A review of recent developments in graphene-enabled membranes for water treatment
Yi
Jiang
 ,
Pratim
Biswas
and
John D.
Fortner
*
,
Pratim
Biswas
and
John D.
Fortner
*
Department of Energy, Environmental, and Chemical Engineering, Washington University in St. Louis, St. Louis, Missouri 63130, USA. E-mail: jfortner@wustl.edu; Fax: +1 314 935 5464; Tel: +1 314 935 9293
First published on 27th September 2016
Abstract
Graphene based materials, including graphene and derivatives such as graphene oxide, have considerable potential as key components in next-generation membrane technologies. Their tunable size, surface chemistry, and structure can be engineered for a spectrum of aqueous filtration purposes ranging from ultrafiltration to reverse osmosis. Among a number of recently developed advanced graphene-enabled membranes, monolayer nanoporous graphene membranes, graphene oxide membranes, and polymeric membranes incorporated with graphene oxide (graphene oxide as nanoscale, material fillers), are currently considered to be the most promising. In this review, the most recent advancements in the development of these three types of membranes are described and discussed, followed by opportunities and challenges presented for graphene-enabled separation technologies. Finally, we conclude the article by providing our perspectives on commercialization potentials based on current technological readiness and cost levels for these graphene-based membranes.
Water impactMembrane technologies have recently experienced considerable nano-technological advancements while growing as key process components towards integrated water treatment and reuse. Novel engineered nanoscale materials such as graphene have the potential to significantly advance the performance of current membrane-based treatment approaches, and may even revolutionize the long-existing polymeric membrane industry. |
1. Introduction
Water quantity and quality underpin major yet interwoven global development and sustainability challenges spanning from human health to the techno-economics of energy production. In line with the rapid development and application of nanoscale material science over the last two to three decades, water treatment technologies have undergone significant advancements. In particular, membrane technologies, which have experienced some remarkable nano-enabled technological breakthroughs, are becoming a key component for integrated water treatment and reuse. For example, thin film nanocomposite (TFN) membranes,1 with preferential water flow paths created by the incorporation of nanoparticles with small, hydrophilic pores, have reached commercialization (LG NanoH2O). More recently, membrane technologies incorporating engineered carbon nanomaterials, such as graphene, carbon nanotubes (CNT) and fullerenes, have demonstrated superior and even unique physical and chemical properties compared to traditional analogues, and may eventually prove economically advantageous, as engineered carbon nanomaterial costs continue to decline with the ongoing development of industrial scale production processes.2Among attractive material properties for separation processes, ultrafast transport of water molecules though confined carbon nanochannels has been demonstrated. Water permeation through CNTs was observed to be 3–5 orders of magnitude higher (faster) than the upper limits predicated by the Hagen–Poiseuille equation due to violation of no-slip boundary conditions.3 Such properties have motivated significant research interests towards engineering well-ordered nanoporous (membrane) structures, which can gate molecular transport through CNT cores. However, scalable fabrication processes for such (ordered CNT) membranes remain a significant challenge. In the past three years, research interests in graphene, a relatively recently discovered two-dimensional (2D) carbon allotrope, and its oxidized derivatives (graphene oxide, GO), has rapidly emerged, as reflected by the large and increasing numbers of related publications and patents each year. Graphene and graphene oxide also have unique material advantages compared to CNTs with regard to separation technologies. GO can be (relatively) more easily fabricated and engineered with regard to size, surface chemistry, and structure. In addition, its manufacturing consumes considerably less energy, thus making it economically competitive (e.g., 500–1000 MJ kg−1 by solvent exfoliation of graphite or chemical reduction of GO, compared to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MJ kg−1 of CNTs).4 Among a number of recently developed graphene-enabled membranes, some of the most promising are monolayer nanoporous graphene desalination membranes, GO membranes, and polymeric membranes incorporated with graphene oxide (GO as nanofillers). In the following sections, we provide a brief introduction of relevant graphene materials; discuss some of the most recent developments in graphene-enabled water treatment membranes; and conclude with a perspective on both opportunities and challenges for such membranes as next-generation water treatment and separation technologies.
000 MJ kg−1 of CNTs).4 Among a number of recently developed graphene-enabled membranes, some of the most promising are monolayer nanoporous graphene desalination membranes, GO membranes, and polymeric membranes incorporated with graphene oxide (GO as nanofillers). In the following sections, we provide a brief introduction of relevant graphene materials; discuss some of the most recent developments in graphene-enabled water treatment membranes; and conclude with a perspective on both opportunities and challenges for such membranes as next-generation water treatment and separation technologies.
