known carcinogens (e.g., in the case of Direct Red 28 (ref. 18)). As with plastics, concerns about their environmental fate warrant further studies of SAL.
While WWTPs retain the majority (e.g., 95–99% (ref. 11–16)) of influent SAL, they are pathways of SAL discharge to aquatic ecosystems. A WWTP in Långeviksverket, Sweden found elevated SAL concentrations in the final effluent as compared to the receiving water body.11 A study conducted at the Viikinmäki WWTP in Helsinki (Finland) reported that the average fiber and particle concentrations in the final effluent were 25 and three times higher, respectively, than in the receiving water body.12 Similarly, a study in Chicago, Illinois (USA) reported higher levels of MP downstream of a discharge point than upstream.10 In the most comprehensive study to date, all 17 WWTPs tested were confirmed to discharge MP, releasing an average of over 4 million MP per facility per day.16
Wastewater is treated through a series of unit processes as it progresses through preliminary treatment (e.g., screening and grit removal), primary treatment (e.g., gravity separation and surface skimming on primary clarifiers), secondary treatment step (e.g., activated sludge and trickling filters), and tertiary treatment (e.g., gravity sand filtration). Few studies have evaluated the potential of different unit processes present in WWTPs to retain or remove SAL and available studies do not always provide information about the types of unit processes used for treatment.11,12 Based on visual assessment of collected particles, the Finnish study at the Viikinmäki WWTP studied the removal efficiency of some unit processes and reported that primary clarifiers removed most of what they identified as textile fibers and a minor amount of synthetic particles, while secondary treatment by activated sludge systems and tertiary filtration removed most of the particles.8 Carr et al. compared MP loads in effluent discharges at one WWTP with secondary treatment and seven WWTPs with tertiary treatment in Los Angeles County, California (USA).13 They concluded that tertiary WWTPs did not discharge MP contaminants. Another recent study conducted a detailed evaluation of the effectiveness of different unit processes in removing MP at a secondary WWTP in Glasgow (Scotland).14 They determined that although 98.4% of MP were removed, 65 million MP are released per day by the plant, which serves a population equivalent of 650![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.14 Detailed assessments of different treatment plant configurations and the effectiveness of different unit processes in removing MP can focus efforts of technological innovation to further reduce loads of MP delivered to the environment by WWTPs.
000.14 Detailed assessments of different treatment plant configurations and the effectiveness of different unit processes in removing MP can focus efforts of technological innovation to further reduce loads of MP delivered to the environment by WWTPs.
The current study sought to determine the removal potential of different size and shape classes of SAL across a spectrum of WWTP unit processes. SAL retention was compared for plants that employ (i) secondary treatment (activated sludge) as the final step, (ii) tertiary filtration (granular sand filtration) as the final step, and (iii) membrane filtration as the final step in a novel membrane bioreactor treatment plant. The inclusion of both conventional and innovative WWTP process configurations in the study provided insights into which unit processes have the greatest potential to remove SAL and can be used in the future to guide reduction of SAL, especially MP, levels in the environment.
Experimental
Wastewater treatment plants and sample collection
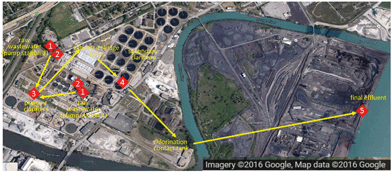
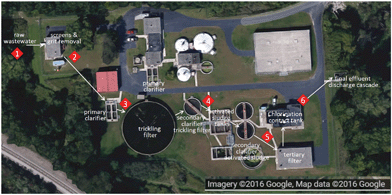
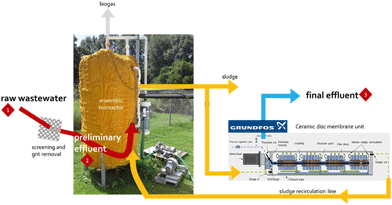
Samples were collected from two full-scale WWTPs, which were selected because of their different treatment trains and unit processes employed, and a novel pilot-scale WWTP. Grab samples were collected in plastic containers that were previously cleaned with deionized water (dH2O) and air-dried. Sample volumes were determined precisely, but varied for different sampling events (raw wastewater [1–2 L], preliminary effluent [1–6 L], primary effluent [10–20 L], secondary effluent [10–20 L], and final effluent [34–38 L]). Samples were transported to the laboratory and stored at 4 °C until further processing. Descriptions of the WWTPs and sampling locations are provided below.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v
1 (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) ratio. Materials removed by the bar screen and the grit chamber are landfilled. The preliminary effluent flows to primary clarifiers where heavy solids settle and are collected as primary sludge. Grease floats to the top and is skimmed from the surface and disposed of by landfilling. Secondary treatment is performed in an activated sludge system. This biological treatment step uses microorganisms to remove organic material in the wastewater and the microorganisms are removed in secondary clarifiers as secondary sludge. The final effluent is chlorinated to kill pathogenic microorganisms, dechlorinated to remove residual chlorine, and finally discharged into the Detroit River. Primary and secondary sludges are thickened with the addition of a polymer, dewatered and either landfilled, used as fertilizer in land applications, or incinerated.
v) ratio. Materials removed by the bar screen and the grit chamber are landfilled. The preliminary effluent flows to primary clarifiers where heavy solids settle and are collected as primary sludge. Grease floats to the top and is skimmed from the surface and disposed of by landfilling. Secondary treatment is performed in an activated sludge system. This biological treatment step uses microorganisms to remove organic material in the wastewater and the microorganisms are removed in secondary clarifiers as secondary sludge. The final effluent is chlorinated to kill pathogenic microorganisms, dechlorinated to remove residual chlorine, and finally discharged into the Detroit River. Primary and secondary sludges are thickened with the addition of a polymer, dewatered and either landfilled, used as fertilizer in land applications, or incinerated.