2. What we talk about when we talk about graphene?
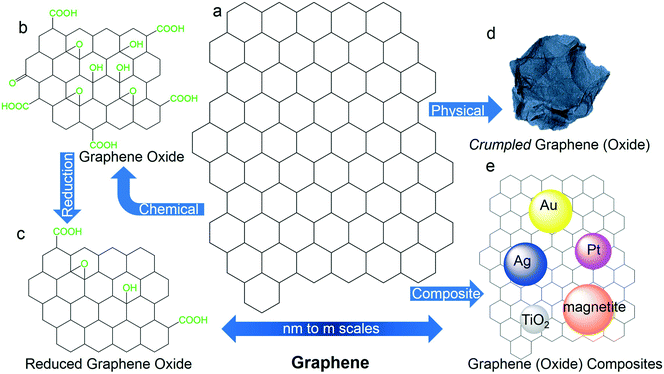
The graphene ‘gold rush’ began with the discovery of a freestanding 2D atomically thin carbon film which earned Geim and Novoselov the 2010 Nobel Prize in Physics.5 Graphene is one layer of carbon atoms packed into honeycomb pattern (Fig. 1a), which is ultrathin (0.35 nm in thickness), while being ultra-strong and exceptionally conductive. Graphene can have different sizes and qualities (i.e., degree of defects), depending on the fabrication methods, such as mechanical exfoliation of graphite,5 chemical vapor deposition (CVD),6 and reduction of graphene oxide (reduced graphene oxide, RGO).7 Material dimensions can span from <20 nm, described as quantum dots, to μm sizes of nanosheets and meter-scale films,8,9 which support application in various fields and correspondingly require different characterization and engineering approaches. Additionally, graphene materials can have a broad variety of surface chemistries resulting from a number of synthesis and/or subsequent chemical functionalization processes.Currently, an important and widely studied family of graphene derivatives is graphene oxide (GO) (Fig. 1b), which is the product of exfoliation of graphite oxide and is the precursor for RGO synthesis by either chemical or thermal reductions.7,10 Detailed chemical structure (surface chemistry) of GO has not been completely resolved due to the pseudo-random chemical functionalization of each layer and variations in composition and size.11,12 In principle, GO partially remains as a one-atom-thick planar sheet with a sp2-bonded carbon structure while being derivatized with oxygen functional groups both on the basal plane (e.g. hydroxyl and epoxy groups) and at the sheet edges (e.g. carboxyl and carbonyl, etc.) (a generic structure shown in Fig. 1b). Compared to graphene, GO has the distinctive feature of being water-dispersible due to electrostatic repulsions between deprotonated carboxyl groups.13 This feature allows for convenient processing of graphene materials as water can be used in lieu of organic solvents. For technical applications, oxygen-based functionality allows for enhanced surface hydrophilicity and aqueous stability, which are discussed later. Further, graphene oxide can undergo various physical transformations. For example, 2D GO was structurally engineered to have various crumpled morphologies to give specific properties (e.g., aggregation-resistance). These morphologies include paper ball-like spheres13–15 and corrugated surfaces (Fig. 1d),16 among others.
Another attractive feature of graphene is its intrinsic antimicrobial properties, which has led to applications in anti-microbial coatings and antifouling membranes. Proposed mechanisms of bacterial inactivation/cell membrane damage includes physical disruption,17 oxidative stress,18 and extraction of phospholipids from cell membranes.19
Graphene has been incorporated into a variety of composite materials,20 due to extremely high specific surface area(s) and ease of broad functionalization, which offer abundant ‘anchoring’ sites for various functional nanoparticles, including magnetic Fe3O4,21 photo-reactive TiO2,22 antimicrobial Ag23 and Au,24 and multifunctional nanocomposites such as graphene–TiO2–magnetite,25 graphene–Au–magnetite.26 (Fig. 1e).
As discussed, graphene/graphene derivatives are a large, diverse family of materials. Size, surface chemistry, morphology, and cost can be drastically different, even by orders of magnitude (e.g., size by 109, surface oxidation degree by 102). However, it is such diversity that has also underpinned potential applications in a wide range of water separation applications.