Sample processing and microplastic quantification
The procedure for sample processing and the identification and counting of SAL was modified from previous reports20 to be consistent with previous assessments of treatment plants published at the time of study design.6,11–14 In brief, five sieves with differing mesh size (U.S. Standard Sieve Series, A.S.T.M. E-11, Dual Manufacturing Co., Inc., Franklin Park, IL) were rinsed with dH2O and stacked: 4.75 mm (top), 0.85 mm, 0.3 mm, 0.106 mm, 0.02 mm (bottom). The sieve stack was placed in a laboratory sink and a complete sample was poured into the sieve stack slowly to prevent clogging of sieves and loss of sample due to overfill. Particles collected in each individual sieve were backwashed with a small amount of dH2O into a clean plastic bin. The collected water and particles were then poured into a small polypropylene plastic container using a funnel, and any remaining particles were transferred into the container with a small amount of dH2O. The above steps were repeated for every sieve size and the complete sieving process was completed for every sample. The processed samples were stored at 4 °C.All sieved fractions were processed in small subsets that were poured into a petri dish and observed through a stereomicroscope (7-30X StereoZoom, Bausch & Lomb, Rochester, NY). Identified SAL were removed from the sample individually using tweezers, counted per shape category: fragments (rough, irregularly shaped), fibers (both single filaments and threads of multiple twisted filaments), paint chips, microbeads (perfectly spherical), and other. Particles classified as non-plastic by observation (based on shape, size, color, and texture) were excluded from the counts.
During the sieving process, it was observed that some particles would not pass through the sieves even if sufficiently small due to their irregular shapes and the orientation of fibers. Therefore, the data from the different sieve size fractions were deemed unreliable, and were not reported (except for the Northfield WWTP samples collected on 19 October 2015, see ESI†). Rather, the number of SAL in the different size fractions were added and the total number of SAL in each shape category were reported for each sample.
To control for potential contamination by sample processing, e.g., from instrument contamination or environmental deposition, a blank control sample consisting of 20 L of dH2O was collected in a plastic bottle and processed in parallel with experimental samples. This blank control passed through identical sieving, container passage, and counting steps that the experimental samples experienced, as described above. Only one fiber was found in the 20 L control; no adjustments were made in the reported count data for samples.
All raw count data and R code21 generated to create figures and perform calculations are freely available on a public github repository and hyperlink (see ESI†).
Results and discussion
Microplastic and other SAL removal depended on wastewater treatment plant configuration
Based on the spring sampling, each of the unit processes at the Detroit WWTP removed a fraction of the SAL from the influent (133.0 ± 35.6 MP L−1) with the preliminary and primary treatment steps removing the largest amount of SAL (Fig. 4a and Table 1). Secondary treatment removed 12.9 MP L−1. The SAL concentration in the final effluent was lower than that in the secondary effluent suggesting that transport through chlorination and dechlorination and to the final outfall removed some additional SAL. The overall removal of SAL was 95.6% and the final effluent contained 5.9 SAL per L.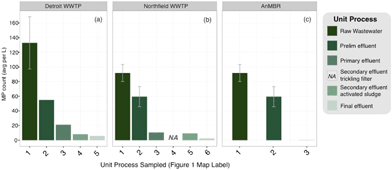 | ||
| Fig. 4 SAL removal profiles (SAL per L) along unit processes of the Detroit WWTP, error bars represent the 6 individual samples at the incoming raw wastewater sites (a), Northfield WWTP, error bars represent the 4 replicate samples taken at the raw and preliminary effluent sample points (b), and Northfield Pilot AnMBR, error bars represent the 4 replicate samples taken at the raw and preliminary effluent sample points (c) from Spring sampling. Numbers on the x-axis refer to sample points depicted in Fig. 1–3. Where present, error bars indicate standard deviations of replicate samples. Where error bars are absent, only one grab sample was collected. | ||
| Treatment step | Detroit WWTP | Northfield WWTP | AnMBR |
|---|---|---|---|
| Preliminary treatment | 58.6% | 35.1% | 35.1% |
| Primary treatment | 84.1% | 88.4% | N/A |
| Secondary treatment | 93.8% | 89.8% | N/A |
| Tertiary treatment | N/A | 97.2% | 99.4% |
As with the Detroit WWTP, the greatest removal of SAL at the Northfield WWTP took place during the preliminary and primary treatment steps, with lesser removal from secondary treatment (Table 1). Tertiary filtration at the Northfield WWTP provided removal to a degree beyond what was possible by secondary treatment alone (Table 1). The MP concentration in the final effluent was 2.6 SAL per L. The pilot-scale AnMBR system removed the highest percentage of incoming MP (Table 1), releasing 0.5 SAL per L in the final effluent.
The SAL removal rate by treatment step was calculated to assess their relative contributions to the overall removal from each by the final treatment steps at Detroit and Northfield did not contribute a large absolute removal (Fig. 4a and b, Table 1), the final steps at each plant (Detroit’s secondary treatment by activated sludge and Northfield’s tertiary treatment by granular sand filtration) still removed 60.9 and 72.7% of SAL remaining in the process, respectively (Table 2). The tertiary treatment (membrane filtration) for the AnMBR pilot process removed 99.1% of the remaining SAL, outperforming removal rates of final treatment in both the Detroit and Northfield plants (Table 2).
| Treatment step | Detroit WWTP | Northfield WWTP | AnMBR |
|---|---|---|---|
| Preliminary treatment | 58.6% | 35.1% | 35.1% |
| Primary treatment | 61.6% | 82.1% | N/A |
| Secondary treatment | 60.9% | 11.9% | N/A |
| Tertiary treatment | N/A | 72.7% | 99.1% |
In summary, the WWTPs in this study removed the majority (93.8–99.4%; Table 1) of SAL present in raw wastewater, consistent with other reports.11–16 Across all plants, most of the SAL were removed during preliminary treatment (screening and grit removal) and primary treatment (gravity separation and surface skimming on primary clarifiers) processes (Fig. 4), with limited additional removal accomplished in the secondary treatment step (activated sludge and trickling filters). This is consistent with a prior assessment that documented the majority (78%) of MP removal happening in the primary treatment phases and another 20% in secondary processing.14 A similar study attributed considerable removal of particles during initial stages of treatment to skimming and settling processes.13 However, tertiary filtration (granular sand filtration or anaerobic membrane bioreactor-based filtration) provided substantial additional polishing (Fig. 4 and Table 1). Previous studies have indicated that tertiary treatment does not consistently ensure notable reduction of SAL in effluent,13,14 but the type of tertiary treatment could influence this. For instance, granular tertiary filtration has been suggested as an ineffective measure for reducing MP loads in effluent.16 Further, in a NY study, four of the 10 WWTPs with advanced filter treatment still released microbeads, but the two plants with membrane filters (as the AnMBR plant here) did not.22
Seasonal variations in influent SAL levels did not result in variation in effluent SAL levels
Though raw wastewater samples were not collected for fall and winter samples at the Northfield WWTP (Fig. 5), the comparison of preliminary effluent across the three seasons suggests that the SAL levels in the raw wastewater were two-fold higher during the spring sampling event (Fig. 5). Despite different inferred levels of SAL in the incoming wastewater, primary treatment brought MP levels to a similar range and by the final effluent, SAL levels were 2.2, 1.4, and 2.6 SAL per L for fall, winter, and spring samples, respectively.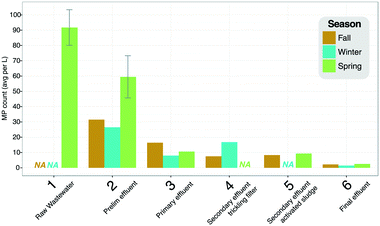 | ||
| Fig. 5 SAL removal profiles (SAL per L) measured in fall, winter, and spring, across the different unit processes at the Northfield WWTP. Error bars represent the 4 replicate samples taken at the raw and preliminary effluent sample points during the Spring sampling. Numbers on the x-axis correspond to sampling locations depicted in Fig. 2. | ||
Removal of different SAL shape classes was influenced by the unit processes employed
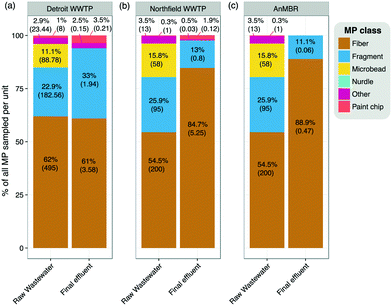
At the Detroit WWTP, 11.1% of the SAL in the raw wastewater was comprised of microbeads, while all microbeads were effectively removed during the plant's preliminary, primary, and secondary treatment processes (Fig. 6a). The Detroit WWTP did not completely remove fibers, fragments, paint chips, and other particles remaining in the final effluent (Fig. 6a).At the Northfield WWTP, microbeads were completely removed during the treatment process, which included preliminary, primary, secondary, and tertiary treatment steps (Fig. 6b). Fibers were the prominent SAL type released, representing almost 80% of the final effluent. While there was a 10-fold reduction in the number of fibers released in the AnMBR treatment, as compared to the conventional Northfield system, the fibers represented a greater percent of SAL in the effluent than in the other plant types. Paint chips, microbeads, and other particles were not detected in the AnMBR effluent. The results of a survey of 17 WWTPs across the U.S. also suggested that tertiary treatment may be most effective at removing fragment-type SAL, as the five fragment-dominated facilities in that study lacked tertiary treatment.16
Microbeads were absent from the final effluent at all plants (Fig. 6). This finding is consistent with a study of eight treatment plants in the San Francisco Bay area where microbeads were detected in the Bay water, but not in WWTP effluents.15 The absence of microbeads in the effluents in these studies was surprising considering the concerns raised about their ubiquity and the global campaigns to encourage action against their use,23,24 which resulted in new legislation, e.g., the Microbead-Free Waters Act signed by President Obama in 2015.25 However, closer examination of the nature of particles in personal care products revealed that the multi-colored perfectly spherical microbeads attributed to rinse-off personal care products15 are only a subset of personal care product-derived MP.26 In fact, most MP in personal care products are rough and irregularly shaped.13,26 Rather than as beads, these particle types were categorized as fragments in the Bay study, as well as here.
Implications of WWTPs as pathways of fibers to the environment
Evidence is mounting that personal care product-derived MP, both spherical and irregular, may represent only a small fraction of total MP in treatment plant effluent. Studies have confirmed that manufactured fibers are introduced to wastewater through the washing of clothing.27,28 In 27 plants across three studies (Fig. 6,15,16), fibers dominated the effluent in all but five U.S. plants. The data reported here suggest a daily release of 9 billion fibers from the Detroit WWTP and 8.9 million fibers from the Northfield WWTP (Table 3). If the Northfield WWTP implemented the novel AnMBR treatment at full scale, the fiber load in the effluent would be reduced ten-fold, yet still 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 fibers would be released per day.