3. Membrane development and application
3.1. Monolayer nanoporous graphene for desalination applications
Despite being only one atom thick, graphene is generally considered to be impermeable to gases and liquids.27 Yet in its nanoporous form, graphene has been hailed as an ideal candidate for reverse osmosis (RO) membranes as it is atomically thin while mechanically robust. In theory, the ultimately thin selective layer can maximize permeability, which scales inversely with selective layer thickness. It is intuitive to imagine ‘knocking out’ carbon atoms from the matrix to form pores for separation purposes, which was initially examined through a series of theoretical studies.28,29 The concept of nanoporous graphene RO membrane was explored by Cohen-Tanugi and Grossman via molecular dynamics (MD) simulations.29 Their MD simulations included two water reservoirs separated by a nanoporous graphene layer, and water and ions were subjected to a driving force across the membrane (i.e. pressure) (Fig. 2a). They estimated that the water permeability could reach as high as ∼103 L m−2 h−1 bar−1, which is 2–3 orders of magnitude greater than that of typical thin film composite RO membranes (∼1–10 L m−2 h−1 bar−1).29 They also revealed that full salt rejection could be achieved with precisely controlled (radius ≤ 0.27 nm) uniform pores. Over the past few years, researchers began to experiment with graphene nanosheets through various methods to explore controlling pore size (basal plane, 0.4–10 nm). In one recent study,30 Surwade et al. successfully developed nanoporous monolayer graphene using an oxygen plasma etching process (Fig. 2b). With 1.5 s exposure to oxygen plasma, pores with size range of 0.5–1 nm and a density of 1012 cm−2 were observed. Resulting membranes exhibited a salt rejection rate of nearly 100% and water flux as high as 70 g m−2 s−1 atm−1 (∼250 L m−2 h−1 bar−1) with osmotic pressure as the driving force.30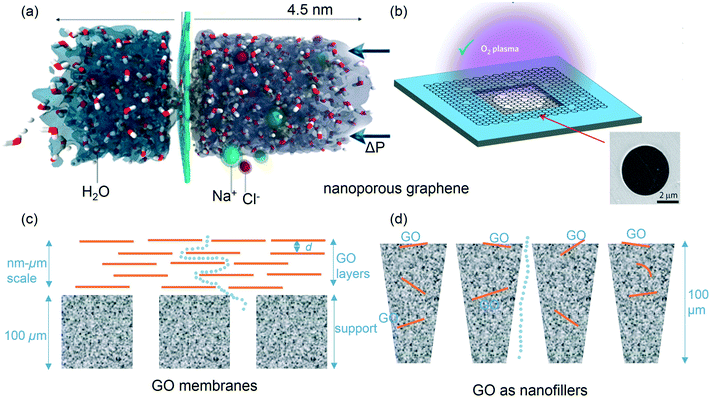 | ||
| Fig. 2 (a) Computational system (molecular dynamics) used in previous study by Cohen-Tanugi and Grossman,29 reprinted with permission from ref. 29, copyright 2012 American Chemical Society; (b) schematic and SEM image of single-layer graphene suspended on a 5 μm-diameter hole. O2 plasma treatment was found to successfully create controlled nanopores in graphene.30 Reprinted with permission from ref. 30, copyright 2015 Nature Publishing Group. (c) Schematic diagram of GO membranes, the deposited GO layers can be GO nanosheets or nanocomposites; (d) schematic diagram of GO as nanofillers in polymeric membranes. | ||
While nanoporous graphene certainly has tremendous potential for desalination membranes (successfully demonstrated in both theoretical and experimental studies), to date, ‘success’ has been accomplished at very small scales (micrometer sizes) and under nearly ideal reaction/processing conditions. Achieving highly uniform, sub-nanometer pores in large-area sheets of graphene remains the critical challenge for nanoporous graphene membranes to be mass produced.31 Despite these challenges, manufacturing of large-area graphene films has made recent progress, and monolayer film as large as >30 inch can be manufactured by a roll-to-roll CVD processes.9 However, defects in CVD graphene sheets from bulk growth and transfer processes, are a likely source of potential problems for desalination applications, which will not only decrease the salt rejections by serving as ion channels, but also compromise the membrane's mechanical integrity. This underpins current needs for technological improvements in fabricating large-area and (near) intact graphene and/or approaches to effectively address unintended defects. While complete elimination of such defects seems improbable in the short term, effectively ‘sealing’ defects may be a technically realistic approach. For example, hafnia and nylon were deposited onto defects-embedded monolayer graphene to block defects, which, when assembled, demonstrated effective reduction of potassium chloride leakage from the membrane.32 Further, new and novel methods to create evenly distributed and uniform nanopores need to be developed, in addition to what has been demonstrated (e.g., electron beam exposure, oxidative etching, and ion/cluster bombardment). The challenges with mass application of nanoporous graphene membranes do not only exist in the fabrication of nanoporous graphene itself, but also in the integrated manufacturing process of the filtration system, such as integration of the graphene-based layer and support, which has yet to be demonstrated. Technically integrating graphene active layers with mechanically appropriate/viable membrane supports in a scalable and cost-effective fashion, either through modification of membrane fabrication procedures or other new procedures, is a key hurdle for commercial reality. In this regard, developing detailed experimental understanding of deformation and fracture (micro-)mechanisms under typical RO conditions is crucial.33
3.2. GO membranes