000 fibers would be released per day.
| Treatment plant | % fiber retention | Flow rate (m3 day−1) | Billion fibers released (day−1) |
|---|---|---|---|
| Central WWTP of Vodokanal (St. Petersburg, Russia)12 | 65.74 | 959![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
153.4 |
| Detroit (Michigan, USA) | 99.28 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
8.94 |
| Seine Centre (Paris, France)29,30 | 88.97 | 240![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
7.68 |
| Viikimäki, Finland8 | 92.33 | 270![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3.73 |
| Northfield (Michigan, USA) | 97.38 | 1700 | 8.9 × 10−3 |
| Lysekil, Sweden11 | 99.96 | 5160 | 2.1 × 10−5 |
These values fall within the range of previously reported effluent fiber loads in studies using analogous methods (Table 3). These studies all have omitted a chemical oxidation step and counts are based on visual inspection—notably, over a dozen recent studies also have relied exclusively on visual identification of MP.16 While comparable, it is possible that not all fibers counted in the studies referenced here (Table 3) were manufactured SAL (petroleum or cellulose derived). In the absence of material type confirmation, fibers derived from non-anthropogenic litter (e.g., decomposing flora and fauna) may be counted. A recent study of wastewater treatment plant effluent found that 48% of all confirmed MP were comprised of polyester and polyamide (e.g., nylon),14 two common synthetic fiber materials—giving credence to their possible dominance in other investigations as well. Further, the positive identification rate of the smallest and most difficult to identify size class of SAL (100–1000 μm) in the research group that conducted the present study is 80% (confirmed by EDS-SEM, Brendon Locke, personal communication). As this field continues to mature and critical data gaps filled, efforts should be invested in developing methods to confirm material composition for these sample types, as current approaches are low throughput,14 can be incompatible with other necessary processing steps, and can be difficult to interpret due to the compositional complexity of manufactured materials.9
Given the size of the populations receiving collection from the Detroit and Northfield plants (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 357
357![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 666 and 9909, respectively19), these data suggest that 3794 and 903 fibers per capita are released in the effluents of these plants each day. In another report, a model was used to estimate the contribution of textile fibers to household effluent.28 Here it was estimated that 9–110 kg of microfibers are discharged per day from a model WWTP serving 100
666 and 9909, respectively19), these data suggest that 3794 and 903 fibers per capita are released in the effluents of these plants each day. In another report, a model was used to estimate the contribution of textile fibers to household effluent.28 Here it was estimated that 9–110 kg of microfibers are discharged per day from a model WWTP serving 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people.28
000 people.28
Based on their assumption of a fiber with an average length of 0.7 mm and linear density of 0.15 mg mm−1 (though contentious, discussed below), these data are consistent with a daily per capita release rate of 857–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 476 fibers. This range spans our estimated per capita load. A report documenting SAL in a Swedish plant serving 12
476 fibers. This range spans our estimated per capita load. A report documenting SAL in a Swedish plant serving 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people found 70% of the 3.25 million particles (>300 μm) entering the per hour to be fibers, 0.04% of which are released in the plant effluent.11 This is equivalent to 1.78 fibers per person per day discharged. While not exclusively fibers, a recent study in Glasgow, Scotland estimated a per capita MP release of 100 MP per person per day.14 These reports span four orders of magnitude, variability that can arise from multiple factors, including (i) differences in data processing and collection (e.g., discrete sizes reported, smallest size class counted, oxidation of labile particles, compositional confirmation), (ii) differences in assumptions and factors, such as mean fiber length and linear density used—a critical but currently poorly constrained value considering the diversity of fiber compositions possible, (iii) dynamic variability in flows and particle loads inherent to the flashiness of wastewater treatment plant systems, and (iv) details of the wastewater treatment plants investigated, e.g., degree of wastewater polishing, whether the plants treat a combined wastewater and stormwater stream, the type of polymers used in solids aggregation and its effectiveness in capturing manufactured particles. It is critical for future studies to reduce these sources of ambiguity as this young field of research matures and mitigation technologies at water treatment facilities are developed and evaluated.
000 people found 70% of the 3.25 million particles (>300 μm) entering the per hour to be fibers, 0.04% of which are released in the plant effluent.11 This is equivalent to 1.78 fibers per person per day discharged. While not exclusively fibers, a recent study in Glasgow, Scotland estimated a per capita MP release of 100 MP per person per day.14 These reports span four orders of magnitude, variability that can arise from multiple factors, including (i) differences in data processing and collection (e.g., discrete sizes reported, smallest size class counted, oxidation of labile particles, compositional confirmation), (ii) differences in assumptions and factors, such as mean fiber length and linear density used—a critical but currently poorly constrained value considering the diversity of fiber compositions possible, (iii) dynamic variability in flows and particle loads inherent to the flashiness of wastewater treatment plant systems, and (iv) details of the wastewater treatment plants investigated, e.g., degree of wastewater polishing, whether the plants treat a combined wastewater and stormwater stream, the type of polymers used in solids aggregation and its effectiveness in capturing manufactured particles. It is critical for future studies to reduce these sources of ambiguity as this young field of research matures and mitigation technologies at water treatment facilities are developed and evaluated.