GO-based membrane demonstrations began with so-called GO paper(s) (here for convenience, we distinguish GO papers as free-standing GO laminates, without polymer support, while GO membranes are typically described as GO-polymeric composite membranes). GO paper laminates are a (surface) deposited collection of micron-sized GO crystallites forming an interlocked layered structure.34,35 For these, scanning electron microscopy (SEM) imaging reveals well-packed layers through the entire cross-section of the GO paper layering. GO layer-to-layer distance (d-spacing) has been estimated to be about 0.83 nm from X-ray diffraction experiments.34 Such spacing is hypothesized to allow for low-friction flow of water, while being able to reject other gases, including helium (i.e. H2O vapor permeates through the membranes at least 1010 times faster than He).36 While such layers are vacuum-tight in a dry state, if immersed in water, they can act as molecular sieves, blocking all solutes with hydrated radii larger than 4.5 angstroms.37 Interestingly, smaller ions permeate through the membranes at rates thousands of times faster than what is expected for simple diffusion, which is attributed to capillary-like high pressures.37 Nevertheless, permeation (flux) through these GO papers remains insufficient to technically compete with current commercial pressure-driven membranes.38GO membranes are GO paper-like selective and/or functional layers on top of porous supports (e.g., polymeric polysulfone (Psf), polyethersulfone (PES) membranes) (Fig. 2c). Conceptually, such membranes are made by deposition of a thin layer of GO or GO nanocomposites (a few nm to μm) onto a relatively thick support membrane (usually >100 μm) via various techniques, such as vacuum filtration39 and layer by layer deposition.40 GO layers deposited onto polymeric supports are typically thinner than the free-standing GO papers. The deposited layer(s) are hypothesized to form nanochannels, which facilitate fast water transport, while also achieving selectivity.23,40
Hu and Mi created a selective surface layer atop a Psf support through a layer-by-layer deposition method with cross-linked flat GO nanosheets.40 As-synthesized membranes showed a 4–10 times higher water flux (∼8–27 L m−2 h−1 bar−1) than that of most commercial, comparable nanofiltration membranes.40 Observed high water flux was partially attributed to the unique water transport properties of the GO nanochannels formed between two horizontally paralleled GO nanosheets. In our recent work,23,41 we designed and demonstrated assemblies of crumpled graphene oxide (CGO) with vertically tortuous nanochannels for ultrafiltration, which have water flux as high as ∼400 L m−2 h−1 bar−1 (3.7 g m−2 deposition), outperforming comparable commercial ultrafiltration membranes. In both studies,23,40 surface charge is hypothesized to play a role in rejection performance, in addition to size exclusion effects. Huang et al. demonstrated pore channels of 3–5 nm in size by sacrificially etching out the copper hydroxide nanostrands (∼2.5 nm in diameter) sandwiched within a GO membrane.38 By ‘opening up’ such channels, higher water permeation was achieved (∼700 L m−2 h−1 bar−1) compared to pristine GO membranes, while still having fairly high rejection of small model foulants (e.g., complete rejection of 5 nm gold nanoparticles).38 Other methods include intercalating carbon nanotubes,39 and carbon dots.42 Further, for bio-fouling control, (partial) coverage of thin-film composite polyamide membranes by GO nanosheets were achieved using amide coupling between carboxyl groups of GO and carboxyl groups of polyamide.43 These membranes demonstrated potentially high antibacterial activity, as 65% E. coli inactivation was observed after 1 h surface contact, without causing detrimental effects to membrane transport properties.43
When combined with other, functional nanoparticles, GO membranes can be further engineered to be photo-reactive23,44 or (more) antimicrobial,23,43 achieving simultaneous filtration, pollutant transformation, and pathogen inactivation. For example, antimicrobial properties of a GO membrane were observed to be even further enhanced through the incorporation of Ag NPs in the GO layer, achieving almost complete inactivation of bacteria.23 Ray et al.24 modified the polyamide membranes with GO and Au nanostars, and showed additional antibacterial properties via photothermal effects of Au upon laser irradiation. In addition, photo-reactive (reduced) GO–TiO2 composite membrane surfaces were created via layer-by-layer deposition44 and vacuum filtration.23,45 In batch mode, membrane coupons have approximately one order of magnitude lower photo-reaction rate constants compared to those of monomeric (suspended) graphene–TiO2 nanocomposites, due to decrease of available surface areas to both light and model organic pollutants.24 These composite membranes showed higher permeate fluxes (and pollutant removals) under UV light irradiation conditions when evaluated in a flow-through mode.23,44,45
Taken together, GO membranes have unique technological and economic comparative advantages over the other two types discussed. Advantages of GO membranes include the simple fact that they do not need to meet the high quality (low defect) requirements of graphene materials required for a 2-D nanoporous graphene membrane. GO membranes can be tuned to cover a broader spectrum of membrane applications from MF to RO. In addition, GO membranes are less material intensive compared to other approaches such as using GO as nanofillers (component impregnation, discussed later), considering the (ultra)thin nature of the surface layer. The top (active) layer can be as thin as a few atomic layers (∼10 nm), corresponding to a mass density of only dozens of mg m−2, compared to component impregnation (into the entire membrane matrix), whereby the material consumption can be tens or hundreds of times higher (g m−2).