Fibers delivered in WWTP effluent represents a considerable mass that is largely unaccounted for on an ecosystem scale. While work is needed to constrain some assumptions in light of the sources of variability listed above, the following exercise demonstrates the possible implications given the current state of the research field. The weighted mean SAL length in the final effluent analyzed in this study was 0.58 mm (ESI† Fig. S1). The fiber weight range of typical synthetic textiles is 0.7–40 dtex for polyester31 and 1.6–35 dtex for polyamide nylon,32 where dtex is a linear density metric representing 1 g per 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m. Applying the median linear density for these common textiles (0.002 mg mm−1), if only 80% of all the fibers released were comprised of such polymers, 6 kg of plastic fibers would be released per day from the Detroit WWTP.
000 m. Applying the median linear density for these common textiles (0.002 mg mm−1), if only 80% of all the fibers released were comprised of such polymers, 6 kg of plastic fibers would be released per day from the Detroit WWTP.
The Detroit WWTP effluent is released into the Detroit River. This river is the influent to Lake Erie, one of the five Laurentian Great Lakes, which together comprise the largest freshwater system on the planet. The mass of fibers in the Detroit WWTP effluent (0.003 mg L−1, when assuming 0.58 mm fibers and adjusting for the 20% false positive rate) is at most one hundredth of the phytoplankton biomass in the western basin of Lake Erie (0.27–2.27 mg L−1).33 If we assume a similar biomass of phytoplankton in the nearby Rouge River, which feeds the Detroit River and has an average annual flow rate of 0.5–10 m3 s−1,34 the Detroit treatment plant effluent may be delivering fibers equivalent to only 0.02% of the phytoplankton mass delivered by the Rouge River to Lake Erie. While this number is low, it captures fiber mass delivered by the WWTP effluent only. Other potential local sources of SAL include atmospheric deposition35 partially treated wastewater that bypasses secondary treatment at the Detroit plant during high-flow storm events (discussed below), and the Detroit River—which includes plastics from rivers draining runoff from the entire Great Lakes watershed.36 Notably, if all 99% of fibers that were diverted from the Detroit treatment plant effluent were retained in biosolids later spread on agricultural fields, and all washed out to the watershed via runoff to Lake Erie, this could result in the delivery of fibers equivalent to 2% of the phytoplankton mass delivered by the Rouge. It is critical to note that these estimates are sensitive to changes in the mean fiber length and the applied linear density. Previous studies have estimated mean fiber lengths of 0.7–5 mm and applied a linear density of 0.15 mg mm−1,28,37 which had been derived from a dtex of 300 for polyester and nylon37—a value we find to be atypically high.31,32 Assessments of the ecosystem-level impacts of the fiber-type SAL hinge upon additional data to constrain this variable.
Studies are needed to quantify the magnitude of these fluxes and the relative contribution of different MP and fiber pathways to focus mitigation efforts at wastewater treatment facilities, as MP and human-sourced fibers may be mistaken for food by resident fauna.38 Studies have found fibers in fish39 and MP in mussels and oysters40 sold or raised for human consumption. To inform innovation in mitigation and prevention technologies, the pathways of SAL into the environment must be further defined, as well as the relative contribution of SAL composition and sizes, with special focus on the dominant fibers.
Ultimate fate of SAL and recommendations
Based on the average wastewater flow of ∼2.5 billion L per day treated at the Detroit WWTP19 and the final effluent SAL concentration determined in this study (5.9 SAL per L), it is estimated that nearly 15 billion potential SAL particles of all shape classes are introduced into the Detroit River each day. Our results suggest that, through the introduction of tertiary filtration, the final effluent load could be reduced to 2.1 SAL per L (based on the average final effluent data for Northfield), thus reducing the number of SAL introduced in the Detroit River three-fold. Further, the AnMBR data (0.5 SAL per L in final effluent) suggest that the implementation of membrane bioreactor treatment could reduce the daily introduction of SAL to the Detroit River from 15 billion to 1.25 billion particles. In addition to a dramatic reduction in the number of SAL introduced, the types would also change. The membrane bioreactor effluent primarily contained fibers, whereas the Detroit WWTP final effluent contained a more diverse spectrum of SAL (Fig. 6).The WWTP reports11,12 and few studies8,13–16 available to date have used widely varying methods for MP and SAL quantification, making it difficult to compare results across studies. Further, most of these studies have not considered the smallest MP and SAL fractions. Misleadingly, one study concluded that “tertiary effluent is not a significant source of [MP] and that [they] are effectively removed during the skimming and treatment processes,”13 which we have shown not to be the case in the plants studied herein. Our results quantified SAL down to 20 μm and indicate that even in the most advanced WWTPs with membrane filtration, SAL, especially fibers, are not completely retained. As to whether those released represent a “significant” source, as pertains to human and environmental health, has yet to be confirmed.
As the vast majority (95–99%) of incoming SAL are removed from WWTP effluents (assuming secondary or more advanced treatment; Table 1),8,11,12,16 we must resolve further the fate of the SAL retained in solids. During preliminary treatment, large debris in raw wastewater is removed by screening and fine grit, sand, and glass are removed in a grit chamber. The solids collected in such preliminary treatment contain a substantial fraction of the MP removed from the raw wastewater; in all plants in this study, the largest bulk removal of SAL occurred in the preliminary treatment step (Fig. 4). These solids are typically disposed of in landfills and, assuming landfills are properly managed, are diverted from entering watersheds. In the primary and secondary clarifiers, solids are removed as primary and secondary sludge, and materials floating on the surface of clarifiers are removed by skimming. Our study suggests that most SAL removed in primary and secondary treatment were removed through adsorption to either sludge or surface solids. Murphy et al. determined that surface grease removed from the primary clarifier contained a substantially greater amount of SAL than the sludge cake produced through processing of primary and secondary sludge.14 Typically, surface solids are landfilled, whereas the fate of SAL in primary and secondary sludge is variable and depends on the sludge management strategy practiced, which can vary seasonally. Sludge is incinerated, sent to landfills, or used for agricultural land application after stabilization (biosolids).
When biosolids are land applied, the 95–99% of incoming SAL removed from raw wastewater will be delivered to the watershed through runoff as non-point source plastic pollution at an undocumented rate. Further research is needed to define the fate of SAL following land application of biosolids. Innovations for complete recovery of SAL, especially fibers, from wastewater processing would prevent their release to aquatic habitats. One possible direction that may be explored for the permanent removal of SAL from the environment is the biodegradation of SAL by microbes in WWTPs (e.g., in activated sludge systems or in anaerobic digesters used for sludge stabilization). A recent study suggests that this may be a possibility.41
Tertiary gravity sand filtration and membrane filtration as part of AnMBR treatment provided substantial additional removal of SAL. However, tertiary filters require regular backwashing. The solids removed through backwashing are typically sent to the beginning of the WWTP, thus re-introducing MP into the liquid stream of the WWTP. Since SAL removed through these treatment processes thus accumulate in the WWTP, there may be an opportunity for permanent removal of the SAL from the environment through biodegradation by microbes in the WWTP.
A currently undocumented possible pathway of SAL to the environment is from stormwater through runoff, the details of which are nontrivial, as stormwater management differs by municipality and many communities employ a combination of management systems. Combined sewer systems (e.g., Detroit19) carry both stormwater and wastewater, as opposed to systems that keep these streams separate (e.g., Northfield19). A massive influx of stormwater can overload the combined system, causing wastewater to bypass treatment or to be partially treated only and enter the environment at points of combined sewer overflows (CSOs) to prevent sewer backups. A previous survey of 17 WWTPs found an association between combined sewers and an increase in numbers of fragments discharged, not fibers.16 These dynamics require more detailed investigation. Separate storm sewer systems are networks intended to transport stormwater exclusively, delivering it untreated into rivers and lakes. The budget of SAL in each of these stormwater pathways has not been documented, but would inform freshwater SAL transport models. Increasingly, stormwater green infrastructure (SGI) is being implemented to reduce flooding and pollutant transfer to the watersheds.42 While communities that employ more SGI show no significant reduction in CSO events,42 the effectiveness of SGI in reducing SAL loads to waterways should be explored.
The U.S. has over 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 publically owned treatment works or municipal WWTPs that treat approximately 32 billion gallons per day (120 billion L per day) and serve 75% of the U.S. population.43 Approximately 10% of this total wastewater flow is treated by tertiary gravity sand filtration and less than 1% is treated by membrane filtration in membrane bioreactor plants (Daigger, G., personal communication, July 1, 2016). Since only a small fraction of WWTPs employ tertiary treatment, the Detroit WWTP, with secondary activated sludge treatment as its final treatment step, is representative of the majority of WWTPs in the U.S. with respect to SAL removal. While the Northfield WWTP is unique with two secondary treatment processes in series (i.e., a trickling filter followed by an activated sludge system), the additional secondary treatment process does not provide substantial additional MP removal (Fig. 4). However, the tertiary treatment step at Northfield increases the overall SAL removal by 7.4%, while final polishing by membrane filtration provides the highest overall SAL removal efficiency (Fig. 4 and Table 1) and serves as a proof of concept gold standard for processing.
000 publically owned treatment works or municipal WWTPs that treat approximately 32 billion gallons per day (120 billion L per day) and serve 75% of the U.S. population.43 Approximately 10% of this total wastewater flow is treated by tertiary gravity sand filtration and less than 1% is treated by membrane filtration in membrane bioreactor plants (Daigger, G., personal communication, July 1, 2016). Since only a small fraction of WWTPs employ tertiary treatment, the Detroit WWTP, with secondary activated sludge treatment as its final treatment step, is representative of the majority of WWTPs in the U.S. with respect to SAL removal. While the Northfield WWTP is unique with two secondary treatment processes in series (i.e., a trickling filter followed by an activated sludge system), the additional secondary treatment process does not provide substantial additional MP removal (Fig. 4). However, the tertiary treatment step at Northfield increases the overall SAL removal by 7.4%, while final polishing by membrane filtration provides the highest overall SAL removal efficiency (Fig. 4 and Table 1) and serves as a proof of concept gold standard for processing.
Conclusion
Retrofitting existing WWTPs with tertiary granular sand filtration and membrane filtration would result in the highest possible removal of MP. Unless regulatory changes take effect, such upgrades are not likely to take place to mitigate SAL pollution. However, there is precedent for change. More established regulatory drivers (e.g., due to nitrogen and phosphorus regulations) have resulted in widespread regional implementation of tertiary filtration. For example, the majority of WWTPs discharging their final effluents in the Chesapeake Bay or in Chesapeake Bay tributaries have now implemented tertiary filtration to meet strict nitrogen standards (Daigger, G., personal communication, July 1, 2016). Innovative solutions to the environmental problem of SAL debris will be most effectively delivered when the sources, sinks, and their fluxes are resolved at an ecosystem scale—thereby informing future research, policy, management, mitigation, and outreach efforts.Acknowledgements
We are grateful to Rachel Cable for providing training for data collection and critically reviewing the manuscript and Krista Wigginton for providing access to the Environmental Biotechnology Laboratories at the University of Michigan. We thank Michael Jurban for assistance with sampling at the Detroit WWTP, Tim Hardesty, Caroline Van Steendam, Bridget Vial, and Nigel Beaton for help with sampling at the Northfield WWTP, and Xunchang Fei for assistance with laboratory equipment. This work was supported by the University of Michigan Water Center, a center of the Graham Sustainability Institute. The Water Center is supported by funds from the Fred A. and Barbara M. Erb Family Foundation and the University of Michigan.Notes and references
- R. C. Thompson, C. J. Moore, F. S. vom Saal and S. H. Swan, Plastics, the environment and human health: current consensus and future trends, Philos. Trans. R. Soc., B, 2009, 364, 2153–2166 CrossRef CAS PubMed.