Nevertheless, synthesizing GO membranes involves fairly complicated chemical processes, including support membrane pre-treatment, cross-linking of GO sheets, and even pre-functionalization of GO sheets. Additional work is needed to understand and develop each individual process/integration. Current technical schemes are focused on vacuum filtration,23,45 or chemical cross-linking using 1,3,5-benzenetricarbonyl trichloride (TMC)40 or amine-based agents.23,43 Approaches like electrostatic layer-by-layer deposition, in situ synthesis are also emerging, with interest focused on simple, reproducible linkages. For (re)active GO membranes (e.g., GO–Ag and GO–TiO2 membranes), it can be difficult to integrate functional materials into current membrane fabrication and application processes. Further, many metal-oxide particles dissolve over time, thus rendering the membrane less effective. Therefore, effective regeneration strategies are needed for dissolution-based mechanism of bacterial inactivation (e.g., GO–Ag membranes).
GO membranes likely have very different water transport mechanisms. For example, it was observed that water flux does not decrease monotonically as the number of flat GO layers increased;40 however, for crumpled GO membranes shown in our recent work,23 the water flux does decrease with the increase of CGO mass/thickness, similar to conventional polymeric membranes. For GO-based membrane surfaces, water transport mechanisms have been proposed based on previous understandings of free-standing GO papers (as discussed earlier), but more evidence is needed to support such unique mechanisms. Additionally, fundamental understanding of ultrathin GO active layers with regard to enhanced membrane performance is needed. Furthermore, separation mechanisms are still not well understood, but likely include some combination of size exclusion, depth filtration and charge-based processes, depending on the membrane material and structure. Looking forward, fundamental understanding of water transport and molecular/ion retention will lead to new design and development of robust yet effective GO membranes.
3.3. GO as (nanoscale) fillers
Incorporation of nanomaterials into polymeric membranes has been extensively studied with nanoparticles such as CNT, TiO2, SiO2, and Ag.46 Nanoparticles can be readily blended into the solvent(s) used in the phase inversion or interfacial polymerization processes for membrane fabrication. In a similar manner, small amounts of GO (usually 0.1–2 wt% with respect to polymer) can be incorporated into conventional polymer structures, including Psf,47,48 PES,49 polyvinylidene fluoride (PVDF)50 ultrafiltration and polyamide RO membranes51,52 (Fig. 2d). GO is hypothesized to migrate to the top (membrane) surface during the phase inversion, making it more hydrophilic, which is supported by the observation of an average decrease of ca. 20° in water contacting angle measurements. In addition to increase in surface hydrophilicity, overall porosity also increased, and as a result, 2–20 fold enhancements in water fluxes have been observed (due to GO additions).48,49 Rejection improvement can vary from a few percent49 to almost 3 times,50 depending on the polymers, GO percentage, and test foulants. Generally, optimal GO percentage balances water permeability and rejection rates, which conforms to the classical trade-off between permeability and selectivity associated with nano- to ultrafiltration membranes. With regard to RO technologies, size-fractionated GO (10–200 nm) was dispersed in the aqueous solution of m-phenylenediamine (MPD) before the interfacial polymerization to make GO-embedded polyamide layers. Water permeability and anti-biofouling property were found to have enhanced by approximately 80% and 98% (based on the biovolume), respectively, without loss of salt rejection.51 More recently, a similar study revealed an about 50% increase in water flux while with only a slight decrease in salt rejection.52To date, most filler-based membranes have been demonstrated via phase inversion processes for the fabrication of UF/NF membranes and only a few focused on impregnation of GO into the polyamide layer with an interfacial polymerization process. The most distinctive advantage for GO as nanofillers is the ease with which it can be readily integrated into current state-of-art technologies for membrane fabrication, including phase inversion or interfacial polymerization. For these, fundamental questions remain, including the relationship(s) of GO properties (size, surface chemistry, etc.) with fabrication processes and the performance, in addition to better dispersion approaches of GO or GO nanocomposites in polymeric solutions. Further, increase in water flux for this type of membrane can, in some cases, arise from passage around the nanomaterial-polymer interface and thus proper solution tuning and material functionalization are needed to minimize such defect-based formation(s) and associated processes. Moreover, top interfacial ‘skin’ layers can be selectively engineered instead of the entire membrane structure to reduce material usage/cost. In general, a detailed understanding of effects of GO addition on thermodynamic and kinetic aspects of the phase inversion and interfacial polymerization processes must be fundamentally elucidated.