- C. M. Rochman, M. A. Browne, A. J. Underwood, J. A. Franeker, R. C. Thompson and L. A. Amaral-Zettler, The ecological impacts of marine debris: unraveling the demonstrated evidence from what is perceived, Ecology, 2016, 97, 302–312 Search PubMed.
- M. Wagner, C. Scherer, D. Alvarez-Muñoz, N. Brennholt, X. Bourrain, S. Buchinger, E. Fries, C. Grosbois, J. Klasmeier and T. Marti, Microplastics in freshwater ecosystems: what we know and what we need to know, Environ. Sci. Eur., 2014, 26, 1–9 CrossRef.
- M. Cole, P. Lindeque, C. Halsband and T. S. Galloway, Microplastics as contaminants in the marine environment: a review, Mar. Pollut. Bull., 2011, 62, 2588–2597 CrossRef CAS PubMed.
- D. Eerkes-Medrano, R. C. Thompson and D. C. Aldridge, Microplastics in freshwater systems: a review of the emerging threats, identification of knowledge gaps and prioritisation of research needs, Water Res., 2015, 75, 63–82 CrossRef CAS PubMed.
- C. M. Rochman, S. M. Kross, J. B. Armstrong, M. T. Bogan, E. S. Darling, S. J. Green, A. R. Smyth and D. Veríssimo, Scientific Evidence Supports a Ban on Microbeads, Environ. Sci. Technol., 2015, 49, 10759–10761 CrossRef CAS PubMed.
- T. Bowmer and P. Kershaw, 2010, Proceedings of the GESAMP International Workshop on microplastic particles as a vector in transporting persistent, bioaccumulating and toxic substances in the oceans, UNESCO-IOC GESAMP Reports and Studies, 2010, p. 82 Search PubMed.
- J. Talvitie, M. Heinonen, J. P. Pääkkönen, E. Vahtera, A. Mikola, O. Setälä and R. Vahala, Do wastewater treatment plants act as a potential point source of microplastics? Preliminary study in the coastal Gulf of Finland, Baltic Sea, Water Sci. Technol., 2015, 72, 1495–1504 CrossRef CAS PubMed.
- F. Remy, F. Collard, B. Gilbert, P. Compère, G. Eppe and G. Lepoint, When Microplastic Is Not Plastic: The Ingestion of Artificial Cellulose Fibers by Macrofauna Living in Seagrass Macrophytodetritus, Environ. Sci. Technol., 2015, 49, 11158–11166 CrossRef CAS PubMed.
- A. McCormick, T. J. Hoellein, S. A. Mason, J. Schluep and J. J. Kelly, Microplastic is an abundant and distinct microbial habitat in an urban river, Environ. Sci. Technol., 2014, 48, 11863–11871 CrossRef CAS PubMed.
- K. Magnusson and F. Norén, Screening of microplastic particles in and down-stream a wastewater treatment plant, IVL Swedish Environmental Research Institute Report, 2014, C 55 Search PubMed.
- J. Talvitie and M. Heinonen, Preliminary study on synthetic microfibers and particles at a municipal waste water treatment plant, Baltic Marine Environment Protection Commission HELCOM, 2014, pp. 1–14 Search PubMed.
- S. A. Carr, J. Liu and A. G. Tesoro, Transport and fate of microplastic particles in wastewater treatment plants, Water Res., 2016, 91, 174–182 CrossRef CAS PubMed.
- F. Murphy, C. Ewins, F. Carbonnier and B. Quinn, Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment, Environ. Sci. Technol., 2016, 50, 5800–5808 CrossRef CAS PubMed.
- R. Sutton, S. A. Mason, S. K. Stanek, E. Willis-Norton, I. F. Wren and C. Box, Microplastic contamination in the San Francisco Bay, California, USA, Mar. Pollut. Bull., 2016, 109, 230–235 CrossRef CAS PubMed.
- S. A. Mason, D. Garneau, R. Sutton, Y. Chu, K. Ehmann, J. Barnes, P. Fink, D. Papazissimos and D. L. Rogers, Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent, Environ. Pollut., 2016, 218, 1045–1054 CrossRef CAS PubMed.
- A. L. Lusher, M. McHugh and R. C. Thompson, Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel, Mar. Pollut. Bull., 2013, 67, 94–99 CrossRef CAS PubMed.
- International Agency for Research on Cancer (IARC) Web site for Benzadine. https://monographs.iarc.fr/ENG/Monographs/vol100F/mono100F-7.pdf (accessed 25 September 2016).
- EPA Clean Watersheds Needs Survey 2012 Data and Reports, https://Ofmpub.Epa.Gov/apex/cwns2012/f?P=cwns2012:1:, (accessed July 2016) Search PubMed.