4. Conclusion
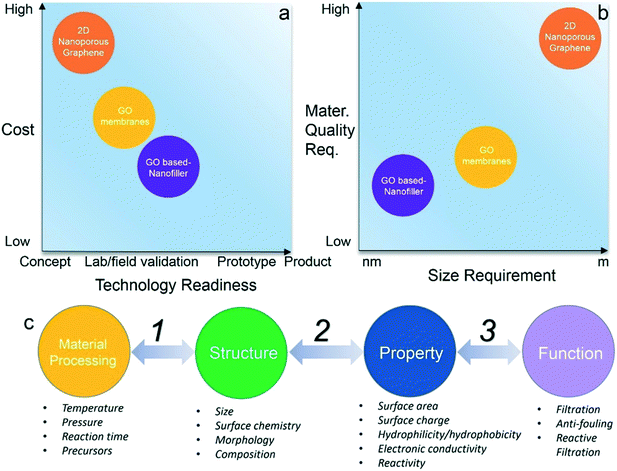
The annual production of graphene materials is estimated to increase by over 10 times to reach a thousand-ton level within the next 5 years.53 Early-stage market demands will likely come from niche applications that present low barriers to adoption, while meeting unmet technology needs. For graphene and graphene oxides, such opportunities will likely include membranes and filtration system for water purification, like most recently the commercialization of TFN membranes.53 Although most graphene-enabled water membrane technologies are still in the research & development stage, it is expected that pilot level demonstrations aimed towards the commercial market will grow rapidly (Fig. 3a). Although maturation and commercialization of technologies are difficult to predict – and nanoporous graphene membranes are at the earliest stage of all technologies discussed – we view them as the most promising of these technologies. However, due to comparatively low cost and relatively easier system integration, GO-based membrane strategies are expected to have more immediate market potential.To reach stated potential, economic and large-scale production of graphene materials that are tailorable for membrane applications is necessary. These include nanoporous graphene, GO, and GO nanocomposites, with varied size and quality requirements (Fig. 3b). More data/information is needed to establish the understanding of material processing, material structure and properties, membrane applications, and their relationships (steps 1–3 in Fig. 3c). For example, correlation of material structures with desired properties, and further translation of these properties into practical applications through integration of graphene and support membranes. Finally, additional research is needed to evaluate the cost-effectiveness of large scale membrane fabrication, and monitoring of the long-term stability of membranes under practical application conditions.
Acknowledgements
This work was supported by the National Science Foundation's CAREER Award (CBET 1454656) and the Mindlin Foundation.References
- B.-H. Jeong, E. M. V. Hoek, Y. Yan, A. Subramani, X. Huang, G. Hurwitz, A. K. Ghosh and A. Jawor, J. Membr. Sci., 2007, 294, 1–7 CrossRef CAS.
- W. Ren and H.-M. Cheng, Nat. Nanotechnol., 2014, 9, 726–730 CrossRef CAS PubMed.
- J. K. Holt, H. G. Park, Y. Wang, M. Stadermann, A. B. Artyukhin, C. P. Grigoropoulos, A. Noy and O. Bakajin, Science, 2006, 312, 1034–1037 CrossRef CAS PubMed.
- R. Arvidsson, D. Kushnir, B. A. Sandén and S. Molander, Environ. Sci. Technol., 2014, 48, 4529–4536 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- X. Li, W. Cai, J. An, S. Kim, J. Nah, D. Yang, R. Piner, A. Velamakanni, I. Jung, E. Tutuc, S. K. Banerjee, L. Colombo and R. S. Ruoff, Science, 2009, 324, 1312–1314 CrossRef CAS PubMed.
- D. Li, M. B. Muller, S. Gilje, R. B. Kaner and G. G. Wallace, Nat. Nanotechnol., 2008, 3, 101–105 CrossRef CAS PubMed.
- K. A. Ritter and J. W. Lyding, Nat. Mater., 2009, 8, 235–242 CrossRef CAS PubMed.
- S. Bae, H. Kim, Y. Lee, X. Xu, J.-S. Park, Y. Zheng, J. Balakrishnan, T. Lei, H. Ri Kim, Y. I. Song, Y.-J. Kim, K. S. Kim, B. Ozyilmaz, J.-H. Ahn, B. H. Hong and S. Iijima, Nat. Nanotechnol., 2010, 5, 574–578 CrossRef CAS PubMed.
- K. A. Mkhoyan, A. W. Contryman, J. Silcox, D. A. Stewart, G. Eda, C. Mattevi, S. Miller and M. Chhowalla, Nano Lett., 2009, 9, 1058–1063 CrossRef CAS PubMed.
- W. Cai, R. D. Piner, F. J. Stadermann, S. Park, M. A. Shaibat, Y. Ishii, D. Yang, A. Velamakanni, S. J. An, M. Stoller, J. An, D. Chen and R. S. Ruoff, Science, 2008, 321, 1815–1817 CrossRef CAS PubMed.
- A. Bagri, C. Mattevi, M. Acik, Y. J. Chabal, M. Chhowalla and V. B. Shenoy, Nat. Chem., 2010, 2, 581–587 CrossRef CAS PubMed.
- Y. Jiang, R. Raliya, J. D. Fortner and P. Biswas, Environ. Sci. Technol., 2016, 50, 6964–6973 CrossRef CAS PubMed.
- J. Luo, H. D. Jang, T. Sun, L. Xiao, Z. He, A. P. Katsoulidis, M. G. Kanatzidis, J. M. Gibson and J. Huang, ACS Nano, 2011, 5, 8943–8949 CrossRef CAS PubMed.
- W.-N. Wang, Y. Jiang and P. Biswas, J. Phys. Chem. Lett., 2012, 3, 3228–3233 CrossRef CAS PubMed.
- M. C. Wang, S. Chun, R. S. Han, A. Ashraf, P. Kang and S. Nam, Nano Lett., 2015, 15, 1829–1835 CrossRef CAS PubMed.
- O. Akhavan and E. Ghaderi, ACS Nano, 2010, 4, 5731–5736 CrossRef CAS PubMed.
- F. Perreault, A. F. de Faria, S. Nejati and M. Elimelech, ACS Nano, 2015, 9, 7226–7236 CrossRef CAS PubMed.
- Y. Tu, M. Lv, P. Xiu, T. Huynh, M. Zhang, M. Castelli, Z. Liu, Q. Huang, C. Fan, H. Fang and R. Zhou, Nat. Nanotechnol., 2013, 8, 594–601 CrossRef CAS PubMed.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- V. Chandra, J. Park, Y. Chun, J. W. Lee, I.-C. Hwang and K. S. Kim, ACS Nano, 2010, 4, 3979–3986 CrossRef CAS PubMed.
- H. Zhang, X. J. Lv, Y. M. Li, Y. Wang and J. H. Li, ACS Nano, 2010, 4, 380–386 CrossRef CAS PubMed.
- Y. Jiang, W.-N. Wang, D. Liu, Y. Nie, W. Li, J. Wu, F. Zhang, P. Biswas and J. D. Fortner, Environ. Sci. Technol., 2015, 49, 6846–6854 CrossRef CAS PubMed.
- J. R. Ray, S. Tadepalli, S. Z. Nergiz, K.-K. Liu, L. You, Y. Tang, S. Singamaneni and Y.-S. Jun, ACS Appl. Mater. Interfaces, 2015, 7, 11117–11126 CAS.
- Y. Jiang, W.-N. Wang, P. Biswas and J. D. Fortner, ACS Appl. Mater. Interfaces, 2014, 6, 11766–11774 CAS.
- Y. Chen, F. Guo, Y. Qiu, H. Hu, I. Kulaots, E. Walsh and R. H. Hurt, ACS Nano, 2013, 7, 3744–3753 CrossRef CAS PubMed.
- J. S. Bunch, S. S. Verbridge, J. S. Alden, A. M. van der Zande, J. M. Parpia, H. G. Craighead and P. L. McEuen, Nano Lett., 2008, 8, 2458–2462 CrossRef CAS PubMed.