- J. Masura, J. E. Baker, G. Foster, C. Arthur and C. Herring, Laboratory methods for the analysis of microplastics in the marine environment: recommendations for quantifying synthetic particles in waters and sediments, NOAA Technical Memorandum NOS-OR&R-48, 2015, pp. 1–31 Search PubMed.
- Team R. C., R: A language and environment for statistical computing. R Foundation for Statistical Computing (Austria). http://www.R-project.org, (accessed July 2016) Search PubMed.
- NYS AOG, Discharging Microbeads to our Waters: An Examination of Wastewater Treatment Plants in New York, New York State Office of the Attorney General, Environmental Protection Bureau, 2015, pp. 1–11 Search PubMed.
- Why Illinois has banned exfoliating face washes, New Scientist, https://www.newscientist.com/article/dn25773-why-illinois-has-banned-exfoliating-face-washes/, (accessed June 2016) Search PubMed.
- Beat the Microbead, https://www.beatthemicrobead.org/, (accessed October 2015) Search PubMed.
- Microbead-Free Waters Act of 2015 (USA). https://www.congress.gov/bill/114th-congress/house-bill/1321/all-info, (accessed June 2016) Search PubMed.
- I. E. Napper, A. Bakir, S. J. Rowland and R. C. Thompson, Characterisation, quantity and sorptive properties of microplastics extracted from cosmetics, Mar. Pollut. Bull., 2015, 99, 178–185 CrossRef CAS PubMed.
- M. A. Browne, P. Crump, S. J. Niven, E. Teuten, A. Tonkin, T. Galloway and R. Thompson, Accumulation of microplastic on shorelines woldwide: sources and sinks, Environ. Sci. Technol., 2011, 45(21), 9175–9179 CrossRef CAS PubMed.
- Microfiber pollution and the apparel industry, http://brenmicroplastics.weebly.com, (accessed June 2016) Search PubMed.
- J. Gasperi, R. Dris, V. Rocher and B. Tassin, Microplastics in the continental area: an emerging challenge, Norman Bulletin, 2015,(4), 18–19 Search PubMed , http://www.norman-network.net/sites/default/files/files/bulletins/NORMAN%20Bulletin_n4March2015_vfinal.pdf, (accessed September 2016).
- J. Passerat, N. K. Ouattara, J. M. Mouchel, V. Rocher and P. Servais, Impact of an intense combined sewer overflow event on the microbiological water quality of the seine river, Water Res., 2011, 45, 893–903 CrossRef CAS PubMed.
- Barnet Europe Polyester Fibers, http://www.barnet-europe.com/en/fibers/staple-fiber/polyester.html, (accessed 25 September 2016) Search PubMed.
- N. A. Kalebek and O. Babaarslan, Fiber Selection for the Production of Nonwovens, Intech, 2016, pp. 1–32 Search PubMed.
- J. D. Conroy, D. D. Kane, D. M. Dolan, W. J. Edwards, M. N. Charlton and D. A. Culver, Temporal trends in Lake Erie plankton biomass: Roles of external phosphorus loading and Dreissenid mussels, J. Great Lakes Res., 2005, 31, 89–110 CrossRef CAS.
- USGS EDNA derived watersheds for major named rivers, http://Edna.Usgs.Gov/watersheds/kml_index.Htm, (accessed July 2016).
- R. Dris, J. Gasperi, R. Rocher, M. Saad, N. Renault and B. Tassin, Microplastic contamination in an urban area: a case study in Greater Paris, Environ. Chem., 2015, 12, 592e599 Search PubMed.
- A. K. Baldwin, S. R. Corsi and S. A. Mason, Plastic Debris in 29 Great Lakes Tributaries: Relations to Watershed Attributes and Hydrology, Environ. Sci. Technol., 2016, 50, 10377–10385 CrossRef CAS PubMed.
- P. Sundt, P.-E. Schulze and F. Syversen, Sources of microplastic-pollution to the marine environment, Mepex Report for the Norwegian Environment Agency, 2014 Search PubMed.
- S. L. Wright, R. C. Thompson and T. S. Galloway, The physical impacts of microplastics on marine organisms: a review, Environ. Pollut., 2013, 178, 483–492 CrossRef CAS PubMed.
- C. M. Rochman, A. Tahir, S. L. Williams, D. V. Baxa, R. Lam, J. T. Miller, F. C. Teh, S. Werorilangi and S. J. Teh, Anthropogenic debris in seafood: Plastic debris and fibers from textiles in fish and bivalves sold for human consumption, Sci. Rep., 2015, 5, 14340 CrossRef CAS PubMed.
- L. Van Cauwenberghe and C. R. Janssen, Microplastics in bivalves cultured for human consumption, Environ. Pollut., 2014, 193, 65–70 CrossRef CAS PubMed.
- S. Yoshida, K. Hiraga, T. Takehana, I. Taniguchi, H. Yamaji, Y. Maeda, K. Toyohara, K. Miyamoto, Y. Kimura and K. Oda, A bacterium that degrades and assimilates poly(ethylene terephthalate), Science, 2016, 351, 1196–1199 CrossRef CAS PubMed.
- M. J. Pennino, R. I. McDonald and P. R. Jaffe, Watershed-scale Impacts of Stormwater Green Infrastructure on Hydrology, Nutrient Fluxes, and Combined Sewer Overflows in the Mid-Atlantic Region, Sci. Total Environ., 2016, 565, 1044–1053 CrossRef CAS PubMed.
- Water and Wastewater Systems Sector: Department of Homeland Security, https://www.dhs.gov/water-and-wastewater-systems-sector, (accessed June 2016) Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ew00207b |
| This journal is © The Royal Society of Chemistry 2016 |