- D.-E. Jiang, V. R. Cooper and S. Dai, Nano Lett., 2009, 9, 4019–4024 CrossRef CAS PubMed.
- D. Cohen-Tanugi and J. C. Grossman, Nano Lett., 2012, 12, 3602–3608 CrossRef CAS PubMed.
- S. P. Surwade, S. N. Smirnov, I. V. Vlassiouk, R. R. Unocic, G. M. Veith, S. Dai and S. M. Mahurin, Nat. Nanotechnol., 2015, 10, 459–464 CrossRef CAS PubMed.
- D. Cohen-Tanugi and J. C. Grossman, Desalination, 2015, 366, 59–70 CrossRef CAS.
- S. C. O'Hern, M. S. H. Boutilier, J.-C. Idrobo, Y. Song, J. Kong, T. Laoui, M. Atieh and R. Karnik, Nano Lett., 2014, 14, 1234–1241 CrossRef PubMed.
- K. A. Mahmoud, B. Mansoor, A. Mansour and M. Khraisheh, Desalination, 2015, 356, 208–225 CrossRef CAS.
- D. A. Dikin, S. Stankovich, E. J. Zimney, R. D. Piner, G. H. B. Dommett, G. Evmenenko, S. T. Nguyen and R. S. Ruoff, Nature, 2007, 448, 457–460 CrossRef CAS PubMed.
- A. R. Koltonow and J. Huang, Science, 2016, 351, 1395–1396 CrossRef CAS PubMed.
- R. R. Nair, H. A. Wu, P. N. Jayaram, I. V. Grigorieva and A. K. Geim, Science, 2012, 335, 442–444 CrossRef CAS PubMed.
- R. K. Joshi, P. Carbone, F. C. Wang, V. G. Kravets, Y. Su, I. V. Grigorieva, H. A. Wu, A. K. Geim and R. R. Nair, Science, 2014, 343, 752–754 CrossRef CAS PubMed.
- H. Huang, Z. Song, N. Wei, L. Shi, Y. Mao, Y. Ying, L. Sun, Z. Xu and X. Peng, Nat. Commun., 2013, 4, 2979 Search PubMed.
- Y. Han, Y. Jiang and C. Gao, ACS Appl. Mater. Interfaces, 2015, 7, 8147–8155 CAS.
- M. Hu and B. Mi, Environ. Sci. Technol., 2013, 47, 3715–3723 CrossRef CAS PubMed.
- Y. Jiang, D. Liu, M. Cho, S. S. Lee, F. Zhang, P. Biswas and J. D. Fortner, Environ. Sci. Technol., 2016, 50, 2514–2521 CrossRef CAS PubMed.
- W. Wang, E. Eftekhari, G. Zhu, X. Zhang, Z. Yan and Q. Li, Chem. Commun., 2014, 50, 13089–13092 RSC.
- F. Perreault, M. E. Tousley and M. Elimelech, Environ. Sci. Technol. Lett., 2013, 1, 71–76 CrossRef.
- Y. Gao, M. Hu and B. Mi, J. Membr. Sci., 2014, 455, 349–356 CrossRef CAS.
- L. M. Pastrana-Martínez, S. Morales-Torres, J. L. Figueiredo, J. L. Faria and A. M. T. Silva, Water Res., 2015, 77, 179–190 CrossRef PubMed.
- A. Lee, J. W. Elam and S. B. Darling, Environ. Sci.: Water Res. Technol., 2016, 2, 17–42 CAS.
- B. M. Ganesh, A. M. Isloor and A. F. Ismail, Desalination, 2013, 313, 199–207 CrossRef CAS.
- C. A. Crock, A. R. Rogensues, W. Shan and V. V. Tarabara, Water Res., 2013, 47, 3984–3996 CrossRef CAS PubMed.
- S. Zinadini, A. A. Zinatizadeh, M. Rahimi, V. Vatanpour and H. Zangeneh, J. Membr. Sci., 2014, 453, 292–301 CrossRef CAS.
- J. Zhang, Z. Xu, W. Mai, C. Min, B. Zhou, M. Shan, Y. Li, C. Yang, Z. Wang and X. Qian, J. Mater. Chem. A, 2013, 1, 3101–3111 CAS.
- H.-R. Chae, J. Lee, C.-H. Lee, I.-C. Kim and P.-K. Park, J. Membr. Sci., 2015, 483, 128–135 CrossRef CAS.
- J. Yin, G. Zhu and B. Deng, Desalination, 2016, 379, 93–101 CrossRef CAS.
- A. Zurutuza and C. Marinelli, Nat. Nanotechnol., 2014, 9, 730–734 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